Abstract
Two Arabidopsis thaliana genes have been shown to function in vacuolar sorting of seed storage proteins: a vacuolar sorting receptor, VSR1/ATELP1, and a retromer component, MAIGO1 (MAG1)/VPS29. Here, we show an efficient and simple method for isolating vacuolar sorting mutants of Arabidopsis. The method was based on two findings in this study. First, VSR1 functioned as a sorting receptor for β-conglycinin by recognizing the vacuolar targeting signal. Second, when green fluorescent protein (GFP) fusion with the signal (GFP-CT24) was expressed in vsr1, mag1/vps29, and wild-type seeds, both vsr1and mag1/vps29 gave strongly fluorescent seeds but the wild type did not, suggesting that a defect in vacuolar sorting provided fluorescent seeds by the secretion of GFP-CT24 out of the cells. We mutagenized transformant seeds expressing GFP-CT24. From ∼3,000,000 lines of M2 seeds, we obtained >100 fluorescent seeds and designated them green fluorescent seed (gfs) mutants. We report 10 gfs mutants, all of which caused missorting of storage proteins. We mapped gfs1 to VSR1, gfs2 to KAM2/GRV2, gfs10 to the At4g35870 gene encoding a novel membrane protein, and the others to different loci. This method should provide valuable insights into the complex molecular mechanisms underlying vacuolar sorting of storage proteins.
INTRODUCTION
Higher plants synthesize large amounts of storage proteins that account for almost 80% of the total protein in seeds during seed maturation. In maturing seeds, most storage proteins are synthesized in the endoplasmic reticulum (ER) as precursors, which are transported into protein storage vacuoles (PSVs), where they are converted into mature forms (Hara-Nishimura et al., 1993; Hara-Nishimura and Maeshima, 2000; Shimada et al., 2003b). Multiple pathways are used for the transport of storage proteins into PSVs (Vitale and Hinz, 2005). One of the pathways is an aggregation sorting that is mediated by precursor-accumulating (PAC) vesicles that are involved in the mass transport of storage proteins to PSVs (Hara-Nishimura et al., 2004). A type I membrane protein, PV72, was found on the membrane of the PAC (Shimada et al., 1997, 2002). Arabidopsis thaliana has seven homologs of PV72, which we designated At VSR1 to At VSR7 (for vacuolar sorting receptor of Arabidopsis) (Shimada et al., 2003a). The homolog most closely related to PV72 was designated At VSR1, which is also referred to as ATELP (Ahmed et al., 1997). We demonstrated that VSR1 functions as a receptor to sort seed storage proteins to PSVs (Shimada et al., 2003a). vsr1 missorts storage proteins by secreting them from cells, resulting in an enlarged and electron-dense extracellular space in the seeds. The vsr1 seeds abnormally accumulate the precursors of two major storage proteins, 12S globulin and 2S albumin. These findings show a receptor-mediated transport of seed storage proteins to PSVs in higher plants.
Sorting of vacuolar proteins requires the interaction between a vacuolar targeting signal and a sorting receptor that recognizes the signal (Kirsch et al., 1994, 1996). Despite many articles that discuss the interactions between VSR and a vacuolar targeting signal (Kirsch et al., 1994; Sanderfoot et al., 1998; Ahmed et al., 2000; Laval et al., 2003), there have been only a few in vivo demonstrations of the interaction between VSR and the determined vacuolar targeting signals: between VSR1 and an N-terminal propeptide of either aleurain or sporamin (Ahmed et al., 2000; Watanabe et al., 2004) and between pea (Pisum sativum) BP-80 and the N-terminal propeptide of sporamin (daSilva et al., 2005). Vacuolar targeting signals for PSVs have been found in soybean (Glycine max) β-conglycinin (Nishizawa et al., 2003, 2004), common bean (Phaseolus vulgaris) phaseolin (Frigerio et al., 1998, 2001b), 2S albumin of Brazil nut (Saalbach et al., 1996), barley (Hordeum vulgare) lectin (Muntz, 1998), and castor bean (Ricinus communis) ricin (Frigerio et al., 2001a). These signals exhibit no consensus amino acid sequence. It has been shown that VSR binds to 2S albumin signals (Shimada et al., 2003a; Jolliffe et al., 2004) and the ricin signal (Jolliffe et al., 2004). The question raised is whether VSR binds to these different signals. It was also reported that At RMR1 (for Arabidopsis receptor homology region transmembrane domain ring H2 motif protein1) interacts with the C-terminal sorting domain of phaseolin, which also belongs to the 7S globulin family and is a homolog of β-conglycinin (Park et al., 2005). A reverse genetic analysis with an RMR1-deficient mutant is necessary to demonstrate that RMR1 functions as a sorting receptor for 7S globulins.
Both VSR1 and PV72 bind to peptides derived from storage proteins, including 2S albumin (Watanabe et al., 2002) and 12S globulin (Shimada et al., 2003a), in a Ca2+-dependent manner. Both VSRs have a binding ability not only to the storage protein peptides but also to a vacuolar targeting signal for lytic vacuoles (Watanabe et al., 2002; Shimada et al., 2003a). Overexpression of the ER-localized soluble form of a seed-specific VSR (PV72) interfered with the transport of a lytic vacuolar protein (ALEU) in Arabidopsis leaves (Watanabe et al., 2004). This means that both VSR for PSVs and VSR for lytic vacuoles share a similar mechanism for recognizing a vacuolar targeting signal.
Although VSR is unique to plants, VSR has a short cytosolic tail containing the sequence YMPL, which is similar to Tyr-based motifs (YXXΦ, in which X represents any amino acid and Φ represents a hydrophobic residue with a bulky side chain) of yeast VPS10 (Cooper and Stevens, 1996) and mammalian mannose 6-phosphate receptor (Honing et al., 1997). The Tyr-based motif is recognized by the adapter complex of clathrin-coated vesicles in yeast (Deloche et al., 2001) and animals (Klumperman et al., 1998). Recently, the Tyr-based motif of BP-80 was reported to be required for the transport of vacuolar proteins (daSilva et al., 2006).
We recently isolated a mutant (maigo1 [mag1]) that missorts storage proteins by secreting them out of the seed cells and identified the responsible gene as At3g47810, which encodes a homolog of the yeast retromer component VPS29 (Shimada et al., 2006). MAG1/VPS29 might be involved in recycling VSR1 for the efficient sorting of seed storage proteins. VSR1 and MAG1/VPS29 are the only factors that have been demonstrated in planta to function in vacuolar sorting of seed storage proteins, although VACUOLELESS (Rojo et al., 2001) and DRP2A (ADL6) (Jin et al., 2001) were reported to be involved in trafficking to lytic vacuoles. Forward genetics should be a useful approach to fully clarify the sorting machinery for PSVs. We have isolated vacuolar sorting mutants (mag) that accumulate storage protein precursors in seeds (Shimada et al., 2006; Li et al., 2006). In this study, we established a more efficient and easier method (green fluorescent seed [gfs] method) of isolating vacuolar sorting mutants and identified factors for vacuolar sorting of storage proteins.
RESULTS
VSR1 Is Involved in Vacuolar Sorting of the Exogenously Expressed α′-Subunit of β-Conglycinin by Recognizing the C-Terminal Vacuolar Targeting Signal in Arabidopsis Seeds
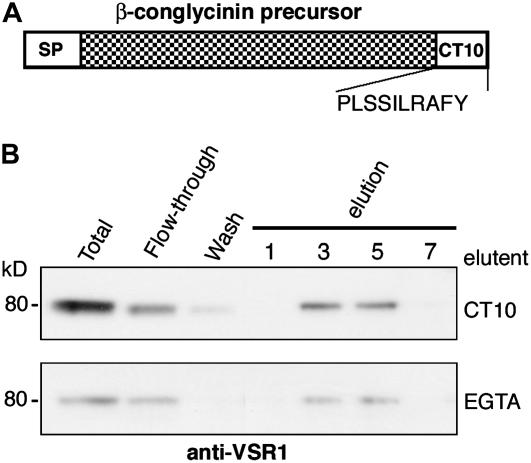
Two vacuolar targeting signals have been identified in 7S globulins. One is the 10–amino acid C-terminal peptide (CT10; Figure 1A) of the α′-subunit of β-conglycinin of soybean (Nishizawa et al., 2003), and the other is the 4–amino acid C-terminal peptide of phaseolin of common bean (Frigerio et al., 1998). In Arabidopsis seeds, CT10 functions as a necessary and sufficient vacuolar targeting signal (Nishizawa et al., 2003). To determine whether VSR binds to the CT10 of β-conglycinin, we applied microsomal proteins from Arabidopsis siliques, which contained VSR1, to an affinity column conjugated with CT10. VSR1 bound to CT10 in the presence of 1 mM CaCl2 and was eluted with CT10 or EGTA (Figure 1B), indicating that VSR1 binds to the vacuolar targeting signal of β-conglycinin in a Ca2+-dependent manner. This means that VSR1 binds to the targeting signal of β-conglycinin, as it binds to the signal of the storage protein 2S albumin (Shimada et al., 2003a), even though the targeting signals of these storage proteins have no consensus amino acid sequences.
Figure 1.
VSR1 Can Bind a Vacuolar Targeting Signal of a Soybean Seed Storage Protein (β-Conglycinin) in a Calcium-Dependent Manner.
