Abstract
Tapetosomes are abundant organelles in tapetum cells during the active stage of pollen maturation in Brassicaceae species. They possess endoplasmic reticulum (ER)–derived vesicles and oleosin-coated lipid droplets, but their overall composition and function have not been established. In situ localization analyses of developing Brassica napus anthers revealed flavonoids present exclusively in tapetum cells, first in an ER network along with flavonoid-3′-hydroxylase and then in ER-derived tapetosomes. Flavonoids were absent in the cytosol, elaioplasts, vacuoles, and nuclei. Subcellular fractionation of developing anthers localized both flavonoids and alkanes in tapetosomes. Subtapetosome fractionation localized flavonoids in ER-derived vesicles, and alkanes and oleosins in lipid droplets. After tapetum cell death, flavonoids, alkanes, and oleosins were located on mature pollen. In the Arabidopsis thaliana mutants tt12 and tt19 devoid of a flavonoid transporter, flavonoids were present in the cytosol in reduced amounts but absent in tapetosomes and were subsequently located on mature pollen. tt4, tt12, and tt19 pollen was more susceptible than wild-type pollen to UV-B irradiation on subsequent germination. Thus, tapetosomes accumulate ER-derived flavonoids, alkanes, and oleosins for discharge to the pollen surface upon cell death. This tapetosome-originated pollen coat protects the haploidic pollen from UV light damage and water loss and aids water uptake.
INTRODUCTION
An anther in a flower usually has approximately four layers of cells enclosing a central locule, in which microspores mature to become pollen (Goldberg et al., 1993; Bewley et al., 2000; Scott et al., 2004). Cells of the outer three layers are highly vacuolated and presumed to be metabolically less active. Cells of the innermost layer, the tapetum, have dense cytoplasm and are metabolically active (Hesse et al., 1993). They regulate maturation of the microspores to become pollen and thus are important in the sexual reproduction process. Yet, we have minimal information on the functioning of the tapetum cells.
The tapetum cells in Brassica and Arabidopsis thaliana of the Brassicaceae family have been characterized to some extent, more so than in other species (Murgia et al., 1991; Owen and Makaroff, 1995; Wu et al., 1997; Platt et al., 1998). Early in development, the cells are involved in the active secretion of molecules into the locule for maturation of the microspores. Late in development, the cells become warehouses for temporary storage. They are packed with two predominant storage organelles, the elaioplasts and tapetosomes. At the conclusion of development, the cells undergo programmed cell death (PCD) and release the storage materials, which will become the coat of mature pollen.
Elaioplasts in the tapetum are specialized plastids derived from proplastids for the temporary storage of steryl esters (Platt et al., 1998; Wu et al., 1999). They have few internal membranes but are packed with globuli of steryl esters enclosed by structural proteins called plastid lipid–associated protein (PAP) (Kim et al., 2001).
Tapetosomes are organelles unique to the tapetum in plants (Wu et al., 1997; Hsieh and Huang, 2005). Each spherical tapetosome of 2 to 3 μm in diameter contains many lipid droplets (0.1 to 0.5 μm each) sequestered among numerous membranous vesicles. Each lipid droplet contains a matrix of neutral lipids, including triacylglycerols (TAGs), enclosed by the amphipathic structural proteins termed oleosins and presumably also phospholipids (PLs). This structure of the lipid droplet is similar to that of a seed oil body.
Tapetosomes are synthesized via a unique mechanism. Early in anther development, the tapetum cells contain massive endoplasmic reticulum (ER) cisternae. Oleosins and TAGs are synthesized in extensive regions of the cisternae and move to many tapetosome-forming centers on the cisternae. At each center, numerous lipid droplets are produced from the ER via a budding mechanism, whereby TAGs accumulate between the PL layers on which oleosins gather. This mode of synthesis of the lipid droplets is identical to that of seed oil bodies (Hsieh and Huang, 2004). Concomitant with the synthesis of lipid droplets, other ER cisternae at the tapetosome-forming center produce numerous vesicles via budding. These membranous vesicles contain calreticulin and binding protein associated with the membrane and unknown molecules in the lumen. Essentially, all of the dozens of tapetosome-forming centers in a tapetum cell are linked to one another via the massive ER cisternae. With the disappearance of the ER immediately before tapetum PCD, tapetosomes, each containing numerous oleosin-coated lipid droplets and membranous vesicles held together by ionic interactions, mature to become solitary entities.
Upon PCD of the tapetum cells at late anther development, steryl esters of the elaioplasts and oleosins of the tapetosomes are discharged to the surface of the almost mature pollen (Wu et al., 1997). They become major ingredients of the coat. Steryl esters waterproof the pollen. Oleosins, being amphipathic, likely emulsify the coat materials, mixing lipids and semi-water-soluble ingredients (Hsieh and Huang, 2004), and may be involved in the uptake of water from the stigma to the pollen for germination (Mayfield and Preuss, 2000).
The coat of mature pollen possesses, in addition to steryl esters and oleosins, abundant flavonoids and alkanes. It has been hypothesized that the coat flavonoids protect the nucleic acids in pollen, which are haploidic, against UV irradiation damage (reviewed in Winkel-Shirley, 2001; Schijlen et al., 2004; Pacini and Hesse, 2005). Coat flavonoids are also involved in pollen germination and tube growth in some species, such as maize (Zea mays) and petunia (Petunia hybrida) (Mo et al., 1992; van der Meer et al., 1992; Napoli et al., 1999), but not Arabidopsis (Burbulis et al., 1996; Ylstra et al., 1996). From biochemical, molecular, and/or genetic studies, pollen coat flavonoids and alkanes are known to have derived from the sporophytic tapetum cells (Stanley and Linskens, 1974; Piffanelli et al., 1998).
The subcellular location of flavonoids in the tapetum and their transfer to the pollen surface have not been examined previously. In general, plants synthesize flavonoids from malonate and coumarate catalyzed by chalcone synthase, the first committed metabolic step (Tuteja et al., 2004; reviewed in Winkel-Shirley, 2001; Schijlen et al., 2004). Chalcone is subsequently converted to different flavonoids. The primary flavonoid synthetic enzymes are present as multienzyme complexes on the cytosolic side of the ER membrane (Winkel, 2004). After synthesis, flavonoids are compartmented in different subcellular locations, including vesicles, vacuoles, and nuclei, or exported to the cell exterior (Mueller and Walbot, 2001; Saslowsky et al., 2005; Taylor and Grotewold, 2005; Yazaki, 2005). The location of flavonoids in different subcellular compartments is plant species– and cell type–specific.
Similarly, the subcellular location of alkanes in the tapetum cells and their discharge to the pollen surface have not been reported previously. In aerial epidermal cells, fatty acids produced in plastids are transported to the ER, where they undergo elongation and other modifications to become cuticular lipids, including alkanes (reviewed in Nawrath, 2002; Kunst and Samuels, 2003; Suh et al., 2005; Yephremov and Schreiber, 2005). The ER cisternae do not appear to be in physical contact with the plasma membrane (PM), and the synthesized lipids in the ER cisternae need a special mechanism for their transport to the PM and then across the PM to the cellular exterior. This transport requires an ATP binding transporter (Kunst and Samuels, 2003; Pighin et al., 2004).
We here report experimental findings on the subcellular location of flavonoids and alkanes in Brassica tapetum cells and the transfer of these molecules to the pollen. Flavonoids located first in the ER and then in ER-derived tapetosomes. Tapetosomes were also the subcellular sites for alkane accumulation. Within the tapetosomes, flavonoids and alkanes located in the ER-derived vesicles and lipid droplets, respectively. After tapetum lysis, the major tapetosome ingredients, including flavonoids, alkanes, and oleosins, were deposited onto the maturing pollen. Thus, tapetosomes function as subcellular metabolic sinks for the coordinated discharge of metabolites to the pollen as the coat upon PCD. Studies with Arabidopsis flavonoid-deficient mutants confirmed these findings and validated the hypothesis that pollen coat flavonoids protect the pollen from UV irradiation damage.
RESULTS
In Developing Brassica Anthers, Levels of Flavonoids and Alkanes Increased and Persisted, Concomitant with the Level of Tapetosome Oleosins
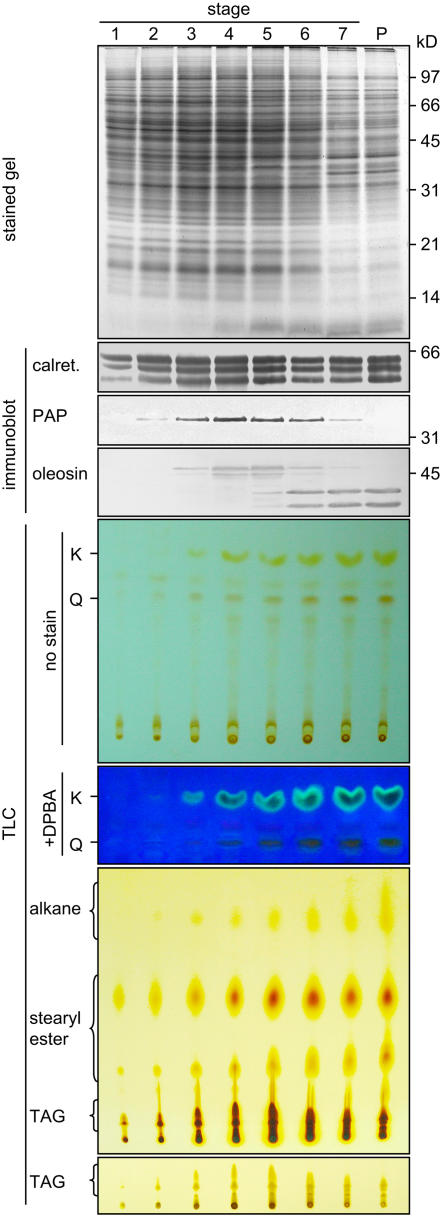
Brassica anthers were divided into seven developmental stages for these studies (see Methods). The tapetum cells became mature at stages 4 and 5 and began to undergo PCD at stage 6 and deposit their contents onto the pollen. Brassica anther extracts (containing materials that derived from both the sporophytic anther cells and the gametophytic microspores) and mature pollen extract (containing materials that originated from the sporophytic coat and the gametophytic interior) were analyzed for contents of various proteins, flavonoids, and alkanes (Figure 1). SDS-PAGE revealed that the level of total anther proteins, estimated from the Coomassie blue–stained polypeptides on the gel, peaked at stages 4 to 5 and then declined. Extracts of stage 6 to 7 anthers contained proteins that were largely those of the pollen.
