Abstract
In immature dendritic cells (DCs), major histocompatibility complex class II molecules accumulate in peptide-loading compartments and, during DC maturation, are exported to the cell surface in response to inflammatory stimuli. Moreover, it has recently been proposed that DCs have specific mechanisms of antigen uptake and delivery into major histocompatibility complex class II-loading compartments. B cells bearing a genetically disrupted invariant chain gene (Ii −/−) show alterations in the transport and function of class II molecules. We herein report that DCs derived from Ii −/− H2k but not Ii −/− H2b mice undergo normal maturation in response to tumor necrosis factor α and show a high degree of class II surface expression. Class II molecules are accumulated in cathepsin D- and H2-M-positive compartments in immature Ii −/− DC and, during DC maturation, are exported to the cell membrane as compact dimers. Ii −/− DCs present putative Ii-dependent hen egg lysozyme-derived epitopes to T cells. These data support the existence of Ii-independent molecular requirements for class II transport and peptide loading in DCs.
Presentation of exogenous antigens to CD4+ T lymphocytes requires antigen internalization and processing in endocytic compartments (1–4). Newly synthesized α and β chains of class II heterodimers associate in the endoplasmic reticulum (ER), with invariant chain (Ii) as a third partner. This complex is transported to specialized compartment(s) along the endocytic route where the loading with antigenic peptides derived from exogenous proteins occurs (5–12). This event is facilitated by the presence of the molecule H-2M, in mice (13–17) and by HLA-DM in humans (18–20), involved in the dissociation of Ii fragments from the class II peptide binding groove (13–20). The intracellular route followed by class II–Ii complexes from the trans-Golgi network to the endocytic pathway remains so far poorly characterized. Among the different models, an initial targeting to early endosomes, possibly via the plasma membrane, has been proposed (1–4, 12, 17, 21–23). A dileucine targeting motif in the cytoplasmic tail of Ii plays a pivotal role in the control of the movements of class II–Ii complexes through the endocytic pathway (for review, see ref. 3). In addition, a leucine-based motive in the tail of the class II β chain has been advocated to be sufficient for the class II entry into early endosomes from the plasma membrane (3). This second pathway is independent of Ii expression and appears to regulate the trafficking, as well as the antigen presentation function, of mature class II molecules (22, 23).
B cells from mice bearing a genetically disrupted Ii gene (Ii −/−) (24–26) show a striking alteration in the intracellular transport and maturation of class II molecules: (i) most complexes are retained into the ER; (ii) few complexes reach the plasma membrane; and (iii) the few complexes expressed at the cell surface do not have the “compact” conformation indicative of tight peptide binding (6, 27). Furthermore, Ii is required for the efficient assembly of class II dimers in allelic variants only: Ii −/− B cells from mice with an H-2b background mostly accumulate free α and β chains in the ER, although Ii −/− class II dimers from H-2k and H-2d background are efficiently assembled (28).
The general health status and growth rate of Ii −/− mice and wild-type (wt) litter mates are similar (24). Although primary IgM responses against nominal antigens were impaired in Ii −/− mice, recall vaccinations induced the production of antigen-specific IgG at levels comparable with those of wt mice (ref. 24 and unpublished results). The production of IgG upon in vivo challenge with antigen requires efficient antigen presentation to class II-restricted CD4+ T cells. Data from several laboratories point out to an essential role of dendritic cells (DCs) in priming and maintenance of T cell responses (29–32). DCs originate from bone marrow CD34+ progenitors (33–35), which include DC colony-forming units that yield homogeneous DC colonies under particular in vitro conditions (36). DC precursors enter the blood and reach peripheral organs where they develop to immature DCs. Immature DCs are capable to capture soluble antigens via macropinocytosis (37, 38) and particulate antigens through phagocytosis (39, 40), to process them to form epitopes that will be subsequently expressed at the cell surface in association with major histocompatibility complex (MHC) class II molecules (41–44). To fully perform their antigen-presentation functions, DCs residing in nonlymphoid tissues need to be activated by stimuli that promote their maturation, including the expression of costimulatory molecules and the rearrangement of the actin-based cytoskeleton that allow their migration to T cell areas of lymphoid organs (45–50), where the priming of naive T lymphocytes occurs (31, 50). Once DCs have interacted with T cells, they complete the differentiation process (50, 51), which is believed to terminate by DC apoptosis (49).
We have recently shown that class II molecules in immature murine DCs are mainly located in internal vesicles and colocalize with the H-2M molecule (49), which have been convincingly implicated in facilitating the loading of antigenic peptides onto class II molecules (14–17) and whose expression has been traced throughout the endocytic pathway, along both conventional and unconventional class II containing compartments (17). Maturation of DCs is characterized by profound changes in antigen-presenting capacity (49, 50, 52), possibly due to modifications of Ii and class II distribution and transport (53–55). This phenomenon possibly reflect the requirement for migratory DCs to retain antigens from the peripheral tissues to the lymphoid organs, where they actually perform the antigen-presentation function (31, 50). In this study we have investigated whether the requirements of Ii to regulate the antigenic peptide loading and presentation by MHC class II molecules apply to DCs from H2k mice, either freshly purified from the spleen or during their in vitro maturation.
MATERIALS AND METHODS
Mice.
