Abstract
The SSX family proteins have been considered new members of the cancer/testis antigens because of the restricted expression in testis among normal tissues and the activation in a wide range of cancers. Thus, they would be potential molecular targets for immunotherapeutic strategies. We have developed a competitive nucleic acid sequence-based amplification (NASBA) assay to analyze SSX mRNA expression in 211 bone and soft tissue tumors. The copy numbers of SSX mRNA per μg of total RNA in tumor tissues were widely distributed, ranging logarithmically from 0.6 to 6.6. We found that malignant tumors showed significantly higher expression of SSX mRNA than benign tumors (P < 0.0001). Further, SSX mRNA expression in stage III tumors was significantly higher than that in stage I or II tumors (P < 0.005). This NASBA assay was also more sensitive compared to immunohistochemistry using newly affinity-purified polyclonal antibody against SSX. Collectively, these results suggest that the SSX quantitative NASBA assay could provide useful information to select eligible patients for SSX-specific cancer vaccines.
The SSX1 and SSX2 genes were initially identified as fusion partners to the SYT gene in human synovial sarcomas carrying a recurrent t(X;18)(p11.2; q11.2) chromosomal translocation.1,2,3 In addition to the well-known five SSX genes (SSX1, −2, −3, −4, and −5),4 there has currently been reported a total of nine SSX genes, all located on the X chromosome and comprise a gene family.5 All nine members of the SSX exhibit close nucleotide homologies (ranging from 87 to 96%) and encode proteins of 188 amino acids (homologies ranging from 73 to 92%) except for SSX8. The most highly conserved domain among these proteins exists in the COOH-terminus of SSX, has been implied in the transcription-repression function and designated as SSX repression domain (SSXRD).6
Using the reverse transcriptase-polymerase chain reaction (RT-PCR) method, four SSX genes (SSX1, −2, −4, and −5) have been found to be ectopically expressed at varying frequencies (0 to 57%) in a number of malignancies.7,8 Among normal human tissues, only the testis expressed SSX1, −2, −3, −4, −5, and −7, whereas SSX6, −8, and −9 were not detected in any normal tissues.5 In addition, the humoral and cellular immune responses against the ectopically expressed SSX2 have been reported in a subset of melanoma patients.9,10 Taken together, the SSX gene products could be categorized as cancer/testis (CT) antigens, and potential molecular targets for the development of cancer immunotherapy. However, because of its extremely high sensitivity, the RT-PCR examination might detect very low transcriptional levels of SSX, thus the corresponding tumor tissues could not be recognized by the autologous cytotoxic T lymphocyte. To select the suitable patients for cancer immunotherapy targeted to SSX, a novel method to quantify the level of SSX mRNA expression is required.
Transcripts of several CT antigens such as MAGE and NY-ESO-1 were reported to be associated with tumor progression and higher malignant potential.11,12 Recently, we have reported that 94% of osteosarcomas expressed at least one of the five SSX genes by RT-PCR.13 By contrast, the human osteoblast cell (NHOst), primary cultured osteoblastoma (benign bone forming tumor), and parosteal osteosarcoma (low-grade osteosarcoma) did not express any SSX genes.13 These results suggested that mRNA expression of SSX might be correlated with disease progression in musculoskeletal tumors. However, those expression rates in musculoskeletal tumors were evaluated by RT-PCR, which could present only qualitative analysis. Moreover, the correlation between SSX expression and tumor progression was not observed in an immunohistochemical study in melanoma.7 Therefore, further studies examining the precise expression level of SSX using quantitative analysis were required.
Nucleic acid sequence-based amplification (NASBA)14 allows us to quantify the level of mRNA expression in surgical specimens, even if the amount of tissue is limited. Unlike real-time RT-PCR, NASBA is reported to specifically amplify RNA but not DNA, because double-stranded DNA is not denatured and consequently amplified in the lower reacting temperature, 41°C.15,16
In this study, we established a competitive NASBA assay to quantify the level of SSX gene transcripts and analyzed a series of 211 bone and soft tissue tumors. The expression levels analyzed by NASBA (NASBA values) in these samples ranged from 0.6 to 6.6 in logarithmic orders (>105-fold difference). In addition, we prepared an anti-SSX2 polyclonal antibody against glutathione S-transferase (GST)-fused SSX2, to detect the SSX protein in paraffin-embedded tissues. Using this antibody, we analyzed more than 30 samples of bone and soft tissue tumors that were confirmed high or low expression of SSX mRNA by the competitive NASBA assay.