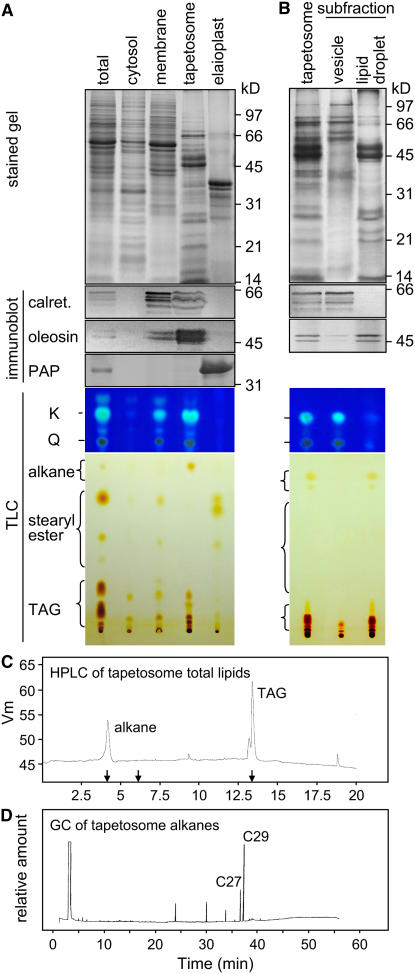
Figure 1.
SDS-PAGE, Immunoblot, and TLC Analyses of Extracts of Brassica Anthers of Seven Developmental Stages and Mature Pollen.
The various extracts applied to the gels or TLC plates represented those from an equal number of anthers. Gels were stained for proteins or subjected to immunoblot analyses with antibodies against calreticulin, elaioplast PAP, or tapetosome oleosin. The two major oleosins of 48 and 45 kD were converted to fragments of 37 and 35 kD, respectively, during anther development; the fragments were more immunoreactive than the originals (Ting et al., 1998). Positions of markers for polypeptide molecular masses are shown at right. The TLC plate after separation of deglycosylated flavonoids was photographed directly (full plate shown) or after being treated with DPBA and placed on top of a UV irradiation source (portion of the plate shown). K and Q denote kaempferol and quercetin, respectively. The TLC plate for lipid analyses was stained with iodine for 24 h to reveal all lipids, including the less reactive alkanes (full plate shown), or for 0.5 h to reveal TAGs more clearly (portion of the plate shown).
Changes in marker proteins of prominent organelles in the tapetum cells during anther development were examined by SDS-PAGE and immunoblot analyses (Figure 1). Several calreticulins (50 to 60 kD) in anther extracts were recognized by antibodies against castor calreticulin, an ER luminal protein (Coughlan et al., 1997). The calreticulins were already present in the anthers at stage 1, and their levels peaked at stages 4 to 5, concomitant with the level of total proteins. Their levels persisted throughout the subsequent development; this pattern is unlike the gradual reduction in levels of total anther proteins. The persistence was shown earlier in Brassica rapa to reflect a decrease of the tapetum calreticulins compensated by the appearance of pollen interior calreticulins (Hsieh and Huang, 2005). The elaioplast marker PAP appeared at stage 2, and its level peaked at stages 4 to 5 and then gradually disappeared. The complete disappearance indicates that the pollen coat or interior did not have detectable PAP. Two major oleosins of 48 and 45 kD (representing those from the AA and CC genomes, respectively, of the amphidiploid Brassica napus used in this study) appeared slightly after PAP did, and their levels increased and persisted throughout the subsequent development. Beginning at stage 5, the 48- and 45-kD oleosins gradually became the smaller 37- and 35-kD fragments, respectively, of the pollen coat. This pattern of appearance and processing of the oleosins in B. napus is similar to that in B. rapa (also known as Brassica campestris; diploid containing the AA genome) reported previously (Ting et al., 1998). The apparent increase in level of the two oleosins (the 48- and 45-kD originals and their 37- and 35-kD fragments) from stages 5 to 7 (Figure 1) was only because the fragments were more immunoreactive than their originals (Ting et al., 1998); in actuality, the level of these two oleosins remained unchanged from stage 4 to stage 7.
Total anther flavonoids after a deglycosylation treatment were resolved by TLC, and individual flavonoids were observed directly or after reaction with diphenylboric acid 2-aminoethyl ester (DPBA) (Figure 1). Two major flavonoids, kaempferol and quercetin, were identified by their colors and comigration with commercial standards on TLC plates with the use of different developing solvents (data not shown). During anther development, the two flavonoids appeared at stage 3, and their levels increased to a plateau at stages 5 to 7. This developmental pattern resembles that of oleosins but not those of calreticulins and PAP.
Total anther lipids were resolved by TLC (Figure 1). Steryl esters in the anther extracts appeared as two major spots on the TLC plate at stage 1, and their levels increased to a plateau at stages 4 to 7. As reported previously (Wu et al., 1999), these steryl esters were located in the tapetum elaioplasts and subsequently transferred to the pollen surface. TAGs in the anther extracts appeared at stages 2 to 3, and their levels peaked at stages 4 to 5 and then declined; they were also present in the total pollen extract (Figure 1). Early in anther development, the anther TAGs were the tapetum tapetosome TAGs, which disappeared during the final maturation of the tapetum. Late in anther development, the anther TAGs were the microspore/pollen oil body TAGs (Wu et al., 1999). The developmental changes of steryl esters and TAGs in B. napus in this study are similar to those in B. rapa (Ting et al., 1998).
The developmental pattern of alkanes, as resolved on a TLC plate, also resembled those of oleosins and flavonoids (Figure 1). The anther alkanes migrated faster than the other lipids on TLC plates and appeared as a smear or one to two spots, as did commercial C-29 and C-30 alkanes, after a prolonged exposure to iodine vapor. They appeared at stage 3, and their levels increased to a plateau at stages 5 to 7; they were also present in the total pollen extract.
A Procedure Was Established for the in Situ Colocalization of Flavonoids and Proteins
DPBA reacts with nonglycosylated but not glycosylated flavonoids and emits fluorescence under UV irradiation. The intensity and spectra of the fluorescence vary with the types and concentrations of flavonoids and the local environments (Sheahan and Rechnitz, 1992). Glycosylated flavonoids are the predominant form of flavonoids in many plant organs, including Arabidopsis leaves (Graham, 1998), flowers, and stamens (Burbulis et al., 1996) and Brassica anthers (see below). Published methods for the in situ localization of flavonoids allowed tissues to react with DPBA without a prior deglycosylation treatment, and fluorescence was then detected by confocal laser scanning microscopy (CLSM) (e.g., applied to roots by Saslowsky and Winkel-Shirley [2001]; Buer and Muday [2004]). Application of these methods to samples such as Arabidopsis leaves and flowers would reveal only very low levels of nonglycosylated flavonoids or background fluorescence. For the separation of total flavonoids in tissue extracts by TLC or HPLC, samples were routinely predeglycosylated in 1 to 2 M HCl at 90 to 100°C for 20 to 150 min. Such a severe condition would hardly be applicable to in situ deglycosylation and the localization of flavonoids with DPBA and proteins with immunodetection.
We sought a relatively mild condition for in situ deglycosylation acceptable for the subsequent localization of flavonoids alone or for colocalization of proteins. We tested in vitro deglycosylation of commercially available rutin (quercetin-3-rhamnose-glucose, a common plant glycosylated flavonoid) in 1 or 2 M HCl at various temperatures for 30 to 60 min. Different degrees of deglycosylation were observed (see Supplemental Figure 1A online). We then examined several of the milder conditions for in situ deglycosylation followed by DPBA staining with Brassica anther sections. We instituted a prior paraformaldehyde fixation step to minimize potential damage of the cells from the acid treatment. After different acid treatments and subsequent DPBA staining, the anther sections were analyzed by CLSM. Exemplified CLSM observations of stage 5 tapetum cells are shown in Supplemental Figure 1C online. After an acid treatment with 1 M HCl at 70°C for 30 min and then DPBA staining, the cell possessed numerous spherical, fluorescent particles (whose identities will be described in subsequent sections). Omission of DPBA or a heated acid treatment gave only background fluorescence. After an acid treatment with 1 M HCl at 100°C for 30 min and then DPBA staining, the cell contained scattered granular fluorescence and several larger, spherical fluorescent particles whose boundaries had surface extensions. The treatment with 1 M HCl at 70°C for 30 min appeared to be the most suitable condition, because afterward the cells were still reactive to antibodies against various organelle markers. The immunodetected organelles, including ER, plastids, tapetosomes, and vacuoles (to be shown throughout this report), had no appreciable difference by CLSM from those in tapetum cells not subjected to acid and DPBA treatment (Hsieh and Huang, 2005). The designated acid condition (1 M HCl at 70°C for 30 min) for deglycosylation was used in all in situ localization of flavonoids in the remaining portion of this report. This acid treatment deglycosylated in vitro approximately half of commercial rutin (see Supplemental Figure 1A online) or glycosylated flavonoids in a crude anther extract (see Supplemental Figure 1B online). For TLC analyses of total flavonoids in anther extracts or organelle fractions (shown in Figure 1 and subsequent figures), we used 2 M HCl at 80°C for 1 h for complete deglycosylation (see Supplemental Figures 1A and 1B online).
DPBA Detected Flavonoids in Situ by CLSM
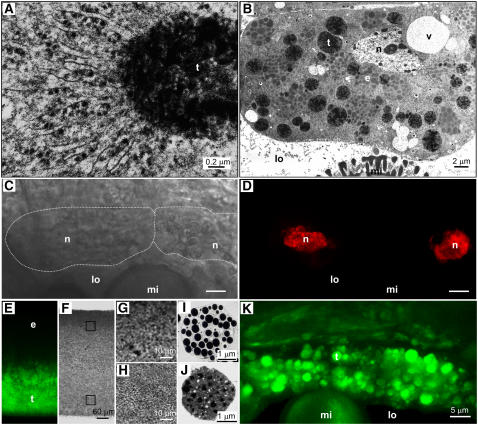
The morphology of the tapetum cells in Brassica and its changes during development by transmission electron microscopy have been described in detail (Platt et al., 1998) and are described here briefly. At a mid stage of anther development (stage 3), each tapetum cell contained abundant solitary elaioplasts, rough ER cisternae, and maturing tapetosomes (1 to 2 μm in diameter). Each maturing tapetosome was attached to massive rough ER cisternae (Figure 2A), and all of the maturing tapetosomes in the cell were interconnected via a network of rough ER cisternae. At a late stage of anther development (stage 5), the tapetum cell possessed abundant solitary tapetosomes (2 to 3 μm in diameter) and elaioplasts (3 to 4 μm), and rough ER cisternae had largely disappeared (Figure 2B). At stages 3 to 5 (Figure 2B) (Platt et al., 1998), each tapetum cell had 10 to 20 vacuoles of various sizes (1 to 6 μm) and 1 or 2 nuclei (Figures 2C and 2D). With this information, we explored the subcellular locations of flavonoids and alkanes.