The C57BL/6 and B10.BR inbred mice bearing a genetically disrupted Ii gene were generously provided by D. Mathis and C. Benoist (Institut de Génetique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) (24). The absence of Ii was tested at the gene level by Southern blot analysis (24) and at the protein level by Western blot analysis using anti-cytoplasmic Ii antibodies (53).
Antibodies.
The anti-MHC class II (IAk) 10.2.16, anti-mouse FcγII/III receptor CD32 2.4G2, anti-DEC205 NLDC-145, anti-Mac1, and anti-CD11c N-418 hybridomas were all from the American Type Culture Collection. The 10.2.16 mAb is capable of precipitating class II dimers regardless of the association of the α/β complexes with stabilizing peptides, with intact Ii, or with Ii cleavage products (3). The anti-cytoplasmic domain Ii (56), anti-cathepsin D, and anti-H2-M β chain cytoplasmic domain antibodies were provided by N. Barois (Centre d’Immunologie de Marseille-Luminy, Marseille, France). The anti-CD45R B220 mAb was from PharMingen. The second-step reagents were from Jackson Immunoresearch.
Cells.
Spleen cells were derived from physical dissociation of the organs (57). After collagenase digestion, an adhesion step was performed (37°C, 2 hours). B cells were retrieved from the nonadherent cells after T lymphocyte depletion (anti-Thy, J1J; anti-CD4, RL172Y; anti-CD8, 31M) and were 85–95% positive for the CD45R marker. Adherent cells were incubated overnight at 37°C. Spleen DCs were obtained from the de-adhered cell population, after centrifugation over a 50% discontinuous Percol gradient (Pharmacia) and were 75–90% positive for the DC marker N418. CD34+ precursors were grown in Iscove’s medium (Sigma) supplemented with 30% NIH 3T3 supernatant containing recombinant murine granulocyte–macrophage colony-stimulating factor (20 ng/ml; Genzyme) (49). At day 3, the granulocyte-enriched nonadherent cells were discarded. To induce DC terminal maturation, 15-day cultured cells were treated for 48 hours with recombinant tumor necrosis factor α (TNF-α) (100 units/ml; Genzyme) before characterization (34, 58). The IAk-restricted T cell hybridomas (3A9, 3B11) (59–61) were provided by L. Adorini (Roche, Milan, Italy). 3A9 cells are specific for the epitope of residues 46–61 of hen egg lysozyme (46–61 HEL) (59), which associate in B cells with newly synthesized IAk molecules only when they are targeted to the loading compartment by Ii. 3B11 T cells recognize the epitope of residues 34–45 of HEL (34–45 HEL) that binds to recycling class II molecules (61). The CTLL-2 cells were from the American Type Culture Collection.
Flow Cytometry.
The samples were stained as described (62). Briefly, the cells were incubated with primary antibodies (1 mg/ml) and secondary reagents (fluorochrome-coupled antibodies or streptavidin) in the presence of 2.4G2 mAb and normal mouse serum to prevent FcγRII/III binding. The samples were analyzed with a FACScan apparatus (Becton Dickinson).
Confocal Microscopy.
Intracellular immunofluorescence was performed on fixed and permeabilized cells as described (49). The confocal laser scanning microscopy was carried out using a Leica TCS 4D instrument (Leica): focal series of four horizontal planes of section were simultaneously monitored for fluorescein isothiocyanate and Texas Red. Unless otherwise specified, a plane of focus 0.8 mm above DC contact to the coverslip was selected. The micrographs were visualized and printed as reported (49). To identify fluorochrome colocalization unambiguously, correlation maps were made by using a statistical method based on the calculation of the local joint moment of standardized images (63). These correlation maps discriminate coincident fluorescence distributions from the superimposition of noncorrelated fluorescence profiles on a local basis. Each pair of red–green images is standardized for contrast and local variation in fluorescence. This correction and the joint moment of red–green images were calculated by considering a 19 × 19 pixel Gaussian window with a half-height width of 5 pixels corresponding to a resolution of 0.5 mm (63). We show herein binarized black and white prints of the correlation maps using a single threshold of 50% of the maximum value.
SDS/PAGE of Surface-Iodinated Class II Complexes.
The cells were labeled by means of lactoperoxidase-catalyzed iodination (24), and the class II molecules were immunoprecipitated with the 10.2.16 mAb. Before electrophoresis on a SDS/12.5% polyacrylamide gel, the immunoprecipitated material was either fully denatured at 95°C for 5 min or incubated for 1 hour at room temperature in SDS/sample buffer containing 5% 2-mercaptoethanol, to preserve the peptide-loaded compact forms of MHC class II heterodimers (64).
Antigen Presentation.
