Abstract
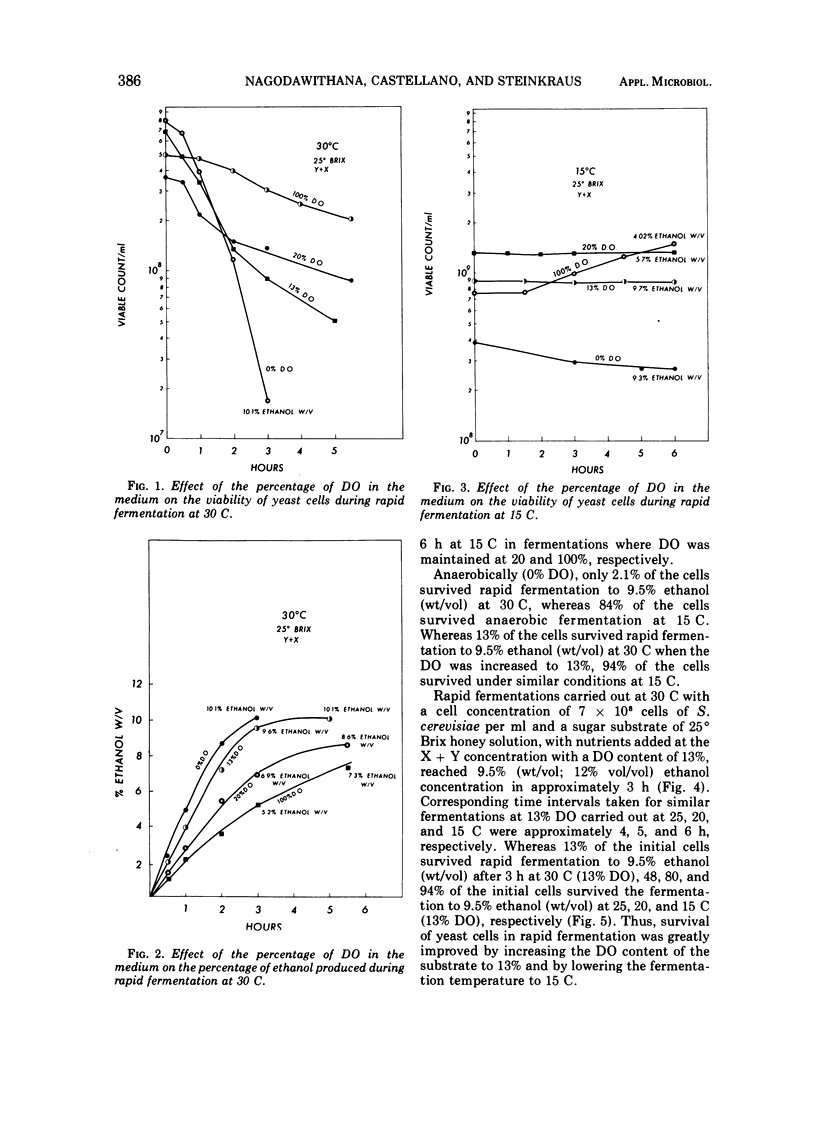
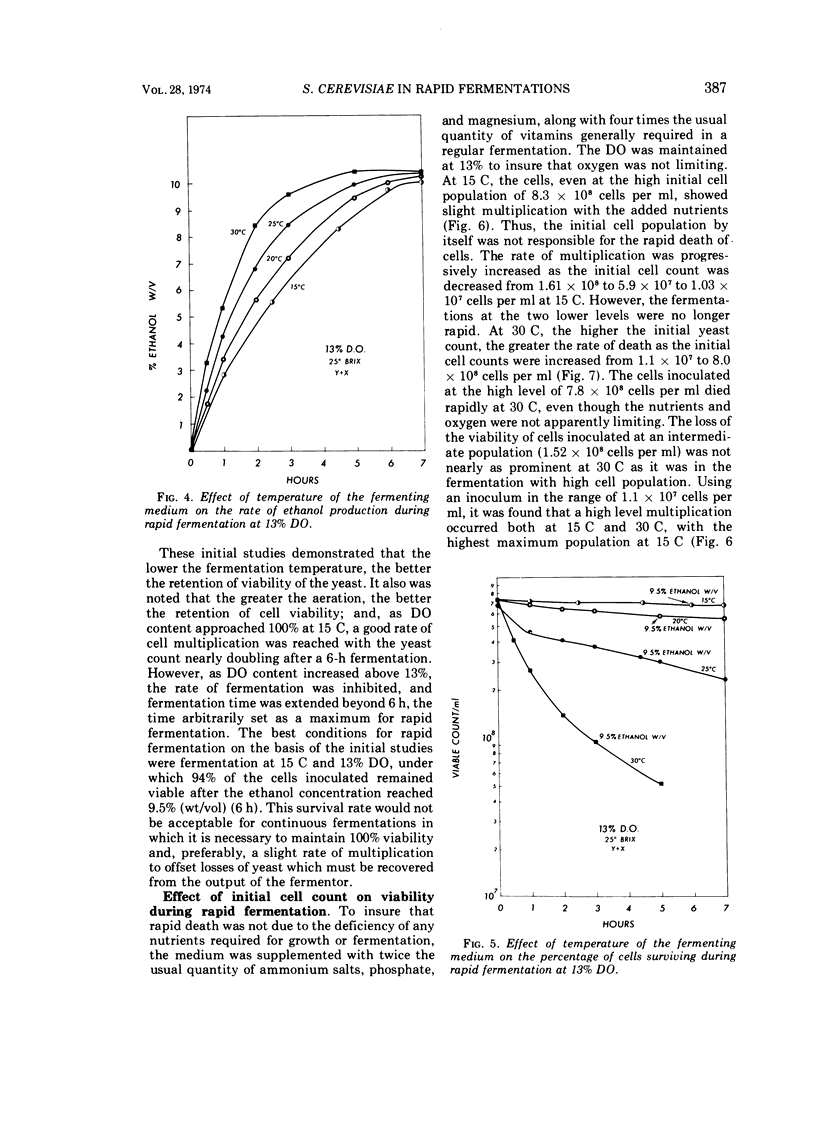
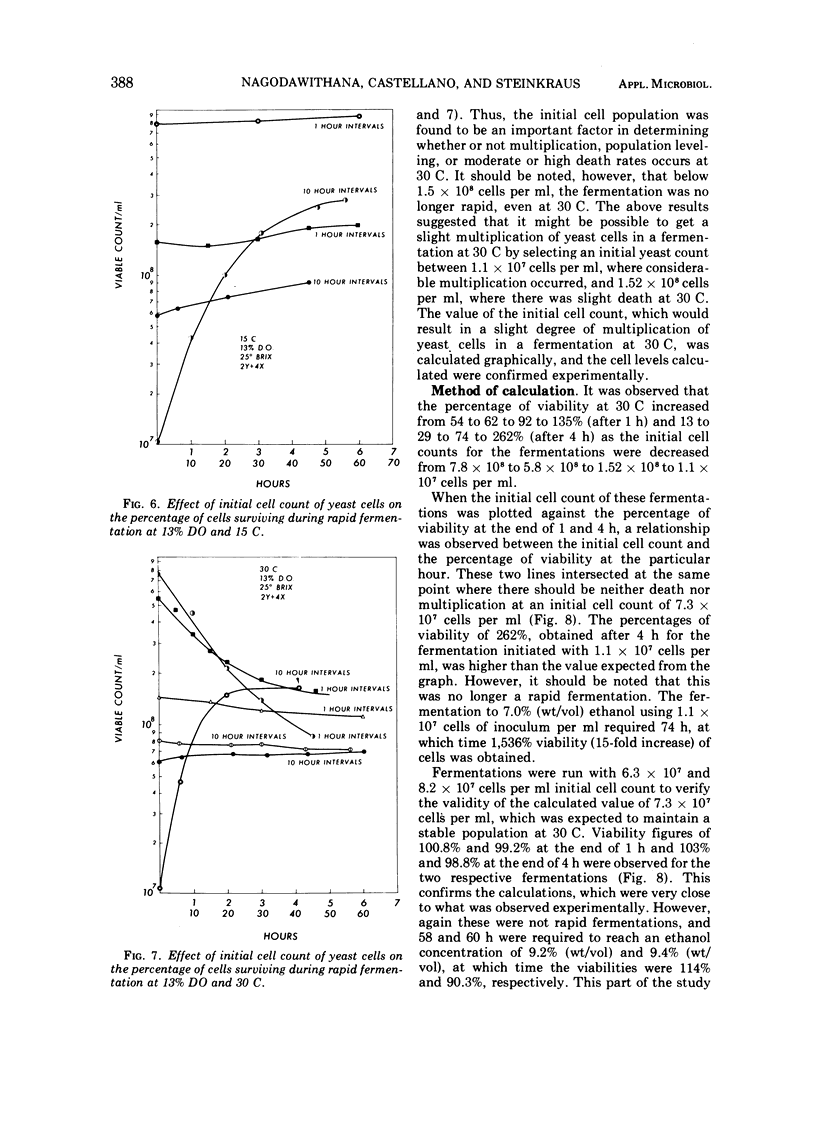
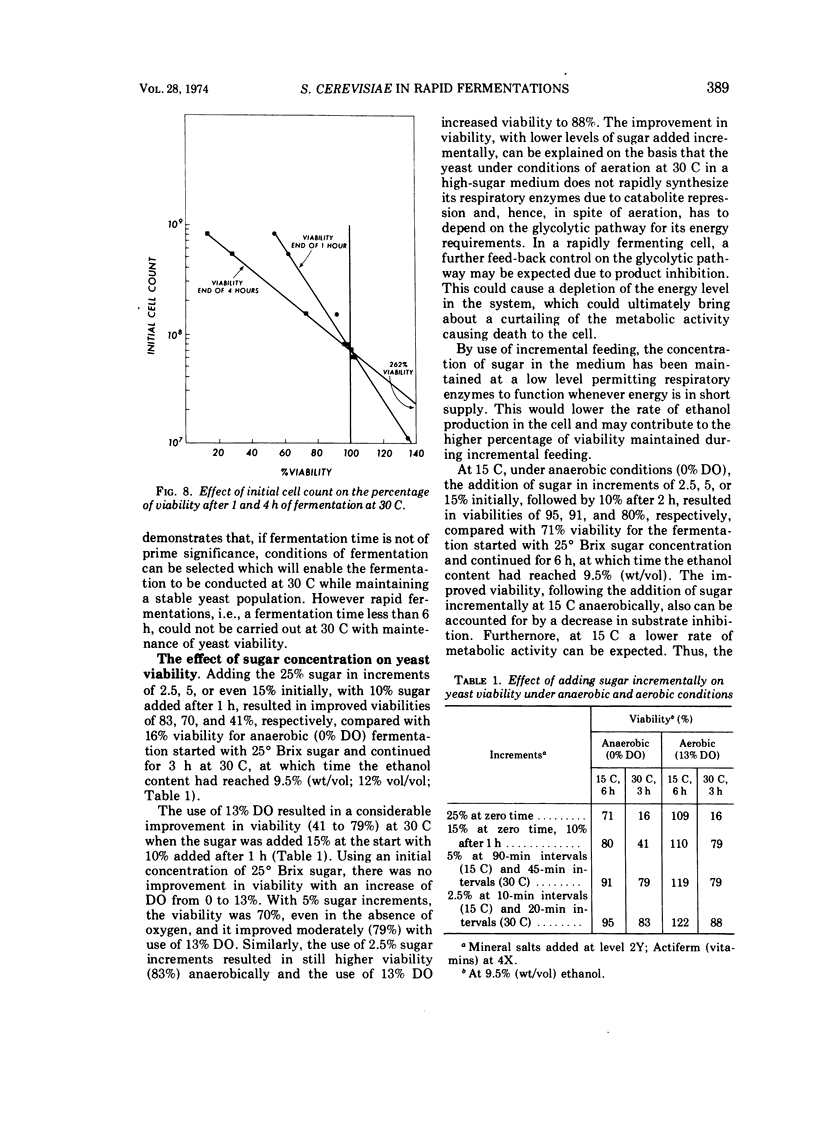
By using 7 × 108 cells of Saccharomyces cerevisiae per ml with which 25° Brix honey solutions were fermented to 9.5% (wt/vol; 12% vol/vol) ethanol in 2.5 to 3 h at 30 C, i.e., rapid fermentation, the death rate was found to be high, with only 2.1% of the yeast cells surviving at the end of 3 h under anaerobic conditions. As the dissolved oxygen in the medium was increased from 0 to 13 to 20 to 100% in rapid fermentations at 30 C, there was a progressive increase in the percentage of cells surviving. The ethanol production rate and total were not seriously affected by a dissolved oxygen concentration of 13%, but fermentation was retarded by 20% dissolved oxygen and still further decreased as the dissolved oxygen content reached 100%. When the fermentation temperature was decreased to 15 C (at 13% dissolved