Materials and Methods
Patients and Samples
Two hundred and eleven samples of bone and soft tissue tumors were obtained from 210 patients who underwent surgical resection between February 2002 and December 2003 at the hospital of Osaka Medical Center for Cancer and Cardiovascular Diseases (Osaka, Japan) under the approved protocol by our local ethical committee. Several normal tissues including fat (two samples), muscle (two samples), cartilage (two samples), synovium (one sample), and bone (one sample) were also obtained from autopsies of three unrelated individuals. Age of patients ranged from 7 to 79 years (median, 47 years), with a male-to-female ratio of 1:1.1. Tumors originated from bone in 71 patients and from soft tissue in 140 patients. Distribution of histological subtypes is summarized in Table 1. According to the Musculoskeletal Tumor Society staging system,17,18 75 malignant bone and soft tissue tumors could divide into 17 stage I tumors, 36 stage II tumors, and 22 stage III tumors. SYT-SSX fusion transcripts were identified in all eight tumors of synovial sarcoma; four tumors had a SYT-SSX1 and four had a SYT-SSX2 fusion transcript. A sample of neurofibroma and another malignant peripheral nerve sheath tumor (MPNST) were excised from a patient suffering type 1 neurofibromatosis.
Table 1.
Histological Distribution of 211 Bone and Soft Tissue Tumors
| Benign | Malignant | ||
|---|---|---|---|
| Bone tumor | |||
| Giant cell tumor | 11 | Osteosarcoma | 11 |
| Enchondroma | 9 | Ewing sarcoma | 5 |
| Chondroblastoma | 5 | Chondrosarcoma | 4 |
| Osteochondroma | 4 | MFH | 1 |
| Fibrous dysplasia | 4 | ||
| Osteofibrous dysplasia | 3 | ||
| Eosinophilic granuloma | 2 | ||
| Nonossifying fibroma | 2 | ||
| Aneurysmal bone cyst | 2 | ||
| Simple bone cyst | 2 | ||
| Chordoma | 2 | ||
| Osteoblastoma | 1 | ||
| Osteoid osteoma | 1 | ||
| Desmoplastic fibroma | 1 | ||
| Hemangioma | 1 | ||
| Total | 50 | 21 | |
| Soft tissue tumor | |||
| Lipoma | 35 | Liposarcoma | 9 |
| Schwannoma | 23 | MPNST | 9 |
| Hemangioma | 10 | Synovial sarcoma | 8 |
| Desmoid | 7 | Leiomyosarcoma | 8 |
| Neurofibroma | 4 | Myxoid liposarcoma | 7 |
| Synovial chondroma | 3 | MFH | 5 |
| GCTTS | 2 | Askin tumor | 1 |
| Neuroma | 1 | Clear cell sarcoma | 1 |
| Eiomyoma | 1 | Hemangiopericytoma | 1 |
| IMT | 1 | ||
| ESOS | 1 | ||
| ESES | 1 | ||
| ESMCS | 1 | ||
| Undifferentiated sarcoma | 1 | ||
| Total | 86 | 54 |
MFH, malignant fibrous histiocytoma; GCTTS, giant cell tumor of tendon sheath; MPNST, malignant peripheral nerve sheath tumor; IMT, inflammatory myofibroblastic tumor; ESOS, extraskeletal osteosarcoma; ESES, extraskeletal Ewing sarcoma; ESMCS, extraskeletal myxoid chondrosarcoma.
During the operation, the dissected tissues were immediately submerged into RNAlater (Ambion, Inc., Austin, TX) to inactivate RNase and stabilize RNA at room temperature. After the solution permeated the tissues, the samples were stored at −80°C until RNA extraction. Part of each tumor sample was fixed in 10% formalin and routinely processed for hematoxylin and eosin (H&E) staining to establish a pathological diagnosis by three of us (Y.T., M.M., S.I.) according to the World Health Organization classification of tumors of soft tissue and bone.19 At the same time, the sample was examined histologically for the presence of tumor cells and was considered suitable for this study if the proportion of the tumor cells was >70%.