The antigen-presentation experiments were performed by incubating 3A9 or 3B11 T hybridoma cells with bone marrow-derived DCs (BMDCs) or spleen B cells, in the presence or absence of 1:10 serial dilutions of the appropriate relevant antigens. When indicated, spleen DCs and spleen B cells were incubated overnight with the various antigens (each at 1 mg/ml). Spleen DCs were further purified over a 50% discontinuous Percole gradient. Serial dilutions of antigen-presenting cells were used. In selected experiments, BMDCs were preincubated for 15 min at 37°C in the presence of 20 μM brefeldin A (BFA) in complete Iscove’s medium before antigen addition (10 μg/ml and 100 μg/ml) in BFA-containing medium (65). After 6 hours of pulse labeling, BMDCs were extensively washed and 3A9 or 3B11 T cells were added (1:2 ratio). Interleukin 2 secretion by hybridoma cells was assessed by using thiazolyl blue MTT (Sigma) to evaluate the growth of the interleukin 2-dependent CTLL-2 cell line. The kinetics of antigen presentation by DCs was performed as described (65, 66). Briefly cells were incubated in the presence or in the absence of HEL (1 mg/ml). At various times (30 min and 1, 2, 4, 6, and 8 hours) cells were extensively washed and either fixed or not fixed with 0.05% glutaraldehyde in PBS for 30 sec at 4°C. The fixation was stopped by adding glycine (200 μM) in PBS for 30 sec, followed by extensive washings. Antigen-pulsed DCs were then incubated for 24 hours in the presence of various amounts of 3A9 and 3B11 T cells.
RESULTS AND DISCUSSION
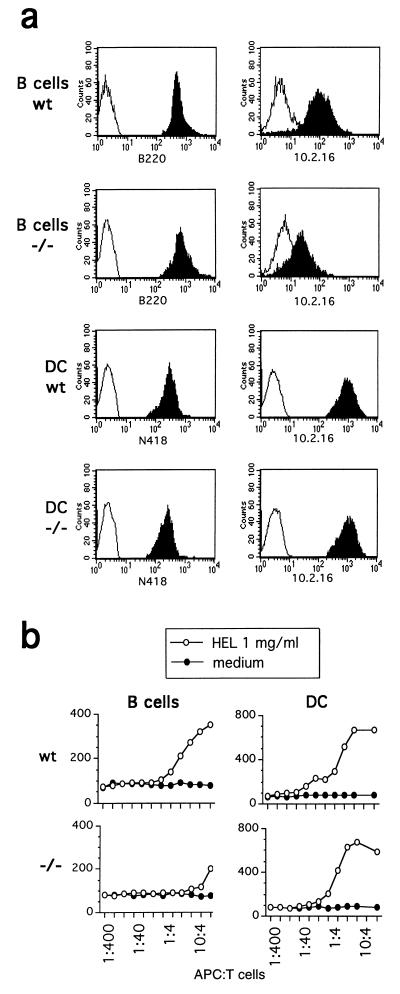
In the H-2k haplotype, the absence of Ii does not compromise class II IA α/β assembly (28); however, the transport of class II dimers to the peptide-loading compartment is greatly affected in Ii −/− B cells (28). We therefore used H-2k Ii −/− mice to analyze class II distribution and peptide loading in immature and mature DCs. The DCs and B cells were purified from whole spleen of wt and Ii −/− mice, and their class II expression was analyzed. Freshly purified N418+ Ii −/− splenic DCs expressed high amounts of membrane class II molecules; on the contrary, as expected, the B220+ Ii −/− B cells had an impaired membrane expression of IAk molecules (Fig. 1a). The analysis of spleen sections in H-2k Ii −/− mice confirmed the presence of highly class II positive DCs in T cell areas (data not shown). Moreover, Ii −/− DCs were able to stimulate IAk-restricted 3A9 T hybridoma cells upon incubation with native HEL protein, whereas B cells from the same mice were not (Fig. 1b).
Figure 1.
Splenic DCs overcome the need for Ii for class II surface expression and antigen presentation. wt and Ii −/− antigen-presenting cells were purified from the spleen and immediately analyzed. (a) Flow cytometric analysis of spleen CD45R+ (B220 mAb) B cells and CD11c+ (N418 mAb) DCs labeled with the 10.2.16 mAb to reveal IAk surface expression (solid histograms). Control stainings (open histograms) were performed with the second-step reagent only. (b) Antigen presentation by B cells and DCs of HEL using the 46–61 epitope-specific 3A9 T cell hybridoma. The assay was performed at the indicated ratios of T cells and antigen-presenting cells, preincubated (○) or not (•) with HEL at 1 mg/ml.
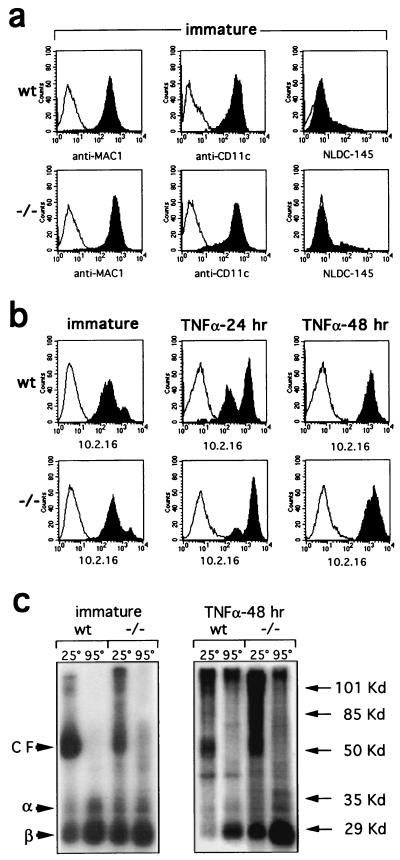
Splenic DCs mainly consist of mature DCs (29). To investigate the role of Ii during DC maturation, we derived immature DC from wt and Ii −/− CD34+ bone marrow cells, grown in the presence of granulocyte–macrophage colony-stimulating factor (34) and fibroblast supernatant (49). These BMDCs shared most of the features of immature DCs (49), including high levels of Mac1 and CD11c molecules (Fig. 2a); only a fraction of the cells expressed the DEC-205 determinant. No major difference in class II expression between wt and Ii −/− immature BMDCs from H2k was detected (Fig. 2b). This feature apparently depends on the H2 haplotype of the strain investigated, because class II expression was reduced in Ii −/− BMDCs derived from H2b mice (data not shown). A small percentage of H2k BMDC exhibited high levels of class II molecules (Fig. 2b) and may represent fully mature DCs. When BMDCs from H2k mice were cultured for 48 hours in the presence of TNF-α at 100 units/ml, [i.e., a cytokine that induces DCs exhaustive maturation (33, 37, 47, 49)], the level of class II membrane expression was enhanced, regardless of the expression of Ii (Fig. 2b).