Figure 2.
Microscopy of Brassica Tapetum Cells and Isolated Tapetosomes and Elaioplasts.
(A) Enlarged electron microscopy image of a maturing tapetosome (t) connected to numerous rough ER cisternae in a tapetum cell (stage 3).
(B) Electron microscopy image of a stage 5 tapetum cell, in which most tapetosomes had become solitary organelles. Nucleus (n), vacuoles (v), tapetosomes (t), and elaioplasts (e) in the tapetum cells, as well as portion of a microspore (mi) in the locule (lo), were present.
(C) and (D) Two tapetum cells stained for nuclei with ethidium bromide and viewed by CLSM with a bright-field (the cell circumference was marked with white dotted lines [C]) or a fluorescence (nuclei in red [D]) setting.
(E) to (H) CLSM images of a lipid pad obtained from a stage 5 anther extract by centrifugation. The lipid pad contained elaioplasts (lighter density) at the top half and tapetosomes at the bottom half. It was treated with DPBA for flavonoid staining, cut perpendicularly along the direction of the original centrifugal force, and viewed by CLSM with a fluorescence (E) or a bright-field ([F] to [H]) setting. Tapetosomes at the bottom half but not elaioplasts at the top half of the lipid pad showed DPBA fluorescence (shown in green). Portions (squares in [F]) of the lipid pad were enlarged to reveal packed particles ([G] and [H] for the top and bottom portions, respectively).
(I) and (J) Electron microscopy images of an isolated elaioplast (I) and tapetosome (J).
(K) Two tapetum cells adjacent to the anther locule (lo) in a stage 5 anther observed by CLSM after DPBA staining. Numerous spherical particles (to be shown as tapetosomes) exhibited DPBA fluorescence (in green).
DPBA was used to detect flavonoids in isolated organelles by CLSM (Figures 2E to 2H). Solitary elaioplasts and tapetosomes of the tapetum in a stage 5 anther extract in a 0.6 M sucrose solution were isolated together as a floating lipid pad by centrifugation (Wu et al., 1997). The lipid pad (Figures 2E and 2F), which contained elaioplasts (density of ∼1.02 g/cm3) at the top half (Figures 2G and 2I) and tapetosomes (density of ∼1.05 g/cm3) at the bottom half (Figures 2H and 2J), could be handled for microscopy treatment. The lipid pad was fixed, acid-treated, and stained with DPBA. DPBA fluorescence was observed only at the bottom half of the lipid pad, which contained the tapetosomes (Figures 2E to 2J).
We proceeded to use DPBA to detect flavonoids in situ in tapetum cells in stage 5 anthers. DPBA fluorescence was located in spherical particles of 2 to 3 μm diameter (Figure 2K). At this developmental stage, the tapetum cells contained tapetosomes and elaioplasts as the two predominant and solitary organelles (Figure 2B). Thus, the fluorescent organelles were most likely to be tapetosomes and not elaioplasts, because in the floating lipid pad (Figures 2E to 2J) flavonoids were present in tapetosomes and not elaioplasts.
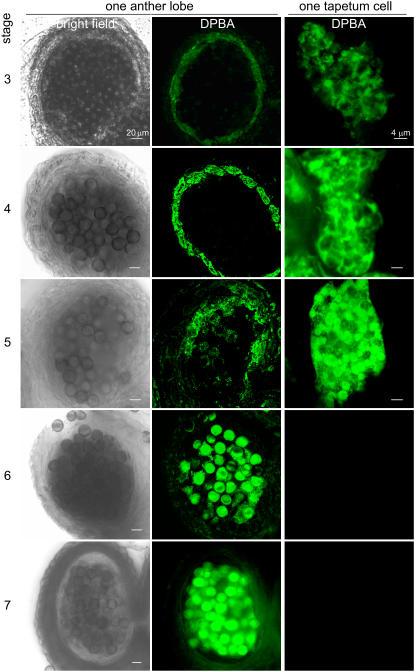
In Developing Anthers, Flavonoids Were Located in Tapetum Cells, First in a Network and Then in Spherical Organelles, and after Tapetum Lysis, on Pollen
The location of flavonoids in anthers during development was further examined by CLSM after DPBA staining (Figure 3). At stage 2 or earlier, DPBA fluorescence was not observable in the anthers. At stage 3, DPBA fluorescence was present mostly in the innermost cell layer, the tapetum, enclosing the locule. The fluorescence in the tapetum increased as development proceeded (stages 3 to 5). After tapetum lysis (stages 6 to 7), the fluorescence in the anther lobe shifted to the microspores. This pattern of appearance and persistence of flavonoids in the anthers, including the shift from the tapetum to microspores (Figure 3), is consistent with the pattern of flavonoid levels in the anthers during development, as detected by TLC (Figure 1).
Figure 3.
CLSM of Brassica Anthers and Tapetum Cells of Different Developmental Stages after DPBA Staining for Flavonoids.
The left and middle columns show CLSM images of one anther lobe of developmental stages 3 to 7 taken with a bright-field or fluorescence setting. Each lobe contained microspores in the locule enclosed by the tapetum and several outer wall layers. At stages 3 to 4, the tapetum cells were intact and possessed most of the DPBA fluorescence (shown in green) in the lobe. At stage 5, the tapetum cells were fully mature, with a few already broken; the microspores began to show DPBA fluorescence. At stages 6 to 7, the tapetum had broken, and fluorescence was present on the microspores. The right column shows CLSM images of one tapetum cell of each developmental stage taken with a fluorescence setting. Fluorescence was present first in a subcellular network and then in spherical particles.
Within the tapetum cells, DPBA fluorescence appeared in a network at stage 3 (Figure 3). At stage 4, the fluorescent network expanded to include spherical particles. Finally, fluorescence was associated only with spherical particles. At stages 6 to 7, no intact tapetum cells were present. Whether the network and spherical particles represented ER and tapetosomes, respectively, was explored by immunofluorescence CLSM and biochemical means.
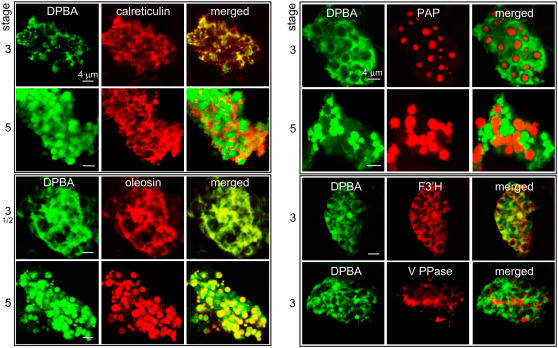
In Tapetum Cells, Flavonoids Were Located Initially in the ER Network with Flavonoid-3′-Hydroxylase and Oleosins and Then in ER-Derived Tapetosomes
Anthers were subjected to DPBA staining and immunodetection with antibodies against protein markers of major organelles in tapetum cells. Flavonoids in the tapetum cells at an early developmental stage (stage 3) were located in a subcellular network, which was also the location of the ER marker calreticulin (Figure 4). Flavonoids (shown in green) and calreticulin (red) were superimposed (yellow) in extensive regions of the network; the superimposition was not complete, which indicates the presence of ER subdomains for tasks not related to flavonoid metabolism. At a later stage (stage 5), flavonoids were present largely in spherical particles (green) and some in the ER (yellow) outside the particles. At developmental stages 3 to 5, flavonoids (green) in the tapetum cells were superimposed (yellow) with oleosins (red) first in most regions of a network (ER cisternae) and subsequently in spherical particles.
Figure 4.
CLSM of Brassica Tapetum Cells of Developmental Stages 3 or 5 to Test for Colocalization of Flavonoids and Organelle-Specific Proteins.
Anthers were treated with DPBA for flavonoid fluorescence (shown in green) and then antibodies against calreticulin (markers of ER), oleosin (tapetosomes), F3′H (a flavonoid-synthesizing enzyme presumed to be located on the ER), V-PPase (vacuoles), or PAP (elaioplasts) for immunodetection (shown in red). Yellow/orange color in the merged images indicates colocalization.
We can now conclude that the flavonoid-containing spherical particles were the tapetosomes on the basis of the following evidence. (1) They exhibited the expected size and abundance of tapetosomes in tapetum cells (Figures 2 and 3). (2) They were associated with the ER at an early developmental stage and became solitary at a late developmental stage (Figures 3 and 4); this is the pattern of tapetosome biogenesis established previously (Hsieh and Huang, 2005). (3) They were also the subcellular locations of oleosins (Figure 4). (4) The tapetosomes in an isolated lipid pad contained flavonoids (Figures 2E to 2J). Additional evidence was obtained with biochemical studies, as described in the next section.
Flavonoids (Figure 4, shown in green) in the ER network in a stage 3 tapetum cell were superimposed (yellow) largely with flavonoid-3′-hydroxylase (F3′H) (red), a key enzyme in the flavonoid synthesis pathway (Winkel-Shirley, 2001). This observation expands the earlier findings that the flavonoid synthetic enzymes, chalcone synthase and chalcone isomerase, are present in the ER in roots (Saslowsky and Winkel-Shirley, 2001). F3′H was not detected in stage 5 tapetum cells or on pollen.
Vacuoles of 1 to 6 μm in diameter were present in each tapetum cell at different stages of anther development (Figure 2B) (Platt et al., 1998). They were detected by immunofluorescence CLSM with antibodies against bean (Phaseolus vulgaris) vacuolar pyrophosphatase (V-PPase) (Sarafian et al., 1992), a vacuole marker protein (Figure 4). V-PPase had subcellular locations distinct from that of ER tapetosomes housing flavonoids; this finding indicates that the flavonoids were not present in cell vacuoles in tapetum cells of Brassica species, as is the case in some cells of other plant species. Flavonoids in ER–tapetosomes were not colocalized with PAP in a stage 3 or stage 5 tapetum cell (Figure 4), a marker of elaioplasts (Kim et al., 2001). The elaioplasts increased in size from 1 to 2 μm to 3 to 4 μm in diameter and were always solitary entities. The distribution of flavonoids in the tapetosomes in tapetum cells did not match the location of the nucleus (two nuclei in some younger cells) (Figures 2K and 2D, respectively); thus, flavonoids were not present in the nuclei.