(A) Scheme of the preproprotein precursor of the soybean β-conglycinin α′-subunit, which contains a signal peptide (SP) at the N terminus and a vacuolar targeting signal composed of 10 amino acids (CT10) at the C terminus.
(B) Affinity chromatography with a CT10-conjugated column. Microsomes were prepared from Arabidopsis siliques including maturing seeds and were applied to the affinity column in the presence of 1 mM CaCl2. The bound VSR1 was eluted competitively with the CT10 peptide and was also eluted with EGTA. Total microsomes, the flow-through fraction, the wash fraction, and the eluted fractions (fractions 1, 3, 5, and 7) were subjected to immunoblot analysis with anti-VSR1 antibody. Molecular masses are given at left.
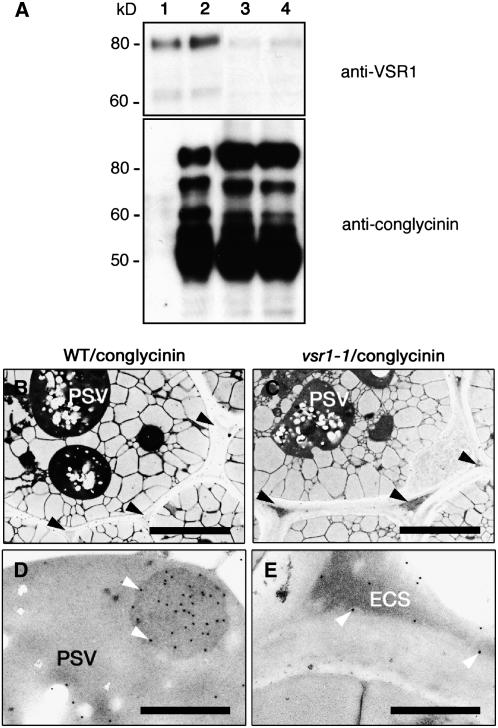
To determine whether VSR is required for vacuolar sorting of β-conglycinin in Arabidopsis seeds, we expressed β-conglycinin exogenously under the control of a seed-specific promoter in the wild type (WT; Columbia) to produce the transformed plant WT/conglycinin and in two vsr1 mutants to produce the transformed plants vsr1-1/conglycinin and vsr1-2/conglycinin. WT/conglycinin seeds gave several bands on an immunoblot with anti-β-conglycinin antibody (Figure 2A, lane 2), although nontransformant seeds gave no signal on the blot (Figure 2A, lane 1). β-Conglycinin, when expressed in Arabidopsis seeds, was shown to be transported to PSVs and to be partially degraded to give several bands on the immunoblot, probably because it is less stable in Arabidopsis PSVs than in soybean PSVs (Nishizawa et al., 2003). The level of the nonprocessed form of β-conglycinin was higher in both vsr1-1 and vsr1-2 seeds than in wild-type seeds, although the protein profiles of β-conglycinin in the mutants and the wild type were similar (Figure 2A).
Figure 2.
β-Conglycinin Is Missorted by Secreting Out of the Cells When It Is Expressed in vsr1 Seeds.
(A) β-Conglycinin was expressed in both the wild type (Columbia) and vsr1 mutants under the control of a seed-specific promoter. Seeds of nontransformant (lane 1), WT/conglycinin (lane 2), vsr1-1/conglycinin (lane 3), and vsr1-2/conglycinin (lane 4) plants were subjected to immunoblot analysis with either anti-VSR1 antibody or anti-β-conglycinin antibody. Molecular masses are given at left.
(B) and (C) Ultrastructures of the seeds of both WT/conglycinin (B) and vsr1-1/conglycinin (C) plants. The extracellular space of the transformed vsr1-1 seeds was abnormally enlarged and filled with electron-dense material. Arrowheads indicate the extracellular spaces. Bars = 5 μm.
(D) and (E) Immunoelectron micrographs of WT/conglycinin (D) and vsr1-1/conglycinin (E) seeds with anti-β-conglycinin antibody. Exogenously expressed β-conglycinin was localized in the electron-dense extracellular space (ECS) of vsr1-1/conglycinin seeds (white arrowheads in [E]), whereas it was localized in PSVs of WT/conglycinin seeds (white arrowheads in [D]). Bars = 1 μm.
The extracellular space of the vsr1-1/conglycinin seeds was abnormally enlarged and filled with electron-dense material (Figure 2C). To determine the localization of β-conglycinin, we performed immunocytochemistry with an anti-β-conglycinin antibody that does not give any signal on nontransformant seeds (Nishizawa et al., 2003). β-Conglycinin was distributed in the electron-dense region of the extracellular space in the vsr1-1/conglycinin seeds (Figure 2E), whereas β-conglycinin was localized in the aggregates within PSVs of WT/conglycinin seeds (Figure 2D). We previously showed that vsr1 mutants missort the storage proteins 12S globulin and 2S albumin by secreting them out of seed cells (Shimada et al., 2003a). Our findings revealed that β-conglycinin, like authentic storage proteins, is abnormally secreted in vsr1 seeds, indicating that VSR is involved in the vacuolar sorting of β-conglycinin in maturing seeds of Arabidopsis.
vsr1 Generates Green Fluorescent Seeds by Secreting the Green Fluorescent Protein Fusion Protein with the Vacuolar Targeting Signal CT24 of β-Conglycinin
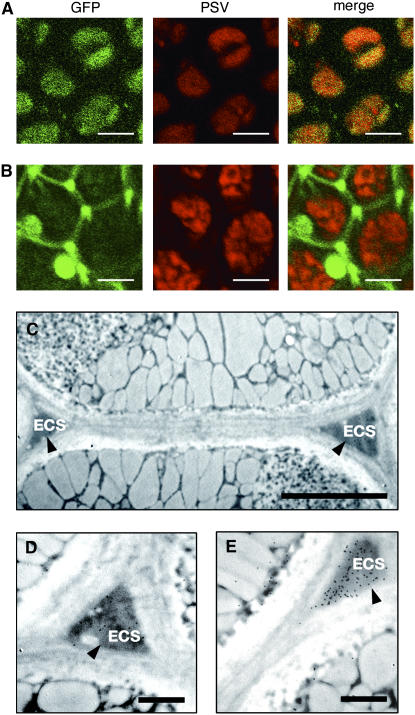
Previously, we generated an Arabidopsis transformant (WT/GFP-CT24) that expressed SP-GFP-CT24, which was composed of a signal peptide and green fluorescent protein (GFP) followed by the β-conglycinin C-terminal 24 amino acids (CT24), including CT10, under the control of a seed-specific promoter (Nishizawa et al., 2003). WT/GFP-CT24 gave GFP fluorescence that was completely merged with the red autofluorescence of PSVs in seeds, showing that GFP-CT24 was transported into PSVs (Figure 3A).
Figure 3.
A GFP Fusion Protein with a Vacuolar Targeting Signal Is Secreted out of the Cells When Expressed in vsr1 Seeds but Is Localized in PSVs When Expressed in Wild-Type Seeds.
(A) and (B) GFP-CT24 was expressed in the wild type (WT/GFP-CT24; [A]) and the vsr1-1 mutant (vsr1-1/GFP-CT24; [B]). The seeds were inspected with a confocal laser scanning microscope to obtain GFP fluorescent images showing the localization of GFP-CT24 (GFP) and autofluorescent image of PSVs. Both images were then merged. GFP fluorescence was detected in PSVs of WT/GFP-CT24 seeds (A), whereas a higher intensity of GFP fluorescence was detected in the extracellular space of vsr1-1/GFP-CT24 seeds (B). Bars = 5 μm.
(C) Immunoelectron micrograph of vsr1-1/GFP-CT24 seeds with anti-GFP antibody, showing that the GFP fusion was localized in the extracellular space (ECS; arrowheads). Bar = 2 μm.
(D) Enlarged image of an immunoelectron micrograph of vsr1-1/GFP-CT24 seeds with anti-GFP antibody, showing the distribution of the GFP fusion in the extracellular space (ECS; arrowhead). Bar = 1 μm.
(E) Immunoelectron micrograph of vsr1-1/GFP-CT24 seeds with anti-12S globulin antibody, showing the secretion of 12S globulin in the vsr1-1 seeds (ECS; arrowhead). Bar = 1 μm.
We generated vsr1-1/GFP-CT24 and vsr1-2/GFP-CT24 by crossing each of two vsr1 mutants (vsr1-1 and vsr1-2) with WT/GFP-CT24, respectively. Figure 3B shows that vsr1-1/GFP-CT24 gave a much higher intensity of GFP fluorescence in the extracellular space than WT/GFP-CT24, suggesting that most of the GFP-CT24 molecules were secreted out of the vsr1 seed cells. Immunocytochemistry revealed that GFP-CT24 (Figures 3C and 3D) and 12S globulin of the authentic storage proteins (Figure 3E) were detected in the electron-dense and enlarged extracellular space of vsr1-1 seeds. vsr1-1 missorted GFP-CT24 by secreting it out of the seed cells together with the authentic storage proteins. vsr1-2 gave the same result as vsr1-1 (data not shown). These results indicate that VSR1 sorts and delivers GFP-CT24 to PSVs by recognizing CT24 of β-conglycinin. Considering that VSR1 binds to CT10 of β-conglycinin in vitro (Figure 1B), CT10 might be sufficient to be recognized by VSR1.