Figure 2.
DC maturation induces high surface levels of class II molecules that are partially compact in the absence of Ii. (a) Immature DCs derived from wt and Ii −/− BMDCs were cultured for 14 days in vitro and characterized by their expression of Mac1, CD11c, and DEC-205 markers (anti-Mac1, N418, and NLDC-145 mAbs) (solid histograms). (b) Immature BMDCs (14 days) were analyzed for class II expression immediately and after 24 and 48 hours of incubation with TNF-α (100 units/ml), which induces class II surface expression (solid histograms). Control stainings (open histograms) were performed with the second-step reagent only. (c) After surface iodination, class II dimers were immunoprecipitated from immature and 48-hour TNF-α-treated BMDCs with the 10.2.16 mAb. Iodinated compact forms (CF) migrating as 55-kDa heterodimers were revealed in nonboiled samples, whereas α and β chains were only detected after boiling.
Access of class II dimers to the peptide-loading compartment leads to peptide association and a stabilization of the class II complex that is visualized as a 50-kDa dimer in SDS/polyacrylamide gels under reducing conditions (64). Immature BMDCs from wt and Ii −/− mice were iodinated and membrane class II molecules were immunoprecipitated with the anti-IAk specific 10.2.16 mAb, which recognizes both the “compact” class II dimers associated with antigenic peptides and empty or unstable dimers (3). SDS/PAGE of the immunoprecipitates demonstrated that class II dimers from immature BMDCs can reach the plasma membrane in a compact conformation, even in the absence of Ii (Fig. 2c). The maturation of BMDCs in response to TNF-α amplifies the proportion of compact dimers in wt and Ii −/− DCs. Of interest, in the absence of Ii, H2k MHC class II complexes that reach the plasma membrane in a SDS-resistant conformation display a broad range of molecular weights between 50 and 120 kDa. This result is reminiscent of the binding between class II α/β dimers and long polypeptides that takes place in Ii-negative HeLa cells transfected with MHC class II molecules (67, 68). The observation that long polypeptides bind in a pre-Golgi compartment to α/β complexes and allow MHC class II localization at the cell surface in Ii-negative DCs (Fig. 2c) and in MHC class II-transfected HeLa cells (67) but not in B cells (ref. 28 and data not shown) may support cell-specific differences in the mechanisms that regulates MHC class II intracellular trafficking (69).
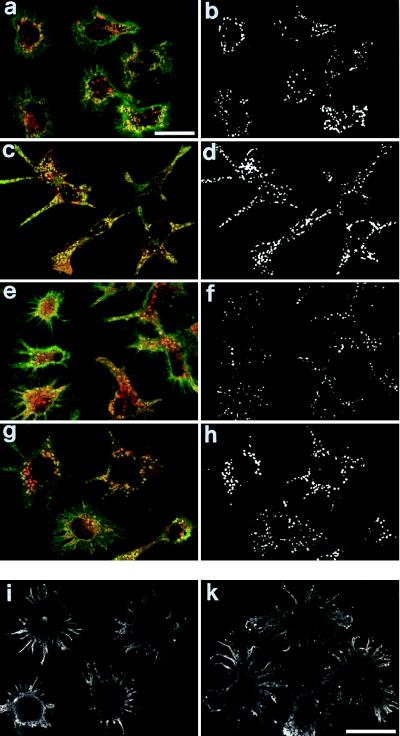
Confocal sections of immature wt and Ii −/− DCs show that H2k class II molecules accumulate in discrete intracellular vesicles (Fig. 3a). These vesicles share features of the peptide-loading compartment(s): e.g., positivity for the lysosomal enzyme cathepsin D and for the peptide-exchange catalyzer H2-M (14–16). Class II (green color) and H2-M and cathepsin D (red color) containing vesicles are shown. We relied on correlation maps (63) to discriminate coincident fluorescence distributions from the superimposition of noncorrelated fluorescence profiles and, therefore, identified the vesicles in which class II and H2-M or cathepsin D were colocalized. Colocalized vesicles were detectable to similar extents in both wt and Ii −/− DCs, thus confirming that Ii is not required for class II accumulation in the putative peptide-loading compartments of immature DCs. Class II molecules were exported to the cell surface in both wt and Ii −/− DCs after treatment with TNF-α (Fig. 3b), suggesting that class II export during DC terminal maturation does not require Ii expression.