Subcellular Fractionation of Tapetum Cells Localized Flavonoids and Alkanes in the Tapetosomes
Subcellular fractionation and biochemical analyses provide further evidence that flavonoids are located in the ER and tapetosomes. An extract of stage 5 anthers, obtained after mild grinding of anther sections and thus extracting largely the tapetum cell contents (see Methods), was separated by sucrose density gradient centrifugation into fractions of cytosol, membranes (largely those of the ER), tapetosomes, and elaioplasts. SDS-PAGE and immunoblot analyses confirmed the authenticity of these fractions (Figure 5A). The membrane fraction (containing ER and ER–tapetosome complexes) possessed calreticulins (several detectable polypeptides) and oleosins (of 48 and 45 kD). The tapetosome fraction also contained calreticulins and oleosins but had a higher proportion of oleosins to calreticulins. It did not contain the more abundant proteins present in the cytosol and elaioplast fractions and thus was considered pure. The elaioplast fraction contained PAP of 30 to 35 kD as the major polypeptides. These subcellular fractions were probed for their content of flavonoids and alkanes.
Figure 5.
SDS-PAGE, Immunoblot, and Chromatography Analyses of Subcellular Fractions of Stage 5 Brassica Anthers and Subfractions of Isolated Tapetosomes.
(A) An anther extract (labeled as total), representing materials mostly from tapetum cells (see Methods), was subfractionated by centrifugation into fractions of cytosol, membranes (densities of 1.10 to 1.12 g/cm), tapetosomes (∼1.04 g/cm), and elaioplasts (∼1.02 g/cm). For clarity in analyses, samples containing approximately equal amounts of proteins were applied to the gel or TLC lanes.
(B) Isolated tapetosomes were treated with 0.1 M Na2CO3 and then subfractionated into vesicle and lipid droplet subfractions. Samples of the two subfractions applied to the gel or TLC lanes represented those from an equal amount of the tapetosome fraction.
For (A) and (B), the analysis methodology and labels are as described for Figure 1.
(C) Lipids in the total tapetosome fraction were separated by HPLC. Arrows on the x axis indicate the elution times of the lipid standards C-29 alkane, cholesteryl palmitate, and triolein (from left to right).
(D) Tapetosome alkanes obtained by TLC were separated by gas chromatography. Alkanes of C-29 and C-27, identified by MS or comigration with C-29 alkane standard, and three unknown alkanes were resolved.
The major flavonoids kaempferol and quercetin and several minor flavonoids were present largely in the tapetosome (∼70%) and membrane (∼30%) fractions and absent in the cytosol and elaioplast fractions (Figure 5A). This distribution of flavonoids in the tapetosome and membrane fractions was similar to that of oleosins. The flavonoids and oleosins in the membrane fraction should represent the nascent molecules in the ER cisternae and the ER–tapetosome complexes. The biochemical findings of flavonoids and oleosins being present largely in the tapetosomes and some in the membrane fraction reflected what we observed in situ by CLSM.
Alkanes were located largely in the tapetosome fraction and some in the membrane fraction (Figure 5A). They were absent in the elaioplast and cytosolic fractions. The lipids in the tapetosome fraction contained mostly TAGs (approximately two-thirds) and alkanes (approximately one-third), as resolved by TLC (Figure 5A) and HPLC (Figure 5C). The silica gel corresponding to the alkane spot on the TLC plate was scraped, and the lipids on the silica gel were extracted and analyzed by gas chromatography. The two major tapetosome alkanes were C-29 (49%) and C-27 (20%) (Figure 5D). Steryl esters were present largely in the elaioplast fraction (Figure 5A), as reported previously (Wu et al., 1999), and at a low level in the membrane fraction (probably containing some broken elaioplasts).
Subfractionation of Isolated Tapetosomes Localized Flavonoids in ER-Derived Vesicles and Alkanes in Oleosin-Coated Lipid Droplets
A tapetosome contains oleosin-coated lipid droplets and ER-derived vesicles held together by ionic forces (Hsieh and Huang, 2005). We explored the subtapetosome locations of flavonoids and alkanes. Isolated tapetosomes were treated with 0.1 M Na2CO3 and then subjected to centrifugation. The resulting lipid droplet and vesicle subfractions were subjected to SDS-PAGE, immunoblot, and TLC analyses (Figure 5B). The two subfractions had distinct protein constituents that were markers of the respective suborganelle compartments. The lipid droplet subfraction possessed the major oleosins of 48 and 45 kD (Figure 5B) and several minor and smaller oleosins (Wu et al., 1997), whereas the vesicle subfraction contained calreticulins and other proteins. The flavonoids kaempferol and quercetin were present exclusively in the vesicle subfraction (Figure 5B). TAGs were present mostly in the lipid droplet subfraction, as reported previously (Hsieh and Huang, 2005). Importantly, alkanes were also present only in the lipid droplet subfraction.
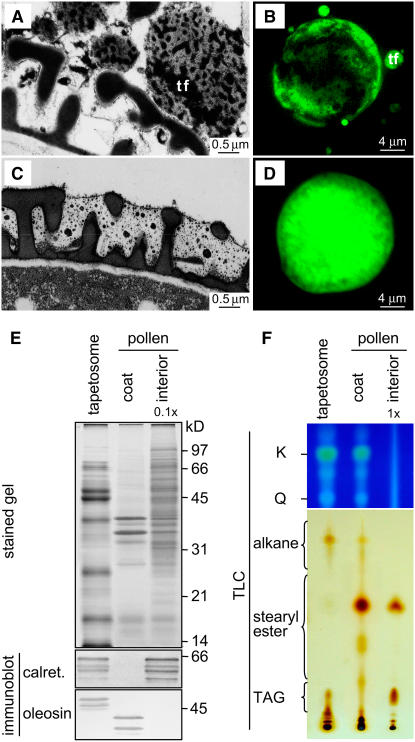
After Tapetum Lysis, the Tapetosome Flavonoids and Alkanes Were Present in the Pollen Coat
After tapetum lysis at a late stage of anther development, tapetosome oleosins in fragmented forms are transferred to the surface of maturing pollen; the tapetosome TAGs, calreticulin, and binding protein disappear (Wu et al., 1997; Hsieh and Huang, 2005). In this study, the fate of the tapetosome flavonoids and alkanes after tapetum lysis was investigated. Observations of late-stage anthers in situ by electron microscopy revealed that immediately after tapetum lysis (stages 5 to 6), the tapetosomes, or their fragmented forms, were located near the surface of maturing microspores, whose exine cavity was still empty (Figure 6A). After the microspores had matured but were still inside the anther locule (stage 7), the coat had a semi-electron-translucent matrix (presumably of lipids including steryl esters and alkanes), which filled the space within the exine and on its surface (Figure 6C). Electron-dense droplets, which could represent flavonoids, were suspended in the semitranslucent matrix. CLSM of mature anthers after DPBA staining showed the progressive increase of flavonoids on the microspore surface from stages 5/6 to 7 (Figures 6B and 6D).
Figure 6.
Microscopy of Brassica Microspores in Anther Locule after Tapetum Lysis, and SDS-PAGE, Immunoblot, and TLC Analyses of Isolated Tapetosomes, Pollen Coat, and Pollen Interior.
(A) Electron microscopy of a stage 6 anther shows tapetosomes or their fragments (tf) adjacent to the surface of a microspore, whose exine cavities had not acquired coat materials.
(C) Electron microscopy of a stage 7 anther shows the exine cavities of a microspore after acquisition of coat materials, which had a semitranslucent matrix embedded with electron-dense droplets.
(B) and (D) Microspores in stage 5/6 (B) and 7 (D) anthers, which had been stained with DPBA for flavonoid fluorescence (shown in green) and analyzed by CLSM with the same CLSM settings (laser power and detection gain).
(E) and (F) SDS-PAGE, immunoblot, and TLC analyses of isolated tapetosomes, pollen coat, and pollen interior. Analysis methodology and labels are as described for Figure 1. The interior fraction applied to the gels for protein analyses and to TLC plates for flavonoid and lipid analyses represented 0.1× and 1×, respectively, the coat fraction from the same amount of mature pollen.
Mature pollen was separated into coat and interior fractions, which, along with isolated tapetosomes, were subjected to SDS-PAGE, immunoblot, and TLC analyses (Figures 6E and 6F). The coat fraction, but not the interior fraction, contained the two fragmented oleosins, which were derived from the intact 48- and 45-kD oleosins of the tapetosomes. The coat contained no calreticulins, which were present in the interior fraction and should represent the gametophytic ER calreticulins. The two flavonoids kaempferol and quercetin were present only in the coat fraction. Apparently, the gametophytic pollen interior did not produce these flavonoids for internal uses.
Alkanes were present only in the coat fraction, whereas steryl esters were present in both the coat and the interior fractions (Figure 6F). The coat contained minimal TAGs originating from the tapetosomes, as reported previously (Wu et al., 1997). TAGs were present in the interior fraction and should be those in pollen oil bodies (Kim et al., 2002).
In an Arabidopsis Mutant Devoid of Chalcone Synthase, the Tapetum Cells, Tapetosomes, and Pollen Coat Contained No Flavonoids
Our findings show that in developing Brassica anthers, flavonoids were present initially in tapetum cells, first in the ER and then in ER-derived tapetosomes, and subsequently on the pollen coat. We further examined this route with Arabidopsis mutants defective in the flavonoid synthesis pathway. All existing information indicates that the tapetum cells in the genera Arabidopsis and Brassica of the Brassicaceae family share similar morphological and molecular characteristics, including tapetosomes and elaioplasts and the clustering of oleosin genes and their expression (Owen and Makaroff, 1995; Ferreira et al., 1997; Kim et al., 2002, 2005).