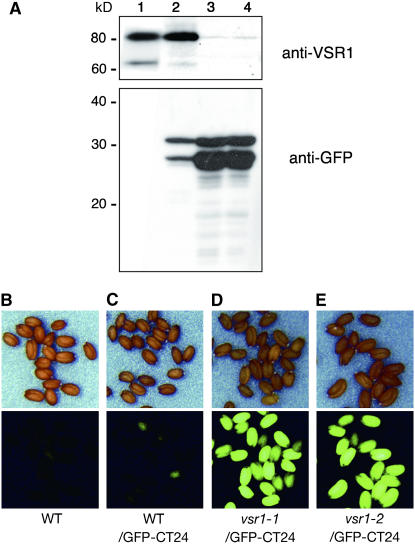
Figure 4A shows an immunoblot of the transformant seeds with anti-GFP antibody to evaluate the amount of GFP. The seeds gave two bands on the blots, which might correspond to 28-kD GFP and 30-kD GFP-CT24. We found that both vsr1-1/GFP-CT24 and vsr1-2/GFP-CT24 accumulated much higher amounts of GFP in their seeds than WT/CT24 (Figure 4A). It is possible that GFP-CT24 is more stable in the extracellular space than in PSVs of Arabidopsis seeds. Interestingly, the high accumulation of GFP in the extracellular space of vsr1 caused the seeds to be fluorescent. Figures 4D and 4E show that all of the seeds of both vsr1-1/GFP-CT24 and vsr1-2/GFP-CT24 exhibited strong GFP fluorescence. On the contrary, the seeds of WT/GFP-CT24, in which GFP was accumulated in PSVs, exhibited extremely low levels of GFP fluorescence (Figure 4C).
Figure 4.
vsr1 Mutants That Express GFP-CT24 Produce Green Fluorescent Seeds by Accumulating a Much Greater Amount of GFP-CT24 in Seeds Than Do Wild-Type Plants That Express GFP-CT24.
(A) Expression levels of GFP-CT24 in the transformed seeds. Seeds of nontransformant (lane 1), WT/GFP-CT24 (lane 2), vsr1-1/GFP-CT24 (lane 3), and vsr1-2/GFP-CT24 (lane 4) plants were subjected to immunoblot analysis with either anti-VSR1 antibody or anti-GFP antibody. Molecular masses are given at left.
(B) to (F) Seeds of wild-type (B), WT/GFP-CT24 (C), vsr1-1/GFP-CT24 (D), and vsr1-2/GFP-CT24 (E) plants were inspected with a binocular microscope (top panels) and a fluorescence microscope (bottom panels). For comparison of the intensities of fluorescence, the photographs were taken under the same conditions. Both vsr1-1/GFP-CT24 and vsr1-2/GFP-CT24 produced green fluorescent seeds.
An Easy Method for Isolating Vacuolar Sorting–Deficient Mutants (gfs)
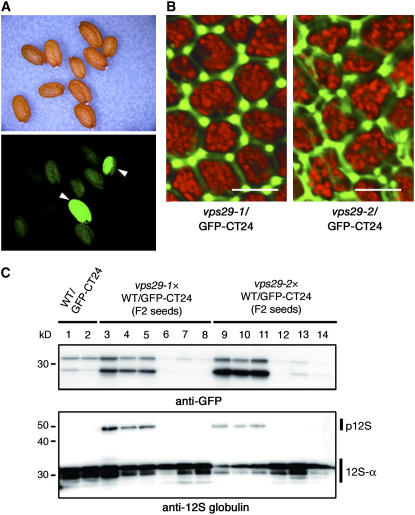
We recently identified another factor, MAG1, for storage protein sorting to PSVs. We found that the mag1 mutant missorts storage proteins by secreting them out of the Arabidopsis seed cells and that MAG1 is a homolog of the yeast retromer component VPS29 (Shimada et al., 2006). To determine whether GFP-CT24 was secreted in mag1/vps29 seeds, we crossed each of two mag1/vps29 alleles (mag1-1/vps29-1 and mag1-2/vps29-2) with WT/GFP-CT24 to produce mag1-1/vps29-1/GFP-CT24 and mag1-2/vps29-2/GFP-CT24, respectively. Figure 5A shows that some seeds of the F2 population fluoresced green. Highly fluorescent seeds of mag1-1/vps29-1/GFP-CT24 and mag1-2/vps29-2/GFP-CT24 exhibited GFP fluorescence in the extracellular space (Figure 5B), as did vsr1/GFP-CT24, suggesting that most of the GFP-CT24 molecules were secreted out of mag1/vps29 seed cells. We selected highly green fluorescent seeds (Figure 5C, lanes 3 to 5 and 9 to 11) and faintly fluorescent seeds (Figure 5C, lanes 6 to 8 and 12 to 14) from the F2 population and subjected them to immunoblot analysis with either anti-GFP antibody or anti-12S globulin antibody. The intensity of GFP fluorescence of the seeds correlated not only with the level of GFP accumulation but also with 12S globulin precursor accumulation (Figure 5C). This finding suggested that the intensity of green fluorescence of the seeds is a useful marker for isolating vacuolar sorting mutant seeds.
Figure 5.
mag1/vps29 Mutants That Express GFP-CT24 Also Produce Green Fluorescent Seeds.
(A) mag1-1/vps29-1 was crossed with WT/GFP-CT24. The seeds of the F2 population were inspected with a binocular microscope (top panel) and a fluorescence microscope (bottom panel). mag1/vps29/GFP-CT24 produced green fluorescent seeds (arrowheads).
(B) Highly fluorescent seeds of mag1-1/vps29-1/GFP-CT24 and mag1-2/vps29-2/GFP-CT24 were inspected with a confocal laser scanning microscope. GFP fluorescence was detected out of the seed cells, showing missorting of GFP-CT24. The GFP fluorescence image was not merged with the autofluorescent image (red) of PSVs. Each image of green and red fluorescence is shown in Supplemental Figure 1 online. Bars = 10 μm.
(C) Selected highly green fluorescent seeds (lanes 3 to 5 and 9 to 11) and faintly fluorescent seeds (lanes 6 to 8 and 12 to 14) from the F2 population were subjected to immunoblot analysis with anti-GFP and anti-12S globulin antibodies. The intensity of the GFP fluorescence of seeds correlated not only with the accumulation levels of GFP but also with the accumulation of the 12S globulin precursor. p12S, 12S globulin precursor; 12S-α, α-subunit of mature 12S globulin. Molecular masses are given at left.
Only two factors, VSR1 and MAG1/VPS29, have been shown in planta to function in vacuolar sorting of storage proteins to PSVs. The results in Figures 4 and 5 show that both vsr1 and mag1/vps29 secreted a vacuole-targeted GFP, resulting in green fluorescent seeds. This finding suggests that vacuolar sorting–deficient mutants would produce green fluorescent seeds if vacuole-targeted GFP was expressed in seeds. This provided an idea for an efficient method for screening vacuolar sorting–deficient mutants. Namely, a mutant seed can be easily identified by the strong green fluorescence that is caused by missorting of GFP-CT24.
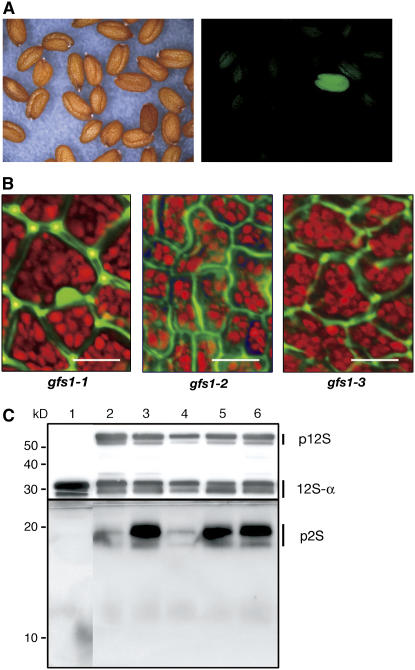
Based on this idea, we mutagenized the seeds of WT/GFP-CT24 with ethyl methanesulfonate to obtain M2 seed populations. Green fluorescent seeds were easily selected from a thousand of the mutagenized M2 seeds with a fluorescence stereomicroscope, as shown in Figure 6A. We obtained >100 mutant lines from ∼3,000,000 lines of M2 seeds and designated them gfs mutants. In this study, we describe 10 of these gfs mutants.
Figure 6.
Selected gfs Mutants Have a Defect in Vacuolar Sorting of Storage Proteins into PSVs.
(A) Seeds of the transformant (WT/GFP-CT24) were mutagenized with ethyl methanesulfonate. The M2 seed populations were inspected with a light microscope (left panel) and a fluorescence microscope (right panel) to select green fluorescent seeds. Finally, ∼100 fluorescent M2 lines were isolated from 3,000,000 M2 seeds and were designated gfs mutants.
(B) Seeds of the selected mutant lines were inspected with a confocal laser scanning microscope. GFP fluorescence was detected out of the seed cells of all gfs1 (gfs1-1, gfs1-2, and gfs1-3) mutants, showing missorting of GFP-CT24. The GFP fluorescence image was not merged with the autofluorescent image (red) of PSVs. Each image of green and red fluorescence is shown in Supplemental Figure 1 online. Bars = 10 μm.