Figure 3.
Intracellular class II molecules are located in cathepsin D- and H2-M-positive compartments in Ii-deficient immature BMDCs. wt (a–d) and Ii −/− (f) immature BMDCs were analyzed by confocal microscopy for the intracellular localization of class II molecules by using the 10.2.16 mAb (a, c, e, and g, green color). The cells were counterstained with mAbs specific for H2-M molecule (the murine equivalent of the human HLA-DM) (a and e, red color) and the cathepsin D lysosomal enzyme (c and g, red color). The superimposition between the two fluorochromes (a, c, e, and g, yellow color) is indicative of the colocalization of class II molecules in vesicles expressing H2-M molecules or cathepsin D. The colocalization was further estimated by calculating local image correlation maps (55), shown next to each picture (b, d, f, and h) using a threshold black and white color scale. Class II molecules labeled with the 10.2.16 mAb were only located at the cell surface of 48-hour TNF-α-elicited wt (i) and Ii −/− (k) BMDCs.
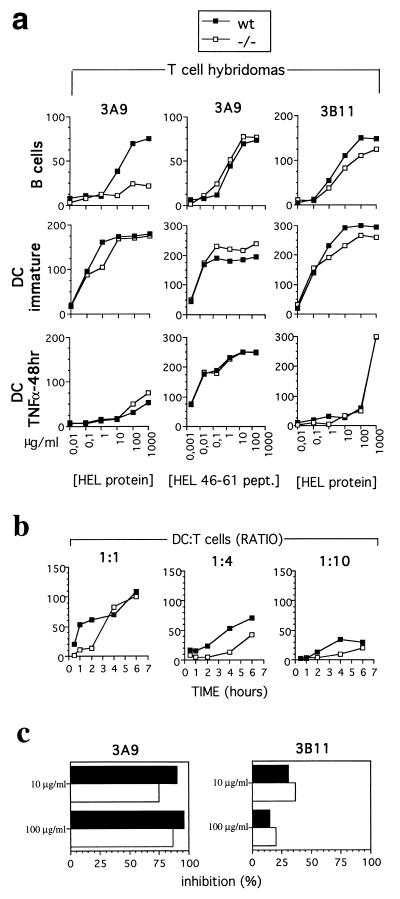
Ii −/− DCs from H2k mice are capable of efficiently presenting epitopes derived from the processing of exogenous antigens to class II-restricted T cells. In particular, Ii −/− immature DCs (but not Ii −/− B cells) efficiently present epitopes from the internalized HEL protein to 3A9 T cells (3, 59, 60) (Fig. 4a). On the contrary, antigen presentation of the 34–45 HEL epitope to 3B11 T cells (3, 60, 61) was not affected in Ii −/− DCs and B cells (Fig. 4a). In immature DCs, exogenous antigens are retained for a long time before efficient MHC class II loading and presentation (66). A pulse of at least 1 hour is required to induce the degradation of the internalized antigen and no presentation of exogenous antigens is detectable before 8 hours of processing (ref. 66, Fig. 4b, and data not shown). In Ii −/− immature BMDCs, presentation is even more delayed: it is not detectable before 4 hours of pulse at high DC/T cell ratios (1:1) and before 6 hours of pulse at lower DC/T cell ratios (1:4–1:10) (Fig. 4b). This delay in the kinetics of antigen presentation could be associated to a difference in the efficiency of H2k MHC class II peptide loading. It is likely that the long polypeptides associated with the MHC class II in H2k Ii −/− DCs are differentially resistant to H2-M-catalyzed peptide exchange. Indeed, in the absence of Ii, HLA-DM expression influences the array of peptides presented by HLA-DR molecules (70). The fact that no difference was observed after long incubation times is most likely due to the higher efficiency of antigen presentation by DCs compared to B cells (Fig. 4a).
Figure 4.
Immature Ii −/− BMDCs can present a larger array of HEL epitopes than B cells. (a) wt (▪) or Ii −/− (□) B cells, immature BMDCs, and TNF-α-elicited mature BMDCs were incubated with serial dilutions of HEL protein or HEL 46–61 synthetic peptides and tested for their ability to stimulate the 46–61 epitope-specific 3A9 T cell hybridoma, which requires Ii in B cells, or the Ii-independent 34–45 epitope-specific 3B11 T cell hybridoma. (b) wt (▪) and Ii −/− (□) immature BMDCs were pulse-labeled with HEL protein at 1 mg/ml for 30 min and 1, 2, 4, and 6 hours (x axis) before extensive washing and then tested for their ability to present the 46–61 epitope to increasing number of 3A9 T cells for 24 hours (ratios tested, 1:1, 1:4, and 1:10). The y axis is the emission at 490 nm. (c) wt (solid bars) and Ii −/− DC (open bars) were preincubated with 20 μM BFA for 15 min before and during 6 hours of pulse labeling with various amounts of the HEL protein (10 μg/ml and 100 μg/ml). After extensive washing, cells were tested for their ability to present the 46–61 HEL epitope to the 3A9 T cells and the 34–45 HEL epitope to the 3B11 T cells. The results are expressed as percentage of inhibition of the BFA treatment calculated as follow: 100 − [(OD490 emission in the presence of BFA × 100)/OD490 emission in the absence of BFA].