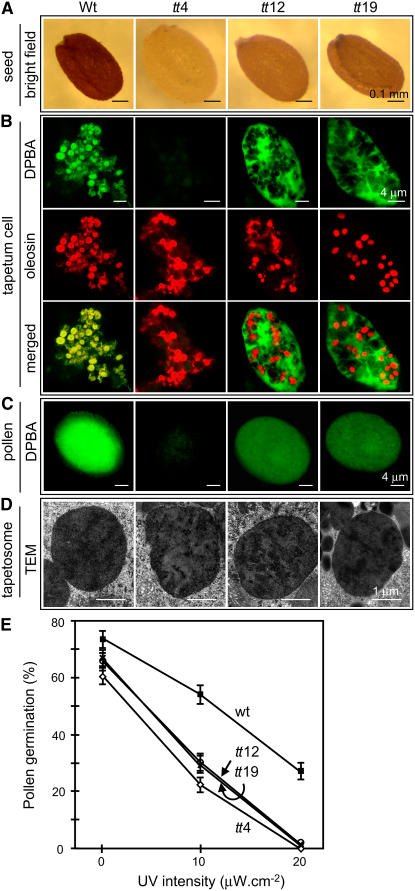
We compared the wild type and the homozygous tt4 mutant, a knockout mutant of the single gene encoding chalcone synthase that catalyzes the first committed step in flavonoid synthesis (Koornneef, 1981). The wild-type seed coat was dark brown, whereas the tt4 seed appeared pale yellow (Figure 7A). Wild-type and tt4 tapetum cells (at a late developmental stage when the tapetosomes had become solitary organelles) were examined for flavonoids and oleosins by CLSM after DPBA staining and immunodetection. Flavonoids in tapetum cells of the Arabidopsis wild type, similar to those in Brassica, were colocalized with oleosins in tapetosomes (Figure 7B). No flavonoid fluorescence was observed in tt4 tapetum cells, which nevertheless possessed oleosin-containing tapetosomes of a size similar to that of wild-type tapetosomes. Flavonoids were found on the wild-type, but not the tt4, pollen surface (Figure 7C).
Figure 7.
Microscopy of Seeds, Tapetum Cells, and Pollen, and Germination of Pollen in the Absence and Presence of UV-B Irradiation of Arabidopsis Wild Type and Homozygous Mutants.
Mutant tt4 has no chalcone synthase, and mutants tt12 and tt19 are deficient in a flavonoid membrane transporter.
(A) Seeds were photographed with a light microscope under an identical bright-field setting.
(B) Tapetum cells in anthers of a late developmental stage, when most of the tapetosomes had become solitary organelles, were stained with DPBA for flavonoids (shown in green) and then treated with antibodies against oleosin for immunodetection (in red) and analyzed by CLSM. Yellow color in the merged images indicates colocalization. Fluorescence imaging of the mutant and wild-type samples for immunodetection of oleosins used the same CLSM settings (laser power and detection gain). Fluorescence imaging of the mutant samples (which had no or reduced flavonoids) for DPBA stain used higher CLSM settings than those of the wild-type samples.
(C) Mature pollen was stained with DPBA (shown in green) and analyzed by CLSM. Fluorescence imaging of the mutant pollen (which had no or reduced flavonoids) for DPBA stain used higher CLSM settings than those of the wild-type pollen.
(D) Stage 5 tapetum cells were observed by transmission electron microscopy (TEM). Each image shows a tapetosome with patches of electron-dense materials.
(E) Pollen was allowed to germinate on agar plates in dim visible light (0.43 μW/cm) without or with supplemented UV-B irradiation of 10 or 20 μW/cm. The Petri dishes were covered with a 0.13-mm-thick cellulose diacetate film. After 2 h of exposure, the agar plates were placed in the dark for 5 h, and germination was observed by light microscopy. The se of each data point (n ≥ 500) is shown.
In Arabidopsis Mutants Devoid of a Membrane Flavonoid Transporter, Flavonoids at Reduced Levels Were Present in the Cytosol but Not in Tapetosomes in the Tapetum
We investigated two related Arabidopsis homozygous mutants, tt12 (Debeaujon et al., 2001) and tt19 (Kitamura et al., 2004), which are devoid of a membrane flavonoid transporter, multidrug secondary transporter–like protein and glutathione S-transferase, respectively. Unlike tt4 seeds, tt12 and tt19 seeds had a brown coat, presumably representing reduced amounts of flavonoids, compared with wild-type seeds (Figure 7A). tt12 and tt19 tapetum cells (at a late developmental stage when the tapetosomes had become solitary organelles) were examined for flavonoids and oleosins by CLSM after DPBA staining and immunodetection. Flavonoids in the tapetum cells of the mutants, unlike those in the wild type, were not associated with the tapetosomes (oleosin as marker) but were present in the cytosol (Figure 7B). The amount of all of the cytosolic flavonoids in a whole tt12 or tt19 tapetum cell was substantially less than that of all of the tapetosome flavonoids in a whole wild-type tapetum cell; for clarity in visualization as presented in Figure 7B, fluorescence imaging of the tt samples used higher CLSM settings (laser power and detection gain) than in the wild-type sample. Thus, in the absence of the flavonoid transporter, flavonoids could not be transported across the ER membrane into the lumen to become tapetosome ingredients. As a consequence, flavonoids accumulated in the cytosol, and the reduction in their levels might have been caused by a metabolic feedback inhibition.
Flavonoids were present on tt12 and tt19 pollen, but at substantially reduced levels, compared with wild-type pollen. This is shown in Figure 7C, in which the CLSM settings with the mutant pollens were deliberately made higher than those with the wild-type pollen to reveal the minimal levels of flavonoids on the mutant pollen. Thus, upon tapetum lysis, the cytosolic flavonoids were released to the locule and then onto the maturing pollen. The reduction in flavonoid amounts in the tapetum cells and pollen coat of tt12 and tt19 (Figures 7B and 7C) is concomitant with that in the mutant seed coats (Figure 7A).
No appreciable difference in the size and number of tapetosomes in the wild type and the tt4, tt12, and tt19 mutants was observed by CLSM (Figure 7B). This was reflected in observations of electron microscopy sections of tapetum cells of the Arabidopsis samples. The four Arabidopsis samples had no appreciable morphological difference in their cytoplasm, and their tapetosomes all possessed patches of electron-dense materials (Figure 7D), as did those in Brassica (Figure 2B). Diameters of tapetosomes (measuring those >1.5 μm on the electron microscopy sections) of the wild type, tt4, tt12, and tt19 were 2.04 ± 0.21, 2.15 ± 0.25, 2.07 ± 0.21, and 2.14 ± 0.25 μm, respectively. Diameters of elaioplasts were 2.45 ± 0.35, 2.49 ± 0.37, 2.51 ± 0.38, and 2.49 ± 0.37 μm, respectively. The differences in the sizes of tapetosomes or elaioplasts among the four Arabidopsis samples were not significant (P > 0.05). The lack of a difference in the size and electron microscopy morphology of tapetosomes with or without flavonoids could be explained as follows. In a Brassica tapetosome, the ER-derived vesicles (shown here to contain flavonoids) in situ are densely packed (Hsieh and Huang, 2005) and thus occupy only a small total organelle volume. In addition, flavonoids in these vesicles apparently did not react sufficiently with the fixation and poststaining reagents to reveal a morphological difference of the whole tapetosomes with or without flavonoids by electron microscopy.
Arabidopsis Mutant Pollen Devoid of Coat Flavonoids Was More Susceptible to UV-B Irradiation Damage Than Wild-Type Pollen
Mutational loss of flavonoid synthesis (tt4) or flavonoid transport into ER–tapetosomes (tt12 and tt19) in the Arabidopsis tapetum led to a deficiency of flavonoids on the pollen surface. Pollen of tt4 had no or only a minimal defect in fertility under experimental conditions (Burbulis et al., 1996; Ylstra et al., 1996). Pollen of diverse plant species contains abundant flavonoids on its surface. Pollen surface flavonoids, which absorb at 280 to 320 nm, could protect the haploidic pollen from UV-B irradiation damage, but a test of this hypothesis has not been reported previously (see a recent extensive review by Pacini and Hesse, 2005). In this study, we subjected wild-type and tt pollen to UV-B irradiation and then examined their ability to germinate on agar (Figure 7E). Wild-type pollen germinated slightly better than the tt pollen (74 versus 61 to 67%) on an agar medium in dim white light of 0.43 μW/cm. When this dim white light was supplemented with UV-B light of 10 μW/cm (approximately similar to that present in average daylight in the United States) and twice this intensity for 2 h, the germination rate of wild-type pollen was reduced, from 74 to 54 to 27%. Pollen of the three types of tt mutants was substantially more sensitive to the UV-B irradiation damage; similar exposure of the mutant pollen to UV-B irradiation reduced their germination rates from 61 to 70% to 26 to 30% and to zero.
DISCUSSION
Colocalization of Flavonoids and Proteins Reveals Intracellular Movement and Storage of Flavonoids and Their Extracellular Transfer
Flavonoid synthetic enzymes are located on the cytosolic side of the ER (Winkel, 2004). After synthesis, the flavonoids are transported to the vacuoles via a membrane transporter (Mueller and Walbot, 2001), the nuclei (Saslowsky et al., 2005), other possible subcellular compartments, or the cellular exterior (Taylor and Grotewold, 2005; Yazaki, 2005). The movement of flavonoids from the ER to these other intracellular sites or the cellular exterior has not been studied previously. Here, we establish a new procedure for the subcellular colocalization of flavonoids and proteins in situ. With this procedure, we show that flavonoids synthesized in the ER in tapetum cells are not released into the cytosol but are translocated into the ER lumen, or at least somehow associated with the ER, and then transported to the ER-derived tapetosomes. We also reveal that in Arabidopsis mutants defective in a flavonoid membrane transporter, the flavonoids reside in the cytosol. Thus, at least in Arabidopsis tapetum cells, the flavonoid transporters reside on the ER membrane instead of the vacuolar membrane. This new in situ procedure should be applied to different plant systems to study subcellular compartmentation and movement of flavonoids.
Tapetosomes Evolved for Storage and the Coordinated Release of Flavonoids, Alkanes, and Oleosins
Tapetosomes in a tapetum cell function as organized metabolic sinks and temporary warehouses of important molecules, including alkanes, oleosins, and flavonoids, for discharge to the pollen surface. Alkanes, along with elaioplast steryl esters, waterproof the pollen; a mutational loss of alkanes (wax) on the pollen surface leads to pollen infertility that can be partially overcome in the presence of high humidity (Preuss et al., 1993; Hulskamp et al., 1995). Oleosins emulsify these lipids and the semi-water-soluble flavonoids to form a semifluidic coat and aid water uptake into the pollen from the stigma. Flavonoids protect the haploidic DNA in the pollen from UV-B irradiation damage, a hypothesis that has not been validated until this study.