(C) Seeds of wild-type (lane 1), vsr1-1 (lane 2), vsr1-2 (lane 3), gfs1-1 (lane 4), gfs1-2 (lane 5), and gfs1-3 (lane 6) plants were subjected to immunoblot analysis with anti-12S globulin and anti-2S albumin antibodies to determine the behavior of endogenous storage proteins in gfs1 seeds. All gfs1 mutants abnormally accumulated precursors of storage proteins in seeds, as did the vsr1 mutant. p12S, 12S globulin precursors; 12S-α, α-subunit of mature 12S globulin; p2S, 2S albumin precursors. Molecular masses are given at left.
Four gfs1 Mutants Are vsr1 Alleles
Four of the mutants were named gfs1 because they were allelic (discussed below). Figure 6B shows confocal laser scanning microscopic images of seeds of three of them (gfs1-1, gfs1-2, and gfs1-3). As expected, GFP fluorescence was observed out of the cells in the seeds of these gfs mutants. An immunoblot revealed that storage protein precursors, pro12S globulin and pro2S albumin, were abnormally accumulated in the seeds of these four allelic lines (gfs1-1 to gfs1-4), as in vsr1 seeds (Figure 6C). The different accumulations of pro2S albumin might be attributable to different growing conditions of the plants and/or different mutation sites. This result suggested that gfs1 mutants have a defect in sorting of the storage proteins into PSVs.
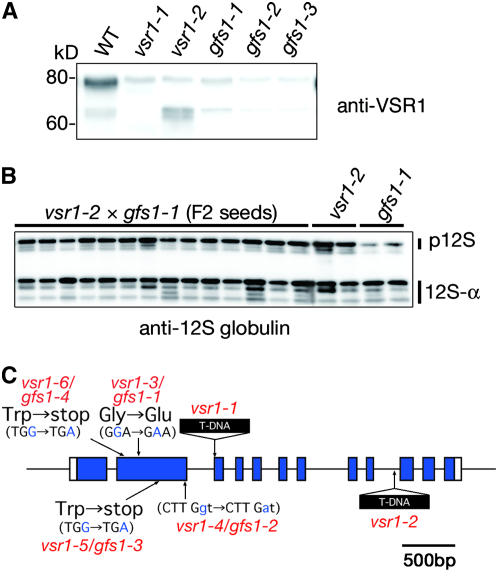
The gene responsible for gfs1 mutants was roughly mapped to a region close to the VSR1 gene (At3g52850). Immunoblot analysis showed that VSR1 was not accumulated in the gfs1 seeds, as in the vsr1 seeds (Figure 7A). The faint signal on the blot of these mutants might be attributable to the cross-reactivity of the antibody with other homologs (VSR2 to VSR7). These results suggested that the gfs1 mutants were allelic to vsr1. For a complementation test, gfs1-1 was crossed with vsr1-2. Fourteen F2 seeds were subjected to immunoblotting with anti-12S globulin antibody (Figure 7B). All of the F2 seeds accumulated 12S globulin precursor, as did vsr1-2 and gfs1-1, suggesting that the gfs1-1 mutant has a defect in VSR1. A complementation test of gfs1-2 gave the same result (data not shown). Finally, we found that each of the gfs1 mutants had a nucleotide substitution in the VSR1 gene (At3g52850), as shown in Figure 7C. gfs1-1 had a nucleotide substitution from GGA (Gly-214) to GAA (Glu). This result suggests that the Gly-214 of gfs1-1 is essential for VSR1 function. gfs1-2 had a nucleotide substitution from CTTGga to CTTGaa at the splicing donor site of the second intron. Both gfs1-3 and gfs1-4 had the nucleotide substitutions from TGG (Trp-105) to TGA (stop) and from TGG (Trp-297) to TGA (stop), respectively. These results show that selecting green fluorescent seeds among a large number of the seeds succeeded in isolating vsr1 alleles.
Figure 7.
Four gfs1 Mutant Lines Have a Mutation in the VSR1 Gene.
(A) Seeds of wild-type, vsr1-1, vsr1-2, gfs1-1, gfs1-2, and gfs1-3 plants were subjected to immunoblot analysis with anti-VSR1 antibody. Faint signals detected on the blot of vsr1 indicate cross-reactivity of the anti-VSR1 antibody with the other VSR homologs in seeds.
(B) We crossed gfs1-1 with vsr1-2 and obtained F2 lines. Seeds of 14 F2 lines (one grain per lane) were subjected to immunoblot analysis with anti-12S globulin antibody. All of the F2 seeds abnormally accumulated 12S globulin precursor, as did vsr1-2 and gfs1-1.
(C) Map-based cloning reveled that four gfs1 mutants had a mutation in the VSR1 gene. Each point mutation site of gfs1-1 to gfs1-4 is shown in a scheme of the VSR1 gene. Closed boxes indicate coding regions, and solid lines indicate introns. The T-DNA insertion sites of vsr1-1 and vsr1-2 are also shown.
Mapping of Other Mutations to Different Loci
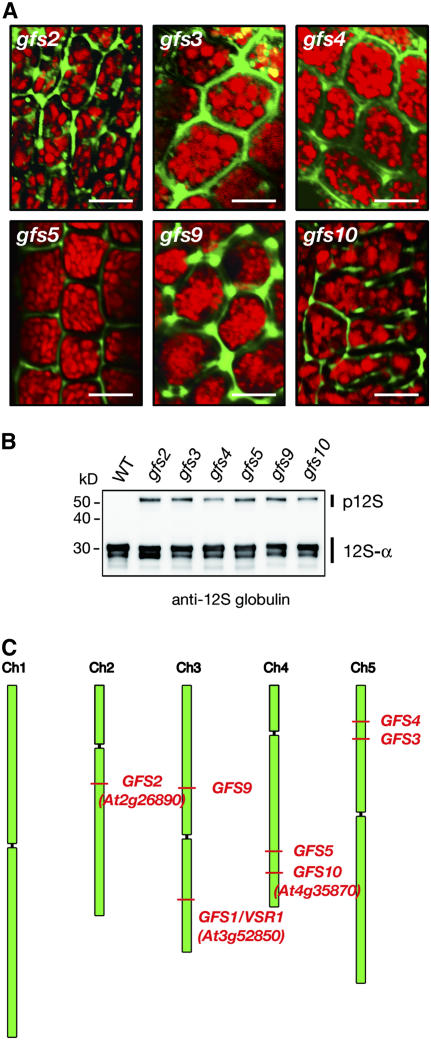
We also analyzed six gfs mutants (gfs2, gfs3, gfs4, gfs5, gfs9, and gfs10). All of them exhibited GFP fluorescence in the extracellular matrix (Figure 8A), suggesting that they secrete vacuole-targeted GFP out of the seed cells. The accumulation levels of GFP in seeds of all of these mutants (data not shown) were similar to those of vsr1/gfs1 (Figure 4A) and vps29/GFP-CT24 (Figure 5C). An immunoblot revealed that these mutants abnormally accumulated storage protein precursor of 12S globulin in their seeds (Figure 8B), suggesting that they have a defect in vacuolar sorting of storage proteins into PSVs. A rough map-based cloning analysis mapped the gfs mutations to different loci (Figure 8C).
Figure 8.
Six Different Mutants (gfs2, gfs3, gfs4, gfs5, gfs9, and gfs10) Other Than vsr1/gfs1 Alleles Were Isolated.
(A) Seeds of six gfs mutants (gfs2, gfs3, gfs4, gfs5, gfs9, and gfs10) were inspected with a confocal laser scanning microscope. GFP fluorescence was detected out of the cells of these mutant seeds, showing missorting of GFP-CT24. The GFP fluorescence image was not merged with the autofluorescent image (red) of PSVs. Each image of green and red fluorescence is shown in Supplemental Figure 1 online. Bars = 10 μm.
(B) Seeds of wild-type, gfs2, gfs3, gfs4, gfs5, gfs6, gfs9, and gfs10 plants were subjected to immunoblot analysis with anti-12S globulin antibody to determine the behavior of endogenous seed storage proteins in these seeds. These gfs mutants abnormally accumulated 12S globulin in precursors in seeds. p12S, 12S globulin precursors; 12S-α, α-subunit of mature 12S globulin. Molecular masses are given at left.
(C) Mutations of gfs1, gfs2, gfs3, gfs4, gfs5, gfs9, and gfs10 were mapped to different loci from each other: gfs1 to At3g52850 (VSR1), gfs2 to At2g26890 (KAM2/GRV2), and gfs10 to At4g35870.