RNA Preparation
Total RNAs were extracted from each frozen specimen by use of the Ultraspec RNA isolation system (Biotecx Laboratories, Inc., Houston, TX). The purity and concentration of recovered RNA samples were spectrophotometrically determined. The prepared RNA was then diluted to 20 ng/μl with nuclease-free water and stored at −80°C until use.
Primers and Probes
The sequences of primers and probes for SSX are listed in Table 2. The sequences of the NASBA forward primers (P2), capture probes, and detection probes were identical to their respective target sequences, whereas the NASBA reverse primers (P1) were composed of a target-specific region and a T7 RNA polymerase promoter region (see the sequence underlined in Table 2). Detection probes were labeled with alkaline phosphatase at the 5′ end. All primers and probes were synthesized by FASMAC Co., Ltd. (Kanagawa, Japan). The sequences of the NASBA forward and reverse primers for SSX were positioned at the downstream of the breakpoint where the SSX gene fuses to the SYT gene, to amplify both native SSX genes and SYT-SSX fusion gene.
Table 2.
Sequences of Primers and Probes Used to Detect SSX mRNA
| Primer/probe | Position in target sequence* | Sequence |
|---|---|---|
| Synovial sarcoma, X-break point (SSX) | ||
| PCR forward primer | 92–106 (exon 1) | 5′-ATGAACGGAGACGAC-3′ |
| PCR reverse primer | 639–658 (exon 6) | 5′-TTACTCGTCATCTTCCTCAG-3′ |
| Reverse primer (P1)† | 619–638 (exon 6) | 5′-aattctaatacgactcactatagggagGGTCGCTGATCTCTTCATAA-3′ |
| Forward primer (P2) | 434–453 (exon 5) | 5′-AAGCCAGCAGAGGAAGGAAA-3′ |
| Capture probe‡ | 553–577 (exon 5/6) | 5′-ATCTGGACCCAAAAGGGGGGAACAT-3′ |
| Detection probe | 587–606 (exon 6) | 5′-CACAGACTGCGTGAGAGAAA-3′ |
| QA capture probe§ | 558–577 (exon 6) | 5′-CTGCAGAGGTGAGGTTACGG-3′ |
Accession numbers: X86175 (H. sapiens mRNA for SSX2 protein).
The T7 RNA polymerase promoter recognition sequences are underlined in lower case.
Detection probe was labeled with alkaline phosphatase (ALP) at the 5′ end.
Capture probe for QA was used to detect the competitor signal.
Nucleic Acid Sequence-Based Amplification
Quantitative NASBA assay comprises a competitive NASBA and a sandwich hybridization/chemiluminescence-based Hybrigene detection system.20 Competitive NASBA was performed by adding a constant amount of competitor (QA) RNA to the unknown samples and to a set of wild-type (WT) RNA calibrators. QA RNAs were identical to WT RNA calibrators except for the 20-base-length capture sequence, hence it can be amplified by the same set of NASBA primers. The co-amplified sample contained two types of anti-sense RNAs; the amount of WT and QA product was analyzed on the Hybrigene detection system. The logarithmic ratio of signals from WT and QA [log (WT/QA)] indicates the ratio of the WT and QA RNAs in the samples.
WT and QA RNAs were synthesized by in vitro transcription, as following. Human SSX2 full-length cDNA (GenBank accession no. X86175) obtained from an osteosarcoma sample with RT-PCR was subcloned into the plasmid vector pSP65 (Promega, Madison, WI). After confirming the sequence, the plasmids were linearized at the 3′ end of the insert, and transcribed to sense strand RNAs using MEGAscript SP6 Kit (Ambion). The synthesized WT RNAs were extracted with phenol-chloroform followed by ethanol precipitation, then spectrophotometrically quantified.
The QA RNA was synthesized from the plasmids substituted with the QA sequence (5′-ctgcagaggtgaggttacgg-3′) at the region of capture sequence of the WT RNAs. The plasmids containing the QA sequence were obtained by recombinant PCR procedure. The QA RNA was prepared by the similar method for the WT RNAs.