The tapetosome system represents a higher order of subcellular compartmentation compared with a system of numerous small flavonoid-containing vesicles and alkane-plus-oleosin droplets scattered in the cytoplasm. Whether tapetosomes are present in plant families other than Brassicaceae is unknown. Putative lipid-rich particles in tapetum cells of diverse species can be seen in electron microscopy images (Hesse et al., 1993), and flavonoids and neutral lipids are present universally on pollen of diverse species. Yet, tapetosomes are clearly absent in maize and other cereals (Bih et al., 1999; Wu et al., 2002). The maize pollen coat does not have oleosins, and its major constituent is an endoxylanase that is synthesized and stored in the tapetum cell cytosol for release upon PCD. The mechanisms whereby tapetum cells in maize and other species synthesize and store flavonoids (secretory vesicles, vacuoles, cytosol, etc.) and neutral lipids (solitary or ER-linked droplets, etc.) and transfer them to the pollen surface remain to be elucidated.
We envision that the tapetosome evolved in tapetum cells in the following manner. The tapetum cells produced flavonoids, which were synthesized in the ER and stored in ER-derived vesicles, which released the flavonoids to the locule either continuously by exocytosis or upon PCD. They also produced ER-synthesized neutral lipids, which were somehow transported to the cellular exterior, perhaps by a mechanism similar to that of lipid secretion in epidermis (Kunst and Samuels, 2003). Subsequently, the neutral lipids were temporarily stored in cytoplasmic lipid droplets, which were stabilized by surface oleosins. These ER-derived flavonoid-containing vesicles and lipid droplets present as separate entities may still exist in the tapetum cells of some plant species, such as maize and other cereals. In Brassicaceae species, the flavonoid-containing vesicles and lipid droplets became associated via ionic binding to become tapetosomes (Hsieh and Huang, 2005). This association confers an advantage of organized storage and simultaneous release.
Tapetum Exports Neutral Lipids by a Mechanism Different from That in Aerial Epidermis
The transfer of neutral lipids such as alkanes from the tapetum to the cellular exterior after PCD in Brassica differs from that in aerial epidermis (Kunst and Samuels, 2003). In epidermal cells, fatty acids synthesized in plastids are transported to the ER, where they undergo elongation and other modifications to become cuticular lipids, including alkanes. The ER cisternae are not in physical contact with the PM; thus, the synthesized lipids in the ER cisternae need to be transported to the PM and then the cellular exterior. The movement of lipids across the PM requires an ATP binding cassette transporter (Pighin et al., 2004).
Alkanes and flavonoids of the tapetosomes are transferred to the adjacent pollen surface upon tapetum PCD. This tapetum system apparently does not operate in epidermal cells but may exist in some cells that undergo PCD (Wu and Cheung, 2000; Gray, 2003). The system of accumulation and temporary storage of alkanes, oleosins, and flavonoids in tapetum cells and their coordinated release to the locule can be used as a working model to test whether its modified forms exist in other cells that undergo PCD.
Pollen Coat Flavonoids Protect the Haploidic Pollen from UV Irradiation Damage and May Serve Other Purposes
Pollen of Arabidopsis tt mutants germinated almost as well as wild-type pollen on an agar medium with no UV irradiation (Figure 7). This finding is consistent with the following results of in vivo fertilization of homozygous recessive tt mutants. (1) Pollen of tt4 was fertile in in vivo selfing under experimental conditions (Burbulis et al., 1996; Ylstra et al., 1996). (2) The ABRC provided us with mostly homozygous but viable tt4, tt12, and tt19 seeds, which presumably were generated from selfing of homozygous tt mutants. (3) When we propagated the tt mutants, we let homozygous mutant plants undergo selfing and obtained viable homozygous tt seeds. Nevertheless, the tt pollen was more susceptible to UV irradiation damage in its ability to germinate in vitro than was wild-type pollen (Figure 7).
Flavonoids are present on the pollen of essentially all land plants. We suggest that flavonoids on the pollen surface provided a long-term evolutionary advantage in protecting the haploidic pollen from UV irradiation, especially for plant species that are not self-pollinating within the same flower. The pollen of maize and petunia mutants devoid of flavonoids is sterile in vivo under experimental conditions (Mo et al., 1992; van der Meer et al., 1992; Napoli et al., 1999). Apparently, flavonoids on the pollen of these latter plant species exert additional physiological functions, perhaps regulating auxin transport (Jacobs and Rubery, 1988; Buer and Muday, 2004) in the pollen tube. These additional physiological functions of the pollen coat flavonoids might have been acquired late during evolution.
METHODS
Plant Materials
Seeds of Brassica napus, an amphidiploid possessing the AA and CC genomes, were obtained from Calgene. Arabidopsis thaliana ecotype Col-0 wild type and mutants tt4 (ethyl methanesulfonate–induced mutation at At5g13930), tt12 (T-DNA insertion at At3g59030), and tt19 (T-DNA insertion at At5g17220) seeds were obtained from the ABRC. Plants were grown from seeds to flowering in greenhouses maintained at 26/18°C in a 14-/10-h day/night cycle. For developmental studies of the flowers, Brassica florets were divided into seven stages according to the flower bud length (Hsieh and Huang, 2005). For stages 1 through 6, the lengths of the buds were 2, 3, 4, 5, 6, and >6 mm, respectively. Stage 7 anthers, including mature pollen, were collected from just-opened flowers, and mature pollen from opened flowers.
Preparation of Anther Extracts, Pollen Coat, and Pollen Interior
Anthers and mature pollen were homogenized vigorously with the use of a mortar and pestle in an SDS-PAGE loading solution (0.06 M Tris-HCl, pH 6.8, 2% SDS, 5% 2-mercaptoethanol, 0.002% bromophenol blue, and 10% glycerol) for protein analyses or in 0.05 M HEPES-NaOH, pH 7.5, for lipid and flavonoid analyses. Light microscopy examination of the homogenates revealed that most anther wall cells and microspores were broken.
Mature pollen was fractionated by washing with diethyl ether to yield a coat fraction and by homogenizing the leftover pollen as an interior fraction with SDS-PAGE loading solution for protein analyses (Hsieh and Huang, 2005) or with 0.05 M HEPES-NaOH, pH 7.5, for lipid analyses. For flavonoid analysis, pollen was fractionated by washing with chloroform and then methanol to yield a coat fraction and by homogenizing the leftover pollen with 0.05 M HEPES-NaOH, pH 7.5, to produce an interior fraction.
Organelle Isolation and Tapetosome Subfractionation
The procedure followed that described previously (Hsieh and Huang, 2005). All solutions contained 0.05 M HEPES-NaOH, pH 7.5. Stage 5 anthers were chopped in a 0.6 M sucrose solution at 4°C with a razor blade. The homogenate was filtered through a Nitex cloth (20 × 20 μm pore size), which retained most microspores (∼25 μm in diameter) and unbroken outer anther cells that were more difficult to break than the wall-depleted tapetum cells. The filtrate, regarded as crude extract, represented materials mostly from tapetum cells. It was subjected to sedimentation centrifugation, with 2 mL placed on a step gradient in a 5-mL tube consisting of 1.5 mL of 1.0 M and 1.5 mL of 0.8 M sucrose solution. The gradient was centrifuged at 35,000 rpm for 4 h in a Beckman SW55 rotor. Materials remaining in the 0.6 M fraction and locating at the interface between the 0.8 and 1.0 M solutions were regarded as the cytosol fraction and the membrane fraction, respectively. The crude extract was also subjected to floatation centrifugation, with 5 mL placed in an 18-mL tube and overlaid with 4-mL solutions each of 0.4, 0.2, and 0 M sucrose. The gradient was centrifuged at 9000 rpm for 2 h in a Beckman SW28 rotor. Elaioplast and tapetosome fractions at the interfaces between the 0 to 0.2 M and 0.2 to 0.4 M sucrose solutions, respectively, were collected.
The floatation centrifugation described above was modified to yield a floating lipid pad that contained elaioplasts in the top half and tapetosomes in the bottom half (Wu et al., 1997). The crude extract (5 mL) was placed in an 18-mL tube and overlaid with 12 mL of 0.4 M sucrose solution. The gradient was centrifuged at 9000 rpm for 2 h in a Beckman SW28 rotor. The lipid pad was removed with a spatula and used for microscopy localization of flavonoids in the two organelles.
Isolated tapetosomes were subfractionated by a procedure described previously (Hsieh and Huang, 2005). The organelles in 0.3 M sucrose/0.05 M HEPES-NaOH, pH 7.5, were mixed with an equal volume of 0.2 M Na2CO3, pH 11.5/0.9 M sucrose at 4°C for 16 h. The sample (2 mL) was placed in a 5-mL tube and overlaid with successive 1-mL layers of 0.5 M sucrose/0.1 M Na2CO3/0.05 M HEPES, pH 11.5, 0.4 M sucrose/0.1 M Na2CO3/0.05 M HEPES, pH 11.5, and 0.2 M sucrose/0.05 M HEPES-NaOH, pH 7.5. The tube was centrifuged at 35,000 rpm for 2 h in a Beckman SW55 rotor. Floating materials at the top of the gradient were collected with a spatula and regarded as the lipid-droplet subfraction. The 2 mL of materials at the bottom of the tube was collected and diluted with 3 mL of 0.1 M Na2CO3, and the mixture was centrifuged at 45,000 rpm for 2 h in a Beckman Ti60 rotor. The pellet was regarded as the vesicle subfraction.
SDS-PAGE and Immunoblot Analyses
Proteins were resolved by 12.5% SDS-PAGE and then subjected to immunoblot analyses as described (Hsieh and Huang, 2005). For immunoblot analyses, 1:500 rabbit polyclonal antisera against castor calreticulin (Coughlan et al., 1997) and Brassica 45-kD oleosin (Wu et al., 1997), as well as 1:200 chicken yolk antibodies against Brassica PAP (Kim et al., 2001), were used.
Lipid Extraction and Analyses
The pollen coat fraction in ether was allowed to evaporate to dryness; the residue was dissolved in lipid extraction solvents (chloroform:heptane:methanol, 4:3:2; v/v/v) (Wu et al., 1997). Lipids in anther crude extracts, anther subcellular fractions, tapetosome subfractions, total pollen extract, and pollen interior fraction, which were all of aqueous preparations, were extracted twice with 2 volumes of the lipid extraction solvents described above. The lipids were applied to TLC plates (silica gel 60 A; Whatman), which were developed in hexane:diethyl ether:acetic acid (95:5:2, v/v/v), and stained in an iodine chamber for 24 h. Most lipids were stained within 30 min, but alkanes were stained only after a long period of time. Triolein, cholesteryl palmitate, and C-30 and C-29 alkanes (all from Sigma-Aldrich) were used as lipid standards.