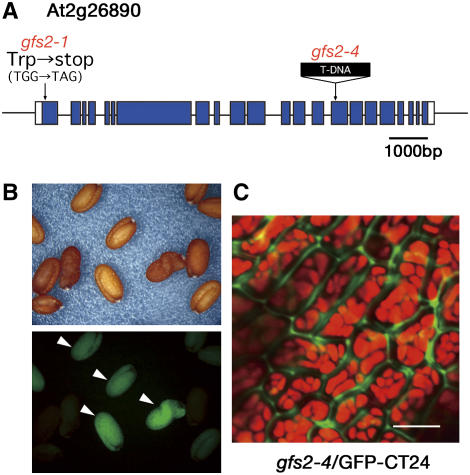
In gfs2, further mapping and DNA sequencing revealed a single base pair mutation from G to A in the At2g26890 gene of gfs2-1, which might cause a nonsense mutation from TGG (Trp-33) to TGA (stop codon) (Figure 9A). To confirm that At2g26890 is the gene responsible for the phenotype conferred by gfs2, we obtained a knockout mutant of the At2g26890 gene (SALK_132563). The mutant, which had T-DNA inserted into an exon of the At2g26890 gene, was named gfs2-4 (Figure 9A). gfs2-4 accumulated precursors of storage proteins, both 12S globulin and 2S albumin (data not shown). We crossed gfs2-4 with WT/GFP-CT24 to generate gfs2-4/GFP-CT24. Some of the F2 seeds were fluoresced (Figure 9B), and gfs2-4/GFP-CT24 exhibited GFP fluorescence in the extracellular space (Figure 9C). This result indicates that the mutation in the At2g26890 gene is responsible for the phenotype conferred by gfs2.
Figure 9.
A gfs2 Mutant Has a Mutation in the GRV2 Gene (At2g26890), Which Encodes a Homolog of Mammalian RME8.
(A) A point mutation site of gfs2-1 is shown in a scheme of the GRV2 gene. Closed boxes indicate coding regions, and solid lines indicate introns. A T-DNA–tagged allele (gfs2-4; SALK_132563) is also shown.
(B) We crossed the T-DNA–tagged mutant gfs2-4 with the transformant WT/GFP-CT24. The F2 seed population was inspected with a binocular microscope (top panel) and a fluorescence microscope (bottom panel). gfs2-4/GFP-CT24 produced green fluorescent seeds (arrowheads).
(C) The gfs2-4/GFP-CT24 seeds were inspected with a confocal laser-scanning microscope. GFP fluorescence was detected out of the cells of these mutant seeds, showing missorting of GFP-CT24. The GFP fluorescence image was not merged with the autofluorescent image (red) of PSVs. Each image of green and red fluorescence is shown in Supplemental Figure 1 online. Bar = 10 μm.
The At2g26890 gene was reported to be responsible for the gravitropism defective2 (grv2) mutant, which is deficient in shoot gravitropism and phototropism (Silady et al., 2004). We recently found that the At2g26890 gene is also responsible for an Arabidopsis mutant, katamari2 (kam2), that had deformed endosomes involving other endosomal compartments and formed abnormally large aggregates with various organelles in the cells (Tamura et al., 2007). kam2 alleles missorted seed storage proteins by secreting them from cells (Tamura et al., 2007), indicating that GFS2/KAM2 is involved in the vacuolar sorting of storage proteins into PSVs. The GFS2/KAM2 gene encodes a homolog of a DnaJ domain–containing RME8 (for receptor-mediated endocytosis8) that is required for endocytosis and the organization of endosomes in Caenorhabditis elegans (Zhang et al., 2001), Drosophila melanogaster (Chang et al., 2004), and human (Girard et al., 2005). KAM2 protein localized on punctate structures, which did not merge with any markers for the Golgi, the trans-Golgi network, endosomes, or prevacuolar compartments, suggesting that GFS2/KAM2 is localized in an uncharacterized endosomal compartment (Tamura et al., 2007). It is possible that DnaJ domain–containing GFS2/KAM2/GRV2 is involved in the folding of protein(s) needed for the proper formation of endosomes that mediate protein trafficking to PSVs and the biogenesis of endosomal compartments, including PSVs.
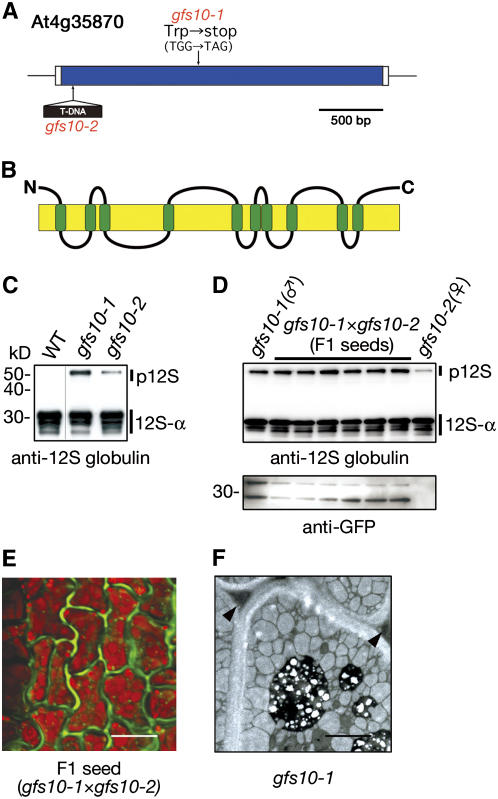
In gfs10, fine-scale mapping and DNA sequencing revealed a single base pair mutation from G to A in the At4g35870 gene of gfs10-1, which might cause a nonsense mutation from TGG (Trp-397) to TGA (stop codon) (Figure 10A). We obtained a T-DNA–tagged knockout mutant of the At4g35870 gene (SALK_139226) and named it gfs10-2 (Figure 10A). An immunoblot showed that gfs10-2 accumulated 12S globulin precursors in seeds, as did gfs10-1 (Figure 10C). We crossed gfs10-2 with gfs10-1 that expressed GFP-CT24 to generate F1 seeds. The expression of GFP in the F1 seeds indicated that the two lines were successfully crossed. Each F1 seed grain accumulated 12S globulin precursor as did both parent seeds (Figure 10D) and exhibited GFP fluorescence in the extracellular matrix of the seed cells (Figure 10E). These results indicate that the mutation in the At4g35870 gene is responsible for the phenotype conferred by gfs10. The ultrastructure of gfs10-1 seeds revealed that the extracellular space was enlarged and filled with electron-dense materials, possibly as a result of the secretion of storage proteins (Figure 10F). The phenotype is very similar to those of the vacuolar sorting mutants vsr1 (Shimada et al., 2003a), mag1/vps29 (Shimada et al., 2006), and gfs2/kam2 (Tamura et al., 2007). The At4g35870 gene, which is an intronless gene, encodes a novel membrane protein of unknown function that has 10 putative transmembrane domains (Figure 10B). The protein is specific to higher plants. Thus, plants appear to have a novel and plant-specific mechanism for the vacuolar sorting of storage proteins. Further identification of the responsible genes of the other gfs mutants should provide valuable insights into the complex molecular mechanisms underlying the vacuolar sorting of storage proteins.
Figure 10.
A gfs10 Mutant Has a Mutation in the At4g35870 Gene, Which Encodes a Novel Membrane Protein.
(A) A point mutation site of gfs10-1 is shown in a scheme of the At4g35870 gene, which is an intronless gene. A T-DNA–tagged mutant allele (gfs10-2; SALK_139226) is also shown.
(B) Scheme of GFS10 with putative 10 transmembrane domains (green boxes).
(C) Seeds of gfs10 were subjected to immunoblot analysis with anti-12S globulin. Two gfs10 alleles abnormally accumulated 12S globulin precursors. p12S, 12S globulin precursors; 12S-α, α-subunit of mature 12S globulin. Molecular masses are given at left.
(D) We crossed gfs10-2 with gfs10-1 that expressed GFP-CT24. Six F1 seeds was subjected to immunoblot analysis with either anti-12S globulin antibody or anti-GFP antibody. The accumulation of GFP in each F1 seed grain indicated that crossing was successful. Each F1 seed accumulated 12S globulin precursors, as did parent seeds.
(E) An F1 seed was inspected with a confocal laser scanning microscope. GFP fluorescence was detected out of the cells, showing missorting of GFP-CT24. The GFP fluorescence image was not merged with the autofluorescent image (red) of PSVs. Each image of green and red fluorescence is shown in Supplemental Figure 1 online. Bar = 10 μm.
(F) Electron micrograph of a gfs1-1 seed. The extracellular space of the gfs1-1 seeds was abnormally enlarged and filled with electron-dense materials (arrowheads). Bar = 5 μm.
DISCUSSION
A VSR Is Involved in Vacuolar Sorting of Storage Proteins into PSVs by Recognizing Their Vacuolar Targeting Signals
It has long been thought that VSR is a sorting receptor for lytic vacuoles but not a sorting receptor for PSVs (Hinz et al., 1999; Li et al., 2002), although a VSR homolog (PV72) was isolated from PAC vesicles of maturing pumpkin (Cucurbita maxima) seeds (Shimada et al., 1997). However, we reported the involvement of VSR1 in the proper sorting of storage protein precursors into PSV cells (Shimada et al., 2003a). Additionally, in this study, we isolated four vsr1 alleles by applying our green fluorescent seed method (Figure 7) and found that all of the vsr1 alleles missorted storage protein precursors in their seeds (Figure 6). It is now clear that VSR1 is involved in the sorting of storage proteins into PSVs.
Arabidopsis has seven members (VSR1 to VSR7) of the VSR family. Previously, we isolated seven mutants, each being deficient in a different VSR gene, and found that only vsr1 accumulated storage protein precursors in the seeds, whereas vsr2 to vsr7 accumulated no precursors in the seeds (Shimada et al., 2003a). By screening >3,000,000 seeds, we isolated four alleles of vsr1 but no alleles of vsr2 to vsr7. These results suggest that VSR1 functions primarily in vacuolar sorting of storage proteins in maturing seeds. This finding is consistent with VSR1 being the most abundant of the seven VSRs in seeds.