Assay procedures were as follows: 5 μl of template (0.1 or 1 μg of total RNA sample, or calibrator RNA) and 5 μl of QA RNA (104 copies) were added to 20 μl of the NASBA reagent, which consisted of 80 mmol/L Tris (pH 8.3); 24 mmol/L MgCl2; 140 mmol/L KCl; 10 mmol/L dithiothreitol; 2 mmol/L each dNTP; 4 mmol/L each ATP, CTP, and TTP; 3 mmol/L GTP; 1 mmol/L ITP; 30% dimethyl sulfoxide; and 0.4 μmol/L each of reverse (P1) and forward (P2) primers. The reaction mixture was heated for 5 minutes at 65°C and, after cooling to 41°C for 5 minutes, 10 μl of NASBA enzyme mixture (17.5 U of AMV-RT, 0.02 U of RNaseH, and 40.7 U of T7 RNA polymerase) was added. The reactions were then incubated for 90 minutes at 41°C, and the amplified products were stored at −80°C until quantification.
Chemiluminescent signals of WT and QA were automatically measured by the Hybrigene system. The instrument software calculates the copy numbers of WT RNA in the sample by extrapolating the signal ratio of the calibration curve. The target gene amount in the sample was expressed as the number of copies per μg of total RNA used. Quantitative NASBA assays were conducted in duplicate for each sample.
Sequencing Analysis
NASBA products from all cases analyzed in the present study were subcloned into PCR-Script Amp SK(+) (Stratagene, La Jolla, CA) cloning vector. All clones were fully sequenced in both orientations using vector-specific oligonucleotides and the ABI Prism Big-Dye Terminator Cycle Sequencing FS Ready Reaction kit (Applied Biosystems, Foster city, CA) and an automated sequencer (ABI Prism 3100, Applied Biosystems).
Bacterial Expression and Generation of Polyclonal Antibody
For bacterial expression of GST fusion protein, full-length cDNA of human SSX2 obtained from RT-PCR product using osteosarcoma RNA was inserted into the plasmid vector pGEX-2T (Amersham Bioscience, Uppsala, Sweden). The correct orientation and in-frame insertion was confirmed by restriction enzyme analysis and DNA sequencing. The GST-SSX2 fusion protein was induced by 0.1 mmol/L of isopropyl-thio-β-galactoside in logarithmically growing Escherichia coli BL21 cells carrying the pGEX-2T-SSX2 constructs, and purified with GST purification modules (Amersham) according to the manufacturer’s instructions. The concentration and purity of the eluted proteins were estimated by conventional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Two Japanese White rabbits were immunized with the purified GST-SSX2 and bled by MBL (Nagoya, Japan). To purify the rabbit anti-sera, we prepared affinity column with Affi-Gel 10 Gel (Bio-Rad, Hercules, CA) coupled with the purified GST or GST-SSX2. To remove antibodies to bind GST portion, the anti-sera were first loaded into the GST affinity column. Then the flow-through fraction was collected and applied to the GST-SSX2 affinity column. The anti-sera bound to the GST-SSX2 column were eluted out with 0.2 mol/L of glycine-HCl (pH 4.0) and immediately neutralized to pH 7.0 with 1 mol/L of Tris. The concentration of eluted anti-SSX2 polyclonal antibody (named JOY-1) was determined with the BCA Protein Assay Reagent kit (Pierce, Rockford, IL) reference to bovine serum albumin as standards.
Immunoblotting Analysis
N-terminus FLAG-tagged full-length cDNAs of human SSX1 and SSX2 were introduced into the mammalian expression vector pcDNA3 (Invitrogen Corp., Carlsbad, CA). These constructs were transfected into COS-7 cells using Lipofectamine Plus (Invitrogen) following the manufacturer’s protocol. Two days after transfection, cells were lysed rapidly in ice with Laemmli’s SDS sample buffer (62.5 mmol/L of Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, and 0.01% phenol red)21 containing 40 mmol/L of dithiothreitol with a cell scraper. These samples were subjected to 12.5% SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Bio-Rad). The membranes were immunoblotted with primary antibodies in T-PBS containing 1% bovine serum albumin (Promega) overnight after 1 hour of blocking with T-PBS containing 3% bovine serum albumin. Anti-SSX2 polyclonal antibody (JOY-1) and anti-FLAG antibody (M2; Sigma, Tokyo, Japan) were used as the primary antibody at the concentration of 16 ng/ml of JOY-1 and 1 μg/ml of M2, respectively. The membrane was incubated for 30 minutes with secondary antibodies (anti-rabbit or anti-mouse IgG Fc alkaline phosphatase conjugate; Promega) at 1:7500 dilution after wash for 10 minutes in T-PBS three times. Immunoreactive bands were visualized by incubation of the membrane with the mixture of nitro blue tetrazolium (Promega) and 5-bromo-4-chloro-3-indolyl-phosphate (Promega) in alkaline phosphatase buffer (100 mmol/L of Tris-HCl, pH 9.5, 100 mmol/L of NaCl, and 5 mmol/L of MgCl2).