Fig. 4 shows that Ii dependence of different epitopes for H2k MHC class II loading and presentation can vary in different cell types (61). It has been previously reported that the 34–45 HEL epitope, recognized by the 3B11 T cell hybridoma, and the 116–129 HEL epitope can be efficiently presented in the absence of Ii by Rat-2 fibroblasts transfected with MHC class II molecules (61), but these epitopes are not presented by Ii −/− B cells (21). The efficient presentation of the 34–45 HEL epitope by Ii −/− DCs is not affected by BFA, i.e., by an agent that compromises newly synthesized class II complexes egress from the ER, supporting the hypothesis that it can be due to MHC class II complexes recycling from the plasma membrane (ref. 61 and Fig. 4c). In contrast, the 46–61 HEL epitope presentation to the 3A9 T cells is severely inhibited by BFA treatment after pulse labeling Ii −/− immature BMDC with HEL protein (Fig. 4c). This was not the case when the cells are pulse-labeled with the 46–61 HEL synthetic peptide (data not shown). This result implicates that newly synthesized MHC class II are involved in the presentation of 46–61 HEL epitope to T cells (71) and is in agreement with the existence of molecular constraints other than Ii expression in the presentation of Ii-dependent HEL epitopes by immature DCs.
Fully mature DC inefficiently presented the HEL protein (Fig. 4a). This is associated with the reduced ability of wt and Ii −/− mature DCs to internalize soluble antigens (data not shown) and with the redistribution of most of the MHC class II molecules at the cell surface (Fig. 3b). The HEL 46–61 peptide, which does not require internalization, was presented with high efficiency by both DC populations (Fig. 4a), and this event was not affected by BFA treatment (data not shown).
DCs are the most potent antigen-presenting cells for CD4+ T cells (31): to induce similar rates of T cell activation, B cells required a 100-fold higher concentration of HEL antigen (Fig. 4a). The molecular basis of this phenomenon can reside in (i) the ability of immature DCs to efficiently present soluble antigens internalized by several mechanisms to CD4+ T cells, including macropinocytosis and receptor-mediated endocytosis (37, 38), whereas B cells efficiently present only antigens internalized through their antigen receptor (72); and (ii) the higher levels of MHC class II and costimulatory molecules in DCs (ref. 49 and Figs. 1 and 3).
This report shows that in H2k Ii −/− DCs, class II dimers apparently overcome the requirement of Ii for loading with antigenic peptides but that B cells require an intact Ii polypeptide for efficient presentation. This implicates that at least in the H2k haplotype DCs, newly synthesized MHC class II complexes are capable of reaching the cathepsin D- and H2-M-positive intracellular compartment(s) in the absence of Ii targeting. The strict haplotype dependence of the phenomenon implicates that a more complex regulation must exist in different murine strains. This pathway is constitutively operating in Ii −/− H2k DCs and may underlie the conserved IgG response to nominal antigens reported in B10.BR Ii −/− mice.
Acknowledgments
We are indebted to Drs. D. Mathis and C. Benoist (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Strasbourg, France) for the gift of the Ii −/− mice. We thank Nicole Brun and Marc Barad for help with the flow cytometry analysis, Nicolas Barois for the anti-Ii antibodies, and Lee Leserman for discussions and critical reading of the manuscript. P.R. is supported by Fondazione Italiana per la ricerca sul cancro. This work was supported by institutional grants from Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, and by Grant 60 58 from the Association pour la Recherche sur le Cancer and the Ligue Nationale de Lutte contre le Cancer. Parts of the work were supported by the Ministry of Health (AIDS project, Grant 9403-82 and MS Grant).