Examination of several plants revealed >10 vacuolar targeting signals for different vacuolar proteins, about half of which are storage proteins of various plants (Vitale and Hinz, 2005). It is possible that VSR functions in the sorting of storage proteins into PSVs by recognizing the signals in various maturing seeds. This notion is supported by the findings that VSR1 is responsible for the sorting of 2S albumin and 12S globulin into PSVs of Arabidopsis (Shimada et al., 2003a) and that VSR-like receptors are involved in the sorting of 2S albumin and ricin into PSVs in castor bean (Jolliffe et al., 2004).
What about the third major storage protein, 7S globulin? The C-terminal peptide CT10 of β-conglycinin, which belongs to the 7S globulin family, was reported to act as a necessary and sufficient signal for vacuolar targeting, because (1) deletion of the signal from β-conglycinin resulted in secretion of the protein and (2) addition of the signal to a GFP reporter resulted in delivery of the GFP into PSVs (Nishizawa et al., 2003). Here, we demonstrate that the vacuolar targeting signal was recognized by VSR1 for vacuolar sorting of β-conglycinin in Arabidopsis seeds.
As described above, in vivo and in vitro analyses have shown that VSRs have an ability to bind to the vacuolar targeting signals of various storage proteins. It remains to be determined how VSRs can recognize a variety of signal sequences, because these targeting signals have no consensus sequences.
Multiple Transport Pathways to PSVs
All of the gfs mutants that we isolated accumulated both precursor and mature forms of storage proteins (Figures 6 and 8), as did vsr1 (Shimada et al., 2003a) and mag1/vps29 (Shimada et al., 2006). We also found that another vacuolar sorting mutant, mag2, accumulated both precursor and mature forms of storage proteins in seeds. MAG2 might bind to ER-localized t-SNAREs and function in the transport of storage protein precursors between the ER and the Golgi complex in plants (Li et al., 2006). These mutants normally deliver a portion of storage proteins into PSVs, even though they have a defect in vacuolar sorting of storage proteins: vsr1, mag1/vps29, gfs2/kam2/grv2, and gfs10 secreted part of the storage proteins, whereas mag2 developed precursor-accumulating bodies within the seeds (Li et al., 2006). The incomplete secretion of storage proteins in these mutants might not be attributable to the redundancy of each responsible gene, because MAG1/VPS29, MAG2, GFS2/KAM2, and GFS10 do not have closely related homologs in the Arabidopsis genome. Vacuolar sorting mutants that accumulate all storage proteins as only precursor forms have not been isolated. Such mutants may be lethal because they could be affected in all mechanisms of vacuolar sorting. Another possibility is that plants use multiple transport pathways to PSVs.
One of the pathways is aggregation sorting, which is an efficient way of transporting large amounts of protein. Two compartments have been shown to mediate aggregation sorting: ER-derived PAC vesicles in pumpkin (Hara-Nishimura et al., 1993, 1998; Mitsuhashi et al., 2001), castor bean (Hiraiwa et al., 1993), soybean (Mori et al., 2004), and rice (Oryza sativa) (Takahashi et al., 2005) and Golgi-derived dense vesicles in pea (Hinz et al., 1999) and common bean (Chrispeels, 1983). PAC-like vesicles were induced in transgenic soybean seeds that cosuppressed the α-subunit of β-conglycinin (Kinney et al., 2001) and in transgenic Arabidopsis leaves that expressed a fusion protein composed of a small subunit of 2S albumin and phosphinothricin acetyltransferase (Hayashi et al., 1999). PAC vesicles can be regarded as prevacuolar compartments or endosomes in maturing seeds of various plant species.
We proposed a hypothetical model of the multiple pathways that is composed of three steps. Storage proteins that are actively synthesized form aggregates within the ER. As a result, aggregates and free molecules of storage proteins exist together in the ER lumen. This means that a storage protein species takes two forms: an aggregate form and a free molecule form. Aggregates develop to generate the PAC vesicles (first step), whereas free molecules leave the ER for the Golgi complex, where they are trapped by a VSR to be recruited to PAC vesicles (second step). PAC vesicles are incorporated directly into PSVs (third step). VSR1 and MAG1/VPS29 are involved in the second step. MAG2 might function in the exit from the ER in the first or second step. Considering a subcellular localization of GFS2/KAM2/GRV2 in uncharacterized compartments (Tamura et al., 2007), a novel pathway should be involved in vacuolar sorting. Further characterization and subcellular localization of GFS10 will provide a clue to the complex mechanism of vacuolar sorting.
A Powerful Tool for the Screening of Mutants Deficient in Vacuolar Sorting
To understand the molecular mechanism underlying the multiple pathways, forward genetics might be a useful approach. In this study, we developed a simple method (the green fluorescent seed method) to isolate vacuolar sorting mutants using GFP fluorescence as an indicator. Using this method, we succeeded in isolating four vsr1 alleles. We also identified GFS2/KAM2/GRV2 and GFS10 as factors of the sorting of storage proteins to PSVs. This technique must be one of the easiest and most efficient for hunting vacuolar sorting mutants.
We found that mag1/vps29 plants that expressed GFP-CT24 exhibited green fluorescent seeds. MAG1/VPS29 is encoded by a single-copy gene and is expressed ubiquitously in various organs, including leaves, roots, flowers, and developing seeds (Schmid et al., 2005). Thus, the MAG1/VPS29 gene may play an indispensable role in various tissues of Arabidopsis. MAG1/VPS29 might be required for vacuolar protein sorting into not only PSVs but also lytic vacuoles. gfs2/kam2/grv2 mutants exhibit a defect in the endomembrane organization of vegetative tissues (Tamura et al., 2007), and both gfs4 and gfs5 mutants exhibit a defect in the development of vegetative tissues (data not shown). Considering these results, vacuolar sorting mutants for both PSVs and lytic vacuoles could be isolated using our method.
The green fluorescent seed method makes it possible to efficiently screen a large number of seeds and to easily isolate mutant seeds. Selecting 3,000,000 seeds gave us >100 gfs mutants, some of which might be allelic. Identification of the responsible genes of these gfs mutants should provide a valuable insight into the complex molecular mechanisms in the multiple pathways unique to higher plants, as do yeast sec and vps mutants (Bonangelino et al., 2002). Further analysis of these mutants will reveal not only the vacuolar sorting mechanism of PSVs but also that of lytic vacuoles.
METHODS
Plant Materials and Growth Conditions
We used wild-type plants of Arabidopsis thaliana (ecotypes Columbia and Wassilewskijia-2) and the Arabidopsis mutants vsr1-1 and vsr1-2 (Shimada et al., 2003a) and mag1-1/vps29-1 and mag1-2/vps2-2 (Shimada et al., 2006). We also used transgenic plants that were generated previously (Nishizawa et al., 2003): Arabidopsis (ecotype Columbia) that expressed soybean (Glycine max) β-conglycinin α′-subunit (WT/conglycinin) and Arabidopsis (ecotype Columbia) that expressed GFP-CT24 (WT/GFP-CT24). We crossed vsr1-1 and vsr1-2 with WT/conglycinin and WT/GFP-CT24 to generate the transgenic plants vsr1-1/conglycinin and vsr1-2/conglycinin and the transgenic plants vsr1-1/GFP-CT24 and vsr1-2/GFP-CT24, respectively. We crossed vsr1-2 with gfs1-1 for complementation tests. We crossed mag1-1/vps29-1 and mag1-2/vps29-2 with WT/GFP-CT24 to generate two transgenic plants (mag1-1/vps29-1/GFP-CT24 and mag1-2/vps29-2/GFP-CT24, respectively). We obtained T-DNA insertion mutants (SALK_132563 and SALK_139226) from the ABRC at Ohio State University. We crossed the SALK_132563 line (gfs2-4) with the transformant WT/GFP-CT24 to produce gfs2-4/GFP-CT24. We further crossed gfs10-1 with the SALK_139226 line (gfs10-2) to produce F1 seeds. Surface-sterilized seeds were sown onto 0.5% Gellan Gum (Wako) with Murashige and Skoog medium supplemented with 1% sucrose and were grown at 22°C under continuous light.
Affinity Column Chromatography
We chemically synthesized the C-terminal peptide (CT10; PLSSILRAFY) of soybean β-conglycinin α′-subunit and immobilized the peptide (5 mg) to Aminolink coupling gel (2 mL of a bed volume; Pierce). Affinity column chromatography was performed essentially as described (Watanabe et al., 2002). Microsomes were prepared from young Arabidopsis siliques and were applied to the column that had been equilibrated with 20 mM HEPES-NaOH, pH 7.0, 150 mM NaCl, 1% CHAPS, and 1 mM CaCl2. The bound proteins were eluted with either the CT10 peptide or 5 mM EGTA. Each fraction was subjected to immunoblotting with anti-VSR1 antibody.
Specific Antibodies and Immunoblot Analysis
Immunoblot analysis of Arabidopsis seeds was performed essentially as described previously (Shimada et al., 2002), except that dilutions of the antibodies were as follows: anti-VSR1 (1:5000) (Yamada et al., 2005), anti-β-conglycinin (1:5000) (Nishizawa et al., 2003), anti-2S albumin (1:10,000), anti-12S globulin α-subunit (1:10,000) (Shimada et al., 2003b), and anti-GFP (1:5000) (Tamura et al., 2003).