Immunohistochemical Analysis
Tissue specimens were fixed in 3.7% formalin/PBS and embedded in paraffin. Serial sections (3 μm) were cut and subjected to immunohistochemistry. We used human testis as a positive control as previously described.7 Sections were deparaffinized and treated by heating in 0.1 mmol/L sodium citrate buffer (pH 6.0) at 120°C for 10 minutes. Then the slides were adjusted to room temperature and incubated for 18 hours at 4°C with JOY-I polyclonal antibody diluted at 1:200 by ChemMate antibody diluent (DAKO SA, Glostrup, Denmark). Sites of primary antibody binding were detected by incubating slides with horseradish peroxidase-conjugated anti-rabbit IgG followed by reaction with substrate, 3,3′-diaminobenzidine tetrahydrochloride. The immunohistochemical slides were assessed independently by three pathologists for their positivity.
Statistic Analysis
All statistic analysis were performed using a software JMP 4.0.5J (SAS Institute Inc., Cary, NC). Differences in expression levels between groups of samples were assessed with the two-sided Mann-Whitney U-test and judged significant at confidence levels >95% (P < 0.05).
Results
Validation of the Standard Curves and Dynamic Range of NASBA
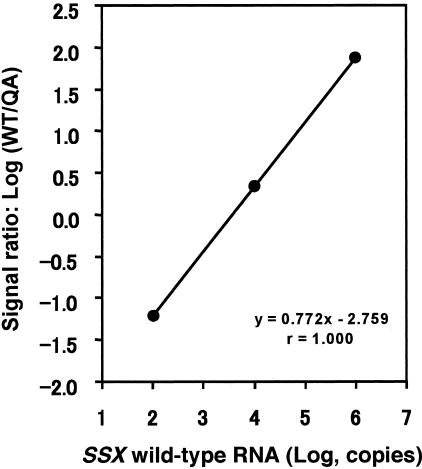
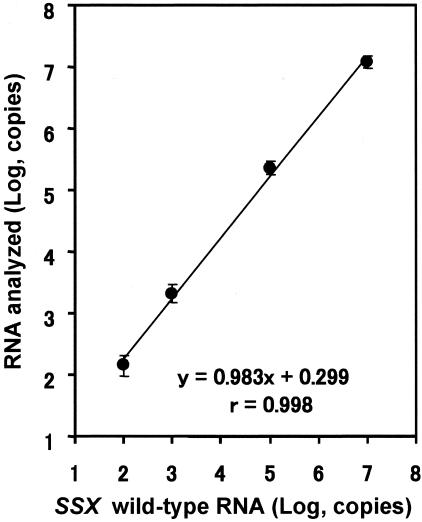
Three different concentrations (102, 104, and 106 copies) of SSX WT calibrator RNA were amplified with a constant amount (104 copies) of QA RNA by competitive NASBA. The calibration curve showed great stringent linearity between the amount of WT calibrator RNA and the signal ratio [log (WT/QA)] (r = 1.000, Figure 1). Four different amounts of SSX WT RNA (102, 103, 105, and 107 copies) were analyzed to test the assay system linearity. A linear relationship ranging from 102 to 107 copies of WT RNA was obtained (r = 0.998, Figure 2). Within-run CV for SSX WT RNA was 1.3 to 7.9% (n = 5), whereas between-run CV at each of three calibrator concentrations was 1.5 to 6.0% (n = 3) (data not shown). These results indicated that competitive NASBA assay for SSX can be used for quantification of 102 to 107 copies of RNA (wide dynamic range) with satisfactory accuracy.