ABBREVIATIONS
- DC
dendritic cell
- Ii
invariant chain
- ER
endoplasmic reticulum
- MHC
major histocompatibility complex
- TNF-α
tumor necrosis factor α
- HEL
hen egg lysozyme
- BFA
brefeldin A
- BMDC
bone marrow-derived DC
- wt
wild type
References
- 1.Germain R N. Cell. 1994;76:287–299. doi: 10.1016/0092-8674(94)90336-0. [DOI] [PubMed] [Google Scholar]
- 2.Cresswell P. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- 3.Germain R N, Castellino F, Han R, Reis e Sousa C, Romagnoli P, Sadegh-Nasseri S, Zhong G-M. Immunol Rev. 1996;151:5–30. doi: 10.1111/j.1600-065x.1996.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 4.Harding C V. Crit Rev Immunol. 1996;16:13–29. doi: 10.1615/critrevimmunol.v16.i1.20. [DOI] [PubMed] [Google Scholar]
- 5.Peters P J, Neefjes J J, Oorschot V, Ploegh H L, Geuze H J. Nature (London) 1991;349:669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- 6.Germain R N, Hendrix L R. Nature (London) 1991;353:134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- 7.Amigorena S, Drake J R, Webster P, Mellman I. Nature (London) 1994;369:113–120. doi: 10.1038/369113a0. [DOI] [PubMed] [Google Scholar]
- 8.Tulp A, Woerd D, Dobberstein B, Ploegh H L, Pieters J. Nature (London) 1994;369:120–126. doi: 10.1038/369120a0. [DOI] [PubMed] [Google Scholar]
- 9.West M A, Lucocq J M, Watts C. Nature (London) 1994;369:147–151. doi: 10.1038/369147a0. [DOI] [PubMed] [Google Scholar]
- 10.Rudensky A Y, Maric M, Eastmen S, Shoemaker L, DeRoos P C, Blum J S. Immunity. 1994;1:585–594. doi: 10.1016/1074-7613(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 11.Peters P J, Raposo G, Neefjes J J, Oorschot V, Leijendekker R L, Geuze H J, Ploegh H L. J Exp Med. 1995;182:325–334. doi: 10.1084/jem.182.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellino F, Germain R N. Immunity. 1995;2:73–88. doi: 10.1016/1074-7613(95)90080-2. [DOI] [PubMed] [Google Scholar]
- 13.Morris P, Shaman J, Attaya M, Amaya M, Goodman S, Bergman C, Monaco J J, Mellins E. Nature (London) 1994;368:551–554. doi: 10.1038/368551a0. [DOI] [PubMed] [Google Scholar]
- 14.Denzin L K, Cresswell P. Cell. 1995;82:155–165. doi: 10.1016/0092-8674(95)90061-6. [DOI] [PubMed] [Google Scholar]
- 15.Sherman M A, Weber D A, Jensen P E. Immunity. 1995;3:197–205. doi: 10.1016/1074-7613(95)90089-6. [DOI] [PubMed] [Google Scholar]
- 16.Roche P A. Immunity. 1995;3:259–262. doi: 10.1016/1074-7613(95)90111-6. [DOI] [PubMed] [Google Scholar]
- 17.Pierre P, Denzin L K, Hammond C, Drake J R, Amigorena S, Cresswell P, Mellman I. Immunity. 1996;4:229–239. doi: 10.1016/s1074-7613(00)80431-8. [DOI] [PubMed] [Google Scholar]
- 18.Kropshofer H, Vogt A B, Stern L J, Hammerling G J. Science. 1995;270:1357–1359. doi: 10.1126/science.270.5240.1357. [DOI] [PubMed] [Google Scholar]
- 19.Vogt A B, Kropshofer H, Moldenhauer G, Hammerling G J. Proc Natl Acad Sci USA. 1996;93:9724–9729. doi: 10.1073/pnas.93.18.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kropshofer H, Hammerling G J, Vogt A B. Immunol Today. 1997;18:77–82. doi: 10.1016/s0167-5699(97)01006-2. [DOI] [PubMed] [Google Scholar]
- 21.Salamero J, Humbert M H, Cosson P, Davoust J. EMBO J. 1990;9:3489–3496. doi: 10.1002/j.1460-2075.1990.tb07557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roche P A, Tetski C L, Stang E, Bakke O, Long E O. Proc Natl Acad Sci USA. 1993;90:8581–8585. doi: 10.1073/pnas.90.18.8581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinet V, Vergelli M, Martin R, Bakke O, Long E O. Nature (London) 1995;375:603–606. doi: 10.1038/375603a0. [DOI] [PubMed] [Google Scholar]
- 24.Viville S, Neefjes J, Lotteau V, Dierich A, Lemeur M, Ploegh H, Benoist C, Mathis D. Cell. 1993;72:635–648. doi: 10.1016/0092-8674(93)90081-z. [DOI] [PubMed] [Google Scholar]
- 25.Bikoff E K, Huang L-Y, Episkopou V, van Meerwijk J, Germain R N, Robertson E J. J Exp Med. 1993;177:1699–1712. doi: 10.1084/jem.177.6.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott E A, Drake J R, Amigorena S, Elsemore J, Webster P, Mellman I, Flavell R A. J Exp Med. 1994;179:681–694. doi: 10.1084/jem.179.2.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadegh-Nasseri S, Germain R N. Nature (London) 1991;353:167–170. doi: 10.1038/353167a0. [DOI] [PubMed] [Google Scholar]
- 28.Bikoff E K, Germain R N, Robertson E J. Immunity. 1995;2:301–310. doi: 10.1016/1074-7613(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 29.Steinman R M, Kaplan G, Witmer M D, Cohn Z A. J Exp Med. 1979;149:1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuler G, Steinman R M. J Exp Med. 1985;161:526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 32.Guéry J C, Ria F, Adorini L. J Exp Med. 1996;183:751–757. doi: 10.1084/jem.183.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caux C, Dezutter-Dambuyant D, Schmitt D, Banchereau J. Nature (London) 1992;360:258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- 34.Scheicher C, Mehlig M, Zecher R, Reske K. J Immunol Methods. 1992;154:253–264. doi: 10.1016/0022-1759(92)90199-4. [DOI] [PubMed] [Google Scholar]