Immunoelectron Microscopy
Dry seeds were cut into slices with a razor blade and then fixed with 4% (w/v) paraformaldehyde, 1% (v/v) glutaraldehyde, 0.05 M cacodylate buffer, pH 7.4, and 10% DMSO. Procedures for immunoelectron microscopic analysis were essentially as described previously (Hara-Nishimura et al., 1993). Ultrathin sections were treated with antibodies against Arabidopsis 2S albumin (dilution, 1:50), Arabidopsis 12S globulin α-subunit (1:50), β-conglycinin (1:50), and GFP (1:50). Sections were examined with a transmission electron microscope (model 120WX; JEOL) at 80 kV.
Confocal Laser Scanning Microscopy
Dry seeds were pressed in glycerol between glass slides and cover strips to push out cotyledons. The cotyledons were inspected with a confocal laser scanning microscope (LSM510 META; Carl Zeiss) using the 488-nm line of a 40-mW Ar/Kr laser and an emission filter (BP 530-505) for GFP fluorescence and the 544-nm line of a 1-mW He/Ne laser and an emission filter (LP 560) for autofluorescence of PSVs with a 40 × 0.75 numerical aperture dry objective. All images were obtained in multitrack mode and analyzed using LSM image-examiner software (Carl Zeiss). The data were exported as eight-bit TIFF files and processed using Adobe Photoshop 5.5 (Adobe Systems).
Isolation of the gfs Mutants
Seeds of the transformed plant that expressed GFP-CT24 (WT/GFP-CT24) were mutagenized by soaking them for 16 h in 0.2 or 0.25% (v/v) methanesulfonic acid ethyl ester (Sigma-Aldrich) and then washed for 11 h in running water to obtain M1 seeds. The M1 seeds were grown after self-fertilization to generate the M2 seed populations. These seeds were inspected with a fluorescence stereomicroscope (MZ-APO; Leica Microsystems), and images were acquired using a digital camera (DS-5ML1; Nikon).
Map-Based Cloning
Each homozygous mutant (background, ecotype Columbia) was crossed with Landsberg erecta wild-type plants to generate a mapping population. Genomic DNA was isolated from leaves of each mutant that was selected from the F2 progeny. The polymorphism between Columbia and Landsberg erecta was analyzed using a combination of cleaved amplified polymorphic sequences and simple sequence length polymorphism markers with data obtained from The Arabidopsis Information Resource (http://www.Arabidopsis.org).
According to our green fluorescent seed method, the mutant seeds can be quickly and easily selected from the F2 progeny. For rough mapping, we selected ∼10 to 20 recombinants of the F2 progeny of each gfs mutant. As a result, all four gfs1 mutations were roughly mapped near the VSR1 gene on chromosome 3 and the other mutations (gfs2, gfs3, gfs4, gfs5, gfs9, and gfs10) were roughly mapped as shown in Figure 8C. For identification of the responsible genes of gfs1 mutants, we subjected the seeds of the four gfs mutants to immunoblot analysis with anti-VSR1 antibody to see no accumulation of VSR1, crossed each gfs1 mutant with vsr1-2 to confirm the responsible gene to be VSR1, and sequenced the VSR1 gene of each gfs1 mutant to determine the mutation site of the gene, as shown in Figure 7. We further performed fine-scale mapping for gfs2 and gfs10 mutants. We selected 120 mutants from the F2 progeny for gfs2 and 100 mutants from the F2 progeny for gfs10 by the green fluorescent seed method and prepared genomic DNAs from the leaves of each mutant for fine-scale mapping. We sequenced the respective genes to determine the mutation sites of gfs2-1 and gfs10-1. Finally, to determine the responsible genes of gfs2-1 and gfs10-1, we obtained T-DNA–tagged lines that had a defect of each gene to examine the phenotype. For gfs2, we had six mutant alleles, all of which exhibited the vacuolar sorting defect (Tamura et al., 2007). For further confirmation of the responsible gene of gfs10-1, we crossed gfs10-1 with gfs10-2, as shown in Figure 10D. Nucleotide sequences were determined from both strands using an ABI Prism Big Dye Terminator Cycle sequence reaction kit (Applied Biosystems) and a DNA sequencer (Prism 3100; Applied Biosystems).
Accession Numbers
Arabidopsis Genome Initiative locus identifiers for the genes mentioned in this article are as follows: GFS1/VSR1 (At3g52850), MAG1/VPS29 (At3g7810), GFS2/KAM2/GRV2 (At2g26890), and GFS10 (At4g35870).
Supplemental Data
The following material is available in the online version of this article.
Supplemental Figure 1. Green Fluorescent and Red Fluorescent Images of Seeds Are Shown Separately for All Transformants in This Study.
Supplementary Material
Acknowledgments
This work was supported by the Core Research for Evolutional Science and Technology program of the Japan Science and Technology Corporation and by Grants-in-Aid for Scientific Research (Grants 16044224, 16085203, and 17107002) and for 21st Century Centers of Excellence Research, Kyoto University (Grant A14), from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We are grateful to the ABRC at Ohio State University for providing the seeds of Arabidopsis T-DNA insertion mutants (SALK_132563 and SALK_139226).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Ikuko Hara-Nishimura (ihnishi@gr.bot.kyoto-u.ac.jp).
Online version contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ahmed, S.U., Bar-Peled, M., and Raikhel, N.V. (1997). Cloning and subcellular location of an Arabidopsis receptor-like protein that shares common features with protein sorting receptors of eukaryotic cells. Plant Physiol. 114 325–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, S.U., Rojo, E., Kovalentina, V., Venkataraman, S., Dombrowski, J.E., Matsuoka, K., and Raikhel, N.V. (2000). The plant vacuolar sorting receptor AtELP is involved in transport of NH2-terminal propeptide-containing vacuolar proteins in Arabidopsis thaliana. J. Cell Biol. 149 1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino, C.J., Chavevz, E.M., and Bonifacino, J.S. (2002). Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol. Biol. Cell 13 2486–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H.C., Hull, M., and Mellman, I. (2004). The J-domain protein Rme-8 interacts with Hsc70 to control clathrin-dependent endocytosis in Drosophila. J. Cell Biol. 164 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels, M.J. (1983). The Golgi apparatus mediates the transport of phytohemagglutinin to the protein bodies in bean cotyledons. Planta 158 140–151. [DOI] [PubMed] [Google Scholar]
- Cooper, A.A., and Stevens, T.H. (1996). Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva, L.L.P., Foresti, O., and Denecke, J. (2006). Targeting of the plant vacuolar sorting receptor BP80 is dependent on multiple sorting signals in the cytosolic tail. Plant Cell 18 1477–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- daSilva, L.L.P., Taylor, J.P., Hadlington, J.L., Hanton, S.L., Snowden, C.J., Fox, S.J., Foresti, O., Brandizzi, F., and Denecke, J. (2005). Receptor salvage from the prevacuolar compartment is essential for efficient vacuolar protein targeting. Plant Cell 17 132–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloche, O., Yeung, B.G., Payne, G.S., and Schekman, R. (2001). Vps10p transport from the trans-Golgi network to the endosome is mediated by clathrin-coated vesicles. Mol. Biol. Cell 12 475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., de Virgilio, M., Prada, A., Faoro, F., and Vitale, A. (1998). Sorting of phaseolin to the vacuole is saturable and requires a short C-terminal peptide. Plant Cell 10 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., Jolliffe, N.A., Cola, A.D., Felipe, D.H., Paris, N., Neuhaus, J.-M., Lord, J.M., Ceriotti, A., and Roberts, L.M. (2001. a). The internal propeptide of the ricin precursor carries a sequence-specific determinant for vacuolar sorting. Plant Physiol. 126 167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio, L., Pastres, A., Prada, A., and Vitale, A. (2001. b). Influence of KDEL on the fate of trimeric or assembly-defective phaseolin: selective use of an alternative route to vacuoles. Plant Cell 13 1109–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard, M., Poupon, V., Blondeau, F., and McPherson, P.S. (2005). The DnaJ-domain protein RME-8 functions in endosomal trafficking. J. Biol. Chem. 280 40135–40143. [DOI] [PubMed] [Google Scholar]
- Hara-Nishimura, I., and Maeshima, M. (2000). Vacuolar processing enzymes and aquaporins. In Vacuolar Compartments in Plants, A.D.G. Robinson and J.C. Rogers, eds (London: Sheffield Academic Press), pp. 20–42.