Figure 1.
Calibration curve in quantitative NASBA for SSX mRNA. Each of three SSX WT calibrators (102, 104, and 106 copies) was amplified with 104 copies of competitor (QA) RNA by competitive NASBA. The calibration curve obtained by plotting the log signal ratios [log (WT/QA)] against the log amount of WT RNAs revealed quite good linearity (r = 1.000).
Figure 2.
Linearity of the synthetic SSX RNA amplified by competitive NASBA. Four different amounts of SSX WT RNA were analyzed to evaluate the linearity and the dynamic range. The copy number of diluted WT RNA was calculated by extrapolating signal ratio to the calibration curve (data points and bars indicate mean ± SD; n = 5). The assay system showed the linearity in the range between 102 and 107 copies. All within-run CVs in this assay were under 5%, except the CV for 102 copies of RNA (7.93%).
Measurement of SSX mRNA Expression in Bone and Soft Tissue Tumors
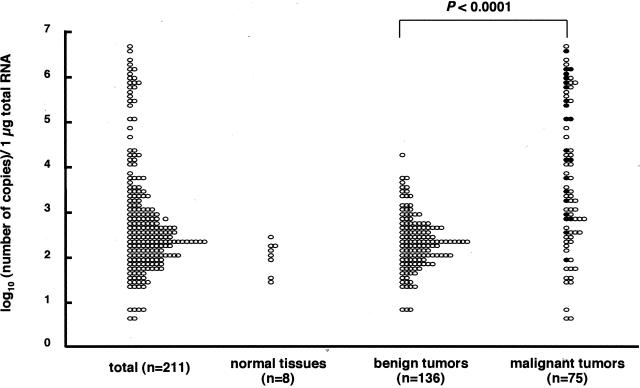
SSX mRNA expression of tumor tissues and normal tissues was quantified by the NASBA system. The amounts of SSX mRNA expression levels were calculated as log10 (number of copies/1 μg of total RNA) in tested tissues. The variation of the duplicate assay was small (data not shown), thus these values were reproducible. The values in 211 samples of bone and soft tissue tumors widely ranged from 0.6 to 6.6 (median, 2.5), whereas those in 8 samples of normal bone and soft tissues ranged from 1.4 to 2.4 (median, 2.0) (Figure 3). According to the pathological findings, we could divide 211 tumors into benign and malignant tumors. The values in 136 samples of benign bone and soft tissue tumors ranged from 0.8 to 4.2 (median, 2.3), whereas those in 75 samples of malignant tumors ranged from 0.6 to 6.6 (median, 3.3) (Figure 3). SSX mRNA expression in the malignant bone and soft tissue tumors was significantly higher than that in the benign tumors (P < 0.0001). Additionally, in the 75 malignant tumors, 22 were subdivided into stage III tumors by the Musculoskeletal Tumor Society staging system17,18 because of the presence of distant metastasis (Figure 3, filled circles). SSX mRNA expression in these 22 stage III tumors (1.9 to 6.5; median, 4.7) was significantly higher than that in the other 53 stage I or II tumors (0.6 to 6.6; median, 3) (P < 0.005).
Figure 3.
Quantification of SSX mRNA in bone and soft tissue tumors (n = 211), normal tissues (n = 8), benign tumors (n = 136), and malignant tumors (n = 75). The NASBA values in 211 bone and soft tissue tumors were widely distributed in the range between 0.6 and 6.6 (median, 2.5). Those in eight normal tissues ranged from 1.4 to 2.4 (median, 2.0). A significantly higher level of SSX mRNA expression was found in malignant tumors than in benign tumors (P < 0.0001). In the 75 malignant tumors, 22 stage III tumors (filled circles) showed higher SSX mRNA expression than the other 53 stage I or II tumors (P < 0.005).