- 35.Caux C, Vanbervliet B, Massacrier C, Dezutter-Dambuyant D, de Saint-Vis B, Jacquet C, Yoneda K, Imamura S, Schmitt D, Banchereau J. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young J, Szabolcs W, Moore M A S. J Exp Med. 1995;182:1111–1120. doi: 10.1084/jem.182.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sallusto F, Lanzavecchia A. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sallusto F, Cella M, Danieli C, Lanzavecchia A. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purè E, Inaba K, Crowley M T, Tardelli L, Witmer-Pack M D, Ruberti G, Fathman G, Steinman R M. J Exp Med. 1990;172:1459–1469. doi: 10.1084/jem.172.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inaba K, Inaba M, Maito M, Steinman R M. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romani N, Koide S, Crowley M, Witmer-Pack M, Livingstone A M, Fathman C G, Inaba K, Steinman R M. J Exp Med. 1989;169:1169–1178. doi: 10.1084/jem.169.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watts C. Nature (London) 1997;388:724–725. doi: 10.1038/41900. [DOI] [PubMed] [Google Scholar]
- 43.Cella M, Engering A, Pinet V, Pieters J, Lanzavecchia A. Nature (London) 1997;388:782–787. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 44.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Nature (London) 1997;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 45.Heufler C, Koch F, Schuler G. J Exp Med. 1988;167:700–712. doi: 10.1084/jem.167.2.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Inaba K, Witmer-Pack M, Inaba M, Hathcock K S, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley P S, Ikehara S, Maramatsu S, Hodes R J, Steinman R M. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roake J A, Rao A S, Morris P J, Larsen C P, Hankins D F, Austyn J M. J Exp Med. 1995;181:2237–2247. doi: 10.1084/jem.181.6.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szabolcs P, Moore M A, Young J W. J Immunol. 1995;154:5851–5861. [PubMed] [Google Scholar]
- 49.Winzler C, Rovere P, Rescigno M, Granucci F, Penna G, Adorini L, Zimmermann V S, Davoust J, Ricciardi-Castagnoli P. J Exp Med. 1997;185:317–328. doi: 10.1084/jem.185.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Austyn J M. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neiß U, Reske K. Int Immunol. 1994;6:61–71. doi: 10.1093/intimm/6.1.61. [DOI] [PubMed] [Google Scholar]
- 54.Nijman H W, Kleijmeer M J, Ossevoort M A, Oorschot V M J, Vierboom M P M, van de Keur M, Kenemans P, Kast W M, Geuze H J, Melief C J M. J Exp Med. 1995;182:163–174. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kleijmeer M J, Ossevoort M A, van Ven C J H, van Hellemend J J, Neefjes J J, Kast W M, Melief C J M, Geuze H J. J Immunol. 1995;154:5715–5724. [PubMed] [Google Scholar]
- 56.Barois N, Forquet F, Davoust J. J Biol Chem. 1997;272:3641–3647. doi: 10.1074/jbc.272.6.3641. [DOI] [PubMed] [Google Scholar]
- 57.Guéry J-C, Adorini L. J Immunol. 1995;154:536–544. [PubMed] [Google Scholar]
- 58.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Maramatsu S, Steinman R M. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allen P M, Unanue O R. J Immunol. 1984;132:1077–1079. [PubMed] [Google Scholar]
- 60.Adorini L, Appella E, Doria G, Nagy Z A. J Exp Med. 1988;168:2091–2104. doi: 10.1084/jem.168.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Momburg F, Fushs S, Drexler J, Busch R, Post M, Hammerling G J, Adorini L. J Exp Med. 1993;178:1453–1458. doi: 10.1084/jem.178.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rovere P, Inverardi L, Bender J R, Pardi R. J Immunol. 1996;156:2273–2279. [PubMed] [Google Scholar]
- 63.Demandolx D, Davoust J. J Microscopy. 1997;185:21–36. [Google Scholar]
- 64.Germain R N, Hendrix L R. Nature (London) 1991;353:134–139. doi: 10.1038/353134a0. [DOI] [PubMed] [Google Scholar]
- 65.Adorini L, Ullrich S J, Appella E, Fuchs S. Nature (London) 1990;346:63–66. doi: 10.1038/346063a0. [DOI] [PubMed] [Google Scholar]
- 66.Lutz M, Rovere P, Kleijmeer M J, Rescigno M, Assmann C U, Oorschot V M J, Geuze H J, Trucy J, Demandolx D, Davoust J, Ricciardi-Castagnoli P. J Immunol. 1997;159:3707–3716. [PubMed] [Google Scholar]
- 67.Busch R, Vturina I Y, Drexler J, Momburg F, Hammerling G J. Eur J Immunol. 1995;25:48–53. doi: 10.1002/eji.1830250110. [DOI] [PubMed] [Google Scholar]
- 68.Busch R, Cloutier I, Sekaly R P, Hammerling G J. EMBO J. 1996;15:418–428. [PMC free article] [PubMed] [Google Scholar]
- 69.Simonsen A, Momburg F, Drexler J, Hammerling G J, Bakke O. Int Immunology. 1993;5:903–917. doi: 10.1093/intimm/5.8.903. [DOI] [PubMed] [Google Scholar]
- 70.Lighstone L, Hargeaves R, Bobek G, Peterson M, Alchinger G, Lombardi G, Lechler R. Proc Natl Acad Sci USA. 1997;94:5772–5777. doi: 10.1073/pnas.94.11.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swier K, Miller J. J Immunol. 1995;155:1851–1861. [PubMed] [Google Scholar]
- 72.Lanzavecchia A. Nature (London) 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]