- Hara-Nishimura, I., Matsushima, R., Shimada, T., and Nishimura, M. (2004). Diversity and functions of ER-derived compartments in plants: Are these compartments specific to plant cells? Plant Physiol. 136 3435–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Shimada, T., Hatano, K., Takeuchi, Y., and Nishimura, M. (1998). Transport of storage proteins to protein-storage vacuoles is mediated by large precursor-accumulating vesicles. Plant Cell 10 825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara-Nishimura, I., Takeuchi, Y., Inoue, K., and Nishimura, M. (1993). Vesicle transport and processing of the precursor to 2S albumin in pumpkin. Plant J. 4 793–800. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., Toriyama, K., Kondo, M., Hara-Nishimura, I., and Nishimura, M. (1999). Accumulation of a fusion protein containing 2S albumin induces novel vesicles in vegetative cells of Arabidopsis. Plant Cell Physiol. 40 263–272. [DOI] [PubMed] [Google Scholar]
- Hinz, G., Hillmer, S., Baumer, M., and Hohl, I.I. (1999). Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 11 1509–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraiwa, N., Takeuchi, Y., Nishimura, M., and Hara-Nishimura, I. (1993). A vacuolar processing enzyme in maturing and germinating seeds: Its distribution and associated changes during development. Plant Cell Physiol. 34 1197–1204. [Google Scholar]
- Honing, S., Sosa, M., Hille-Rehfeld, A., and von Figura, K. (1997). The 46-kDa mannose 6-phosphate receptor contains multiple binding sites for clathrin adaptors. J. Biol. Chem. 272 19884–19890. [DOI] [PubMed] [Google Scholar]
- Jin, J.B., Kim, Y.A., Kim, S.J., Lee, S.H., Kim, D.H., Cheong, G.W., and Hwang, I. (2001). A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. Plant Cell 13 1511–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolliffe, N.A., Brown, J.C., Neumann, U., Vicre, M., Bachi, A., Hawes, C., Ceriotti, A., Roberts, L.M., and Frigerio, L. (2004). Transport of ricin and 2S albumin precursors to the storage vacuoles of Ricinus communis endosperm involves the Golgi and VSR-like receptors. Plant J. 39 821–833. [DOI] [PubMed] [Google Scholar]
- Kinney, A.J., Jung, R., and Herman, E.M. (2001). Cosuppression of the α-subunits of β-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum-derived protein bodies. Plant Cell 13 1165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, T., Paris, N., Butler, J.M., Beevers, L., and Rogers, J.C. (1994). Purification and initial characterization of a potential plant vacuolar targeting receptor. Proc. Natl. Acad. Sci. USA 91 3403–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch, T., Saalbach, G., Raikhel, N.V., and Beevers, L. (1996). Interaction of a potential vacuolar targeting receptor with amino- and carboxyl-terminal targeting determinants. Plant Physiol. 111 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman, J., Kuliawat, R., Griffith, J.M., Geuze, H.J., and Arvan, P. (1998). Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J. Cell Biol. 141 359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laval, V., Masclaux, F., Serin, A., Carriere, M., Roldan, C., Devic, M., Pont-Lezica, R.F., and Galaud, J.P. (2003). Seed germination is blocked in Arabidopsis putative vacuolar sorting receptor (atbp80) antisense transformants. J. Exp. Bot. 54 213–221. [DOI] [PubMed] [Google Scholar]
- Li, L., Shimada, T., Takahashi, H., Ueda, H., Fukao, Y., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (2006). MAIGO2 is involved in exit of seed storage proteins from the endoplasmic reticulum in Arabidopsis thaliana. Plant Cell 18 3535–3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y.B., Rogers, S.W., Tse, Y.C., Lo, S.W., Sun, S.S., Jauh, G.Y., and Jiang, L. (2002). BP-80 and homologs are concentrated on post-Golgi, probable lytic prevacuolar compartments. Plant Cell Physiol. 43 726–742. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi, N., Hayashi, Y., Koumoto, Y., Shimada, T., Fukasawa-Akada, T., Nishimura, M., and Hara-Nishimura, I. (2001). A novel membrane protein that is transported to protein-storage vacuoles via precursor-accumulating vesicles. Plant Cell 13 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, T., Maruyama, N., Nishizawa, K., Higasa, T., Yagasaki, K., Ishimoto, M., and Utsumi, S. (2004). The composition of newly synthesized proteins in the endoplasmic reticulum determines the transport pathways of soybean seed storage proteins. Plant J. 40 238–249. [DOI] [PubMed] [Google Scholar]
- Muntz, K. (1998). Deposition of storage proteins. Plant Mol. Biol. 38 77–99. [PubMed] [Google Scholar]
- Nishizawa, K., Maruyama, N., Satoh, R., Fuchikami, Y., Higasa, T., and Utsumi, S. (2003). A C-terminal sequence of soybean β-conglycinin α′ subunit acts as a vacuolar sorting determinant in seed cells. Plant J. 34 647–659. [DOI] [PubMed] [Google Scholar]
- Nishizawa, K., Maruyama, N., Satoh, R., Higasa, T., and Utsumi, S. (2004). A vacuolar sorting determinant of soybean β-conglycinin β subunit resides in a C-terminal sequence. Plant Sci. 167 937–947. [DOI] [PubMed] [Google Scholar]
- Park, M., Lee, D., Lee, G.J., and Hwang, I. (2005). AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J. Cell Biol. 170 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo, E., Gillmor, C.S., Kovaleva, V., Somerville, C.R., and Raikhel, N.V. (2001). VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis. Dev. Cell 1 303–310. [DOI] [PubMed] [Google Scholar]
- Saalbach, G., Rosso, M., and Schumann, U. (1996). The vacuolar targeting signal of the 2S albumin from Brazil nut resides at the C terminus and involves the C-terminal propeptide as an essential element. Plant Physiol. 112 975–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderfoot, A.A., Ahmed, S.U., Marty-Mazars, D., Rapoport, I., Kirchhausen, T., Marty, F., and Raikhel, N.V. (1998). A putative vacuolar cargo receptor partially colocalizes with AtPEP12p on a prevacuolar compartment in Arabidopsis roots. Proc. Natl. Acad. Sci. USA 95 9920–9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid, M., Davison, T.S., Henz, S.R., Pape, U.J., Demar, M., Vingron, M., Scholkopf, B., Weigel, D., and Lohmann, J.U. (2005). A gene expression map of Arabidopsis thaliana development. Nat. Genet. 37 501–506. [DOI] [PubMed] [Google Scholar]
- Shimada, T., Fuji, K., Tamura, K., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (2003. a). Vacuolar sorting receptor for seed storage proteins in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 100 16095–16100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada, T., Koumoto, Y., Li, L., Yamazaki, M., Kondo, M., Nishimura, M., and Hara-Nishimura, I. (2006). AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 47 1187–1194. [DOI] [PubMed] [Google Scholar]
- Shimada, T., Kuroyanagi, M., Nishimura, M., and Hara-Nishimura, I. (1997). A pumpkin 72-kDa membrane protein of precursor accumulating vesicles has characteristics of a vacuolar sorting receptor. Plant Cell Physiol. 38 1414–1420. [DOI] [PubMed] [Google Scholar]
- Shimada, T., Watanabe, E., Tamura, K., Hayashi, Y., Nishimura, M., and Hara-Nishimura, I. (2002). A vacuolar sorting receptor PV72 on the membrane of vesicles that accumulate precursors of seed storage proteins (PAC vesicles). Plant Cell Physiol. 43 1086–1095. [DOI] [PubMed] [Google Scholar]
- Shimada, T., et al. (2003. b). Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J. Biol. Chem. 278 32292–32299. [DOI] [PubMed] [Google Scholar]
- Silady, R.A., Kato, T., Lukowitz, W., Sieber, P., Tasaka, M., and Somerville, C.R. (2004). The gravitropism defective 2 mutants of Arabidopsis are deficient in a protein implicated in endocytosis in Caenorhabditis elegans. Plant Physiol. 136 3095–3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, H., Saito, Y., Kitagawa, T., Morita, S., Masumura, T., and Tanaka, K. (2005). A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant Cell Physiol. 46 245–249. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Shimada, T., Ono, E., Tanaka, Y., Nagatani, A., Higashi, S.-i., Watanabe, M., Nishimura, M., and Hara-Nishimura, I. (2003). Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J. 35 545–555. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Takahashi, H., Kunieda, T., Fuji, K., Shimada, T., and Hara-Nishimura, I. (2007). Arabidopsis KAM2/GRV2 is required for proper endosome formation and functions in vacuolar sorting and determination of the embryo growth axis. Plant Cell 19 320–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale, A., and Hinz, G. (2005). Sorting of proteins to storage vacuoles: How many mechanisms? Trends Plant Sci. 10 316–323. [DOI] [PubMed] [Google Scholar]
- Watanabe, E., Shimada, T., Kuroyanagi, M., Nishimura, M., and Hara-Nishimura, I. (2002). Calcium-mediated association of a putative vacuolar sorting receptor PV72 with a propeptide of 2S albumin. J. Biol. Chem. 277 8708–8715. [DOI] [PubMed] [Google Scholar]
- Watanabe, E., Shimada, T., Tamura, K., Matsushima, R., Koumoto, Y., Nishimura, M., and Hara-Nishimura, I. (2004). An ER-localized form of PV72, a seed-specific vacuolar sorting receptor, interferes the transport of an NPIR-containing proteinase in Arabidopsis leaves. Plant Cell Physiol. 45 9–17. [DOI] [PubMed] [Google Scholar]
- Yamada, K., Fuji, K., Shimada, T., Nishimura, M., and Hara-Nishimura, I. (2005). Endosomal proteases facilitate the fusion of endosomes with vacuoles at the final step of the endocytotic pathway. Plant J. 41 888–898. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Grant, B., and Hirsh, D. (2001). RME-8, a conserved J-domain protein, is required for endocytosis in Caenorhabditis elegans. Mol. Biol. Cell 12 2011–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.