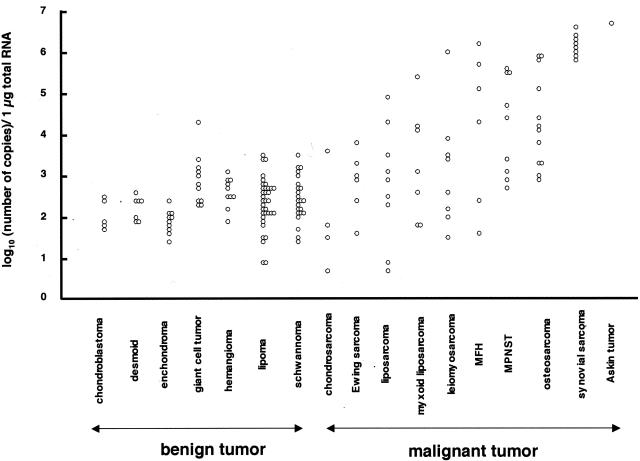
In specific histological types, 12 samples of osteosarcoma showed higher SSX mRNA expression (2.8 to 5.0; median, 4.1), consistent with our previous study13 (Figure 4). In addition, four samples of MPNST, three samples of malignant fibrous histiocytoma, a sample of liposarcoma, myxoid liposarcoma, leiomyosarcoma, and Askin tumor demonstrated higher expression than 4.5, whereas all samples of chondrosarcoma and Ewing sarcoma showed lower expression than 4.0. Notably, eight samples of synovial sarcoma (5.7 to 6.5; median, 6.1) showed markedly higher expression than other bone and soft tissue tumors, and the range of expression levels was quite narrow. The sequences of NASBA products in the samples of synovial sarcoma containing SYT-SSX1 or SYT-SSX2 were identical to SSX1 or SSX2, respectively, whereas those in all other samples but a sample of Askin tumor were identical to SSX2. Even if these eight samples of synovial sarcoma were excluded, SSX mRNA expression in the 67 malignant tumors (0.6 to 6.6; median, 3.2) was still significantly higher than that in the 136 benign tumors (P < 0.0001).
Figure 4.
Quantification of SSX mRNA in each histological type. All benign tumors showed lower SSX mRNA expression (NASBA value <4.0) except for a sample of giant cell tumor (4.2). Eight samples of synovial sarcoma (5.7 to 6.5; median, 6.1) showed extremely higher expression. Several samples of osteosarcoma, MPNST, malignant fibrous histiocytoma, leiomyosarcoma, myxoid liposarcoma, liposarcoma, and Askin tumor demonstrated higher expression (NASBA value >4.5), whereas all samples of chondrosarcoma and Ewing sarcoma showed lower expression than 4.0.
Characterization of the Anti-SSX Polyclonal Antibody
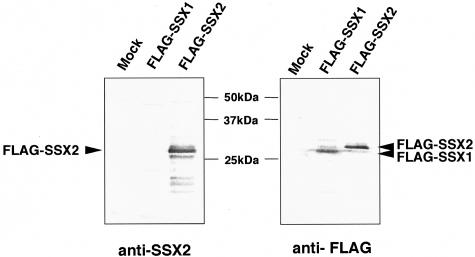
We have already reported the immunohistochemical study using anti-SSX monoclonal antibodies (E3AS),7,13 which mainly recognized the KRAB domain of SSX. For further immunohistochemical analysis, we newly prepared a rabbit polyclonal antibody (JOY-1) against whole GST-SSX2 protein. To determine the specificity of JOY-1, COS-7 cells were transiently transfected with FLAG-tagged SSX expression constructs (SSX1 and SSX2) and subjected to immunoblotting analysis. We found that the JOY-1 polyclonal antibody was able to specifically recognize SSX2 but not SSX1 (Figure 5).
Figure 5.
Immunoblotting analysis of COS-7 cells transiently transfected with FLAG-tagged SSX expression constructs (SSX1 and SSX2) using anti-SSX polyclonal antibody (JOY-1) and anti-FLAG monoclonal antibody (M2). Both FLAG-SSX1- and FLAG-SSX2-specific bands (∼29 kd) were recognized by M2 monoclonal antibody. Notably, two bands showed different electrophoretic mobilities in SDS-PAGE (FLAG-SSX1 protein migrated faster than FLAG-SSX2.). By contrast, JOY-1 polyclonal antibody only detected SSX2 but not SSX1 protein.
Evaluation of SSX Protein Expression in Human Testis and Bone and Soft Tissue Tumors