Total lipids extracted from the isolated tapetosome fraction were analyzed by HPLC with a Chromsep cartridge (LiChrosorb DiOL, 5 μm, 3 × 100 mm; Chrompack) (Singh et al., 2003). The cartridge was attached to a ternary gradient HPLC modular system (HP1050; Hewlett-Parkard) and an evaporative light-scattering detector (Alltec-Varex Mark III) operated at 40°C, with nitrogen used as a nebulizing gas at a flow rate of 1.6 L/min. The mobile phase was a gradient of hexane, 2-propanol, and acetic acid, and the flow rate was constant at 0.5 mL/min. Standards of alkanes (C-30 and C-29), steryl ester (cholesteryl palmitate), and TAG (triolein) were used to mark elution times.
The silica gel corresponding to the alkane spot on a TLC plate after separation of the total lipids of an isolated tapetosome fraction was scraped, and the lipids were extracted three times with chloroform. The chloroform sample was evaporated to dryness under a stream of nitrogen, and the residue was dissolved in N,O-bis[trimethylsily]trifluoroacetamide and heated at 80°C for 1 h. Excess N,O-bis[trimethylsily]trifluoroacetamide was evaporated under a stream of air. The sample was dissolved in iso-octane and injected into a gas chromatograph (Hewlett-Packard 5890) with a DB-1 column, 30 m long and 0.25 mm in diameter, attached to a flame ionization detector and a mass detector (Hewlett-Packard 5971A). The mass spectra of the resolved peak molecules, plus comparisons of the migration of commercially available C-30 and C-29 alkanes, identified the resolved major alkanes to be C-29 and C-27 alkanes.
Flavonoid Extraction and Analyses
In initial tests, rutin (quercetin-3-rhamnose-glucose; Sigma-Aldrich) and Brassica anther extracts were placed in 0 to 2 M HCl at different temperatures for 30 to 60 min. Nonglycosylated and glycosylated flavonoids in the samples were extracted twice with an equal volume of isoamyl alcohol at 80°C. They were dried under a stream of nitrogen gas, and the residues were dissolved in methanol for TLC. After these initial tests, all anther extracts were deglycosylated in 2 M HCl at 80°C for 1 h. TLC plates were developed in toluene:ethylacetate:formic acid:water (50:40:10:5, v/v/v/v) and sprayed with 0.5% (w/v) DPBA (Sigma-Aldrich) in methanol for flavonoid staining (van der Meer et al., 1992). The plates were observed and photographed with a DSC-707 Cyber-shot digital camera (Sony) on top of a UV irradiation source. Quercetin, kaempferol, and rutin (Sigma-Aldrich) were used as flavonoid standards.
CLSM
A procedure was developed and is explained in Results. For flavonoid localization with DPBA, anther sections (cross-sectioned), mature pollen, and the lipid pad (containing elaioplasts on top of tapetosomes) were fixed in 4% paraformaldehyde, 1× PBS (10 mM K-phosphate, pH 7.4, 138 mM NaCl, and 2.7 mM KCl) and 0.15 M sucrose at 20°C for 1 h. Samples were washed with PBST (1× PBS and 0.1% Tween 20) twice and treated with 1 M HCl at 70°C for 30 min. Samples were washed with 10% glycerol twice and stained with a DPBA dye (saturated DPBA [<0.5%, w/v], 0.01% Triton X-100, and 10% glycerol [Buer and Muday, 2004]) at 20°C for 2 h. Samples were washed twice with 10% glycerol.
For double labeling with DPBA for flavonoids and antibodies against organelle marker proteins, the samples after the above fixation and acid treatment were blocked with 3% milk in 1× PBS at 20°C for 1 h. Samples were treated with 1:40 rabbit antibodies against calreticulin, PAP (chicken instead of rabbit antibodies), oleosin (antibodies against these three proteins were described above), F3′H (courtesy of Johnson Wang, Taiwan Agricultural Institute), or V-PPase (courtesy of Philip A. Rea; Sarafian et al., 1992) in 1% milk and 1× PBS at 20°C for 2 h. After two washes with PBST, samples were treated with a mixture of DPBA dye and secondary antibodies (0.5% DPBA, 0.01% Triton X-100, 10% glycerol, 1× PBS, and 1:100 cyanine 5–conjugated goat antibodies against rabbit IgG [product 111-175-003; Jackson ImmunoResearch] or cyanine 5–conjugated rabbit antibodies against chicken IgY [product 303-175-003; Jackson ImmunoResearch]) at 20°C for 2 h. Samples were washed twice with PBST.
For labeling of nucleic acid, stage 5 Brassica anther sections were fixed in 4% paraformaldehyde, 1× PBS, and 0.15 M sucrose at 20°C for 1 h. They were incubated with 4 μg/mL ethidium bromide in 1× PBS at 20°C for 4 h and washed twice with PBST.
Confocal microscopy involved the use of a Leica SP2 microscope fitted with a 63 × 1.2 water-immersion objective (HC × PL APO) and two HeNe and Ar ion lasers. Cyanine 5 was excited with the 633-nm line, and emission was detected at 650 to 750 nm. DPBA flavonoid was excited with 364- to 422-nm lines, and emission was detected at 450 to 550 nm. Ethidium bromide was excited with 364- to 422-nm lines, and emission was detected at 650 to 750 nm. Antifade reagent (S-2828; Molecular Probes) in 1× PBS was used as imaging medium. Sequential confocal images were taken with the use of Leica confocal software (LCS Lite).
UV Irradiation on Arabidopsis Pollen and Germination Test
Pollen of Arabidopsis wild type and tt mutants was collected from open flowers and allowed to dehydrate for 60 min at 24°C. Pollen gains were placed on 9-cm Petri dishes containing an agar germination medium [18% sucrose, 0.01% boric acid, 1 mM MgSO4, 1 mM CaCl2, 1 mM Ca(NO3)2, and 0.5% agar, pH 7] (Li et al., 1999). The Petri dishes were covered with a 0.13-mm-thick cellulose diacetate film and exposed to dim light (0.43 μW/cm, from fluorescent light bulbs; Sylvania Ocgtron 3500) alone or supplemented with UV light for 2 h. For UV supplement, a UV lamp (FS40T12 UV-B bulb; Light Sources) covered with a layer of 0.13-mm-thick cellulose diacetate (Beiza and Lois, 2001) was placed at various distances to allow the Petri dishes to receive 10 and 20 μW/cm UV-B irradiation. Afterward, the Petri dishes were placed in the dark for 5 h. Germination of the pollen was observed by light microscopy. At least 500 pollen grains per treatment per Arabidopsis sample were scored.
Electron Microscopy
Sections of anthers of Brassica and Arabidopsis were fixed with 0.5% paraformaldehyde, 2% glutaraldehyde, and 50 mM K-phosphate, pH 7.0, for 8 h at 4°C (Platt et al., 1998). The samples were washed twice in 100 mM K-phosphate buffer, pH 7.0, and postfixed in 1% OsO4 in 50 mM Na-cadodylate buffer, pH 7.0, for 1.5 h at 20°C. Isolated tapetosomes and elaioplasts were treated similarly, except that all of the solutions contained 0.35 M sucrose. The samples were then dehydrated in ethanol and embedded in Spurr's resin. The samples were sectioned (70 to 90 μm thick) with a Sorvall MT2 ultramicrotome. The sections were poststained with 1% uranyl acetate (15 min) and then 2% lead citrate (1.5 min). They were photographed with a Philips EM400 electron microscope (FEI).
Accession Numbers
The GenBank accession numbers for the mutated genes in Arabidopsis tt4, tt12, and tt19 are NM121396, AJ294464, and NM121728, respectively.
Supplemental Data
The following material is available in the online version of this article.
Supplemental Figure 1. Test of Conditions for Deglycosylation of Rutin, Flavonoids in Brassica Anther Extract in Vitro, and Flavonoids in Tapetum Cells in Situ.
Supplementary Material
Acknowledgments
This research was supported by the National Science Foundation (Grant MCB-0131358) and the U.S. Department of Agriculture (National Research Initiative Competitive Grant 2005-02429). We thank Sherry Wu and Robert Moreau for assistance in lipid analyses, Kathy Platt and Wann-Neng Jane for assistance in electron microscopy, and Rodrigo Lois and Shu Shing Wu for advice on UV irradiation. We greatly appreciate receiving antibodies against calreticulin from Sean Coughlan and Tony Kinney of DuPont; V-PPase from Philip Rea at the University of Pennsylvania, Philadelphia; and F3′H from Johnson Wang of the Taiwan Agricultural Institute.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Anthony H.C. Huang (anthony.huang@ucr.edu).
Online version contains Web-only data.
References
- Bewley, J.D., Hempel, F.D., McCormick, S., and Zambryski, P. (2000). Reproductive development. In Biochemistry and Molecular Biology of Plants, B.B. Buchanan, W. Gruissem, and R.L. Jones, eds (Rockville, MD: American Society of Plant Physiologists), pp. 988–1043.
- Bieza, K., and Lois, R. (2001). An Arabidopsis mutant tolerant to lethal UV-B levels shows constitutively elevated accumulation of flavonoids and other phenolics. Plant Physiol. 126 1105–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bih, F.Y., Wu, S.S.H., Ratnayake, C., Walling, L.L., Nothnagel, E.A., and Huang, A.H.C. (1999). The predominant protein on the surface of maize pollen is an endoxylanase synthesized by a tapetum mRNA with a long 5′ leader. J. Biol. Chem. 32 22884–22894. [DOI] [PubMed] [Google Scholar]
- Buer, C.S., and Muday, G.K. (2004). The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis root to gravity and light. Plant Cell 16 1191–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulis, I.E., Iacobucci, M., and Shirley, B.W. (1996). A null mutation in the first enzyme of flavonoid biosynthesis does not affect male fertility in Arabidopsis. Plant Cell 8 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlan, S.J., Hastings, C., and Winfrey, R. (1997). Cloning and characterization of the calreticulin gene from Ricinus communis L. Plant Mol. Biol. 34 897–911. [DOI] [PubMed] [Google Scholar]
- Debeaujon, I., Peeters, A.J.M., Leon-Kloosterziel, K.M., and Koornneef, M. (2001). The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13 853–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, M.A., de Almeida-Engler, J., Miguens, F.C., van Montagu, M., Engler, G., and deOliveira, D.E. (1997). Oleosin gene expression in Arabidopsis thaliana tapetum coincides with accumulation of lipids in plastids and cytoplasmic bodies. Plant Physiol. Biochem. 35 729–739. [Google Scholar]
- Goldberg, R.B., Beals, T.P., and Sanders, P.M. (1993). Anther development: Basic principles and practical applications. Plant Cell 5 1217–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, T.L. (1998). Flavonoid and flavonol glycoside metabolism in Arabidopsis. Plant Physiol. Biochem. 36 135–144. [Google Scholar]
- Gray, J. (2003). Programmed Cell Death in Plants. (Boca Raton, FL: CRC Press).