oxygen), the rate of fermentation decreased, and the fermentation time to 9.5% ethanol (wt/vol) increased to 6 h. It was found that the higher the temperature between 15 and 30 C, the greater the rate of death as initial cell counts were increased from 1.1 × 107 to 7.8 × 108 cells per ml. At the lowest level of inoculum, 1.1 × 107 cells per ml, there was actual multiplication, even at 30 C; however, the fermentation was no longer rapid. The addition of 15% sugar, initially followed after an hour by the remaining 10%, or addition of the sugar in increments of 2.5 or 5% yielded a better survival rate of yeast cells than when the fermentation was initiated with 25% sugar.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREASEN A. A., STIER T. J. Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Physiol. 1954 Jun;43(3):271–281. doi: 10.1002/jcp.1030430303. [DOI] [PubMed] [Google Scholar]
- Farrell J., Rose A. Temperature effects on microorganisms. Annu Rev Microbiol. 1967;21:101–120. doi: 10.1146/annurev.mi.21.100167.000533. [DOI] [PubMed] [Google Scholar]
- LEMOIGNE M., AUBERT J. P., MILLET J. La production d'alcool et le rendement de croissance de la levure de boulangerie cultivée en aérobiose. Ann Inst Pasteur (Paris) 1954 Oct;87(4):427–439. [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- Swanson W. H., Clifton C. E. Growth and Assimilation in Cultures of Saccharomyces cerevisiae. J Bacteriol. 1948 Jul;56(1):115–124. doi: 10.1128/jb.56.1.115-124.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Uden N., Abranches P., Cabeça-Silva C. Temperature functions of thermal death in yeasts and their relation to the maximum temperature for growth. Arch Mikrobiol. 1968;61(4):381–393. doi: 10.1007/BF00409674. [DOI] [PubMed] [Google Scholar]
- van Uden N., Madeira-Lopes A. Concurrent exponential growth and death of cell populations of Saccharomyces cerevisiae at superoptimal growth temperatures. Z Allg Mikrobiol. 1970;10(7):515–526. [PubMed] [Google Scholar]