- Hesse, M., Pacini, E., and Willemse, M.T.M. (1993). The Tapetum. Cytology, Function, Biochemistry and Evolution. (Vienna, Austria: Springer-Verlag).
- Hsieh, K., and Huang, A.H.C. (2004). Endoplasmic reticulum, oleosins, and oils in seeds and tapetum cells. Plant Physiol. 136 3427–3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh, K., and Huang, A.H.C. (2005). Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J. 43 889–899. [DOI] [PubMed] [Google Scholar]
- Hulskamp, M., Kopczak, S.D., Horejsi, T.F., Kihl, B.K., and Pruitt, R.E. (1995). Identification of genes required for pollen–stigma recognition in Arabidopsis thaliana. Plant J. 8 703–714. [DOI] [PubMed] [Google Scholar]
- Jacobs, M., and Rubery, P.H. (1988). Naturally occurring auxin transport regulators. Science 241 346–349. [DOI] [PubMed] [Google Scholar]
- Kim, H.U., Hsieh, K., Ratnayake, C., and Huang, A.H.C. (2002). A novel group of oleosins is present inside the pollen of Arabidopsis. J. Biol. Chem. 277 22677–22684. [DOI] [PubMed] [Google Scholar]
- Kim, H.U., Li, Y., and Huang, A.H.C. (2005). Ubiquitous and endoplasmic reticulum-located lysophosphatidyl acyltransferase, LPAT2, is essential for female but not male gametophyte development in Arabidopsis. Plant Cell 17 1073–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.U., Wu, S.S.H., Ratnayake, C., and Huang, A.H.C. (2001). Brassica rapa has three genes that encode proteins associated with different neutral lipids in plastids of specific tissues. Plant Physiol. 126 330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura, S., Shikazone, N., and Tanaka, A. (2004). TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 37 104–114. [DOI] [PubMed] [Google Scholar]
- Koornneef, M. (1981). The complex syndrome of TTG mutants. Arabidopsis Inf. Serv. 18 45–51. [Google Scholar]
- Kunst, L., and Samuels, A.L. (2003). Biosynthesis and secretion of plant cuticular wax. Prog. Lipid Res. 42 51–80. [DOI] [PubMed] [Google Scholar]
- Li, H., Lin, Y.K., Heath, R.M., Zhu, M.X., and Yang, Z.B. (1999). Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to the tip-localized calcium influx. Plant Cell 11 1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield, J.A., and Preuss, D. (2000). Rapid initiation of Arabidopsis pollination requires the oleosin-domain protein GRP17. Nat. Cell Biol. 2 128–130. [DOI] [PubMed] [Google Scholar]
- Mo, Y., Nagel, C., and Taylor, L.P. (1992). Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc. Natl. Acad. Sci. USA 89 7213–7217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, L.A., and Walbot, V. (2001). Models for vacuolar sequestration of anthocyanins. Recent Adv. Phytochem. 35 297–312. [Google Scholar]
- Murgia, M., Charzynska, M., Rougier, M., and Cresti, M. (1991). Secretory tapetum of Brassica oleracea L.—Polarity and ultrastructural features. Sex. Plant Reprod. 4 28–35. [Google Scholar]
- Napoli, C.A., Fahy, D., Wang, H.Y., and Taylor, L.P. (1999). white anther: A petunia mutant that abolishes pollen flavonol accumulation, induces male sterility, and is complemented by a chalcone synthase transgene. Plant Physiol. 120 615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrath, C. (2002). The biopolymers cutin and suberin. In The Arabidopsis Book, C.R. Somerville and E.M. Meyerowitz, eds (Rockville, MD: American Society of Plant Biologists), pp. 1–14, doi/10.1199/tab.0021, http://www.aspb.org/publications/arabidopsis/. [DOI] [PMC free article] [PubMed]
- Owen, H.A., and Makaroff, C.A. (1995). Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L) Heynh ecotype Wassilewskija (Brassicaceae). Protoplasma 185 7–21. [Google Scholar]
- Pacini, E., and Hesse, M. (2005). Pollenkitt—Its composition, forms and functions. Flora 200 399–415. [Google Scholar]
- Piffanelli, P., Ross, J.H.E., and Murphy, D.J. (1998). Biogenesis and function of the lipidic structures of pollen grains. Sex. Plant Reprod. 11 65–80. [Google Scholar]
- Pighin, J.A., Zheng, H., Balakshin, L.J., Goodman, I.P., Western, T.L., Jetter, R., Kunst, L., and Samuels, A.L. (2004). Plant cuticular lipid export requires an ABC transporter. Science 306 702–704. [DOI] [PubMed] [Google Scholar]
- Platt, K.A., Huang, A.H.C., and Thomson, W.W. (1998). Ultrastructural study of lipid accumulation in tapetal cells of Brassica napus L. cv. Westar during microsporogenesis. Int. J. Plant Sci. 159 724–737. [Google Scholar]
- Preuss, D., Lemieux, B., Yen, G., and Davis, R.W. (1993). A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signaling during fertilization. Genes Dev. 7 974–985. [DOI] [PubMed] [Google Scholar]
- Sarafian, V., Kim, Y., Poole, R.J., and Rea, P.A. (1992). Molecular cloning and sequence of cDNA encoding the pyrophosphate-energized vacuolar membrane proton pump of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 89 1775–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saslowsky, D., and Winkel-Shirley, B. (2001). Localization of flavonoid enzymes in Arabidopsis roots. Plant J. 27 37–48. [DOI] [PubMed] [Google Scholar]
- Saslowsky, D.E., Warek, U., and Winkel, B.S. (2005). Nuclear localization of flavonoid enzymes in Arabidopsis. J. Biol. Chem. 280 23735–23740. [DOI] [PubMed] [Google Scholar]
- Schijlen, E.G., Ric de Vos, C.H., van Tunen, A.J., and Bovy, A.G. (2004). Modification of flavonoid biosynthesis in crop plants. Phytochemistry 65 2631–2648. [DOI] [PubMed] [Google Scholar]
- Scott, R.J., Spielman, M., and Dickinson, H.G. (2004). Stamen structure and function. Plant Cell 16 (suppl.): S46–S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, J.J., and Rechnitz, G.A. (1992). Flavonoid-specific staining of Arabidopsis thaliana. Biotechniques 13 880–883. [PubMed] [Google Scholar]
- Singh, V., Moreau, R.A., and Hick, K.B. (2003). Yield and phytosterol composition of oil extracted from grain sorghum and its wet-milled subfractions. Cereal Chem. 80 126–129. [Google Scholar]
- Stanley, R.G., and Linskens, H.F. (1974). Pollen pigments. In Pollen. Biology, Biochemistry and Management. (New York: Springer-Verlag), pp. 223–246.
- Suh, M.C., Samuels, A.L., Jetter, R., Kunst, L., Pollard, M., Ohlrogge, J., and Beisson, F. (2005). Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol. 139 1649–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, L.P., and Grotewold, E. (2005). Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 8 317–323. [DOI] [PubMed] [Google Scholar]
- Ting, J.T.L., Wu, S.S.H., Ratnayake, C., and Huang, A.H.C. (1998). Constituents of the tapetosomes and elaioplasts in Brassica campestris tapetum and their degradation and retention during microsporogenesis. Plant J. 16 541–551. [DOI] [PubMed] [Google Scholar]
- Tuteja, J.H., Clough, S.J., Chan, W.C., and Vodkin, L.O. (2004). Tissue-specific gene silencing mediated by a naturally occurring chalcone synthase gene cluster in Glycine max. Plant Cell 16 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer, I.M., Stam, M.E., van Tunen, A.J., Mol, J.N.M., and Stuitje, A.R. (1992). Antisense inhibition of flavonoid biosynthesis in petunia anthers results in male sterility. Plant Cell 4 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel, B.S. (2004). Metabolic channeling in plants. Annu. Rev. Plant Biol. 55 85–107. [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley, B. (2001). Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 126 485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, H.M., and Cheung, A.Y. (2000). Programmed cell death in plant reproduction. Plant Mol. Biol. 44 267–281. [DOI] [PubMed] [Google Scholar]
- Wu, S.S.H., Moreau, R.A., Whitaker, B.D., and Huang, A.H.C. (1999). Steryl esters in the elaioplasts of the tapetum in developing Brassica anthers and their recovery on the pollen surface. Lipids 34 517–523. [DOI] [PubMed] [Google Scholar]
- Wu, S.S.H., Platt, K.A., Ratnayake, C., Wang, T.W., Ting, J.T.L., and Huang, A.H.C. (1997). Isolation and characterization of novel neutral-lipid-containing organelles and globuli-filled plastids from Brassica napus tapetum. Proc. Natl. Acad. Sci. USA 94 12711–12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S.S.H., Suen, D.F., Chang, H.C., and Huang, A.H. (2002). Maize tapetum xylanase is synthesized as a precursor, processed and activated by a serine protease, and deposited on the pollen. J. Biol. Chem. 277 49055–49064. [DOI] [PubMed] [Google Scholar]
- Yazaki, K. (2005). Transporters of secondary metabolites. Curr. Opin. Plant Biol. 8 301–307. [DOI] [PubMed] [Google Scholar]
- Yephremov, A., and Schreiber, L. (2005). The dark side of the cell wall: Molecular genetics of plant cuticle. Plant Biosyst. 139 74–79. [Google Scholar]
- Ylstra, B., Muskens, M., and van Tunen, A.J. (1996). Flavonols are not essential for fertilization in Arabidopsis thaliana. Plant Mol. Biol. 32 1155–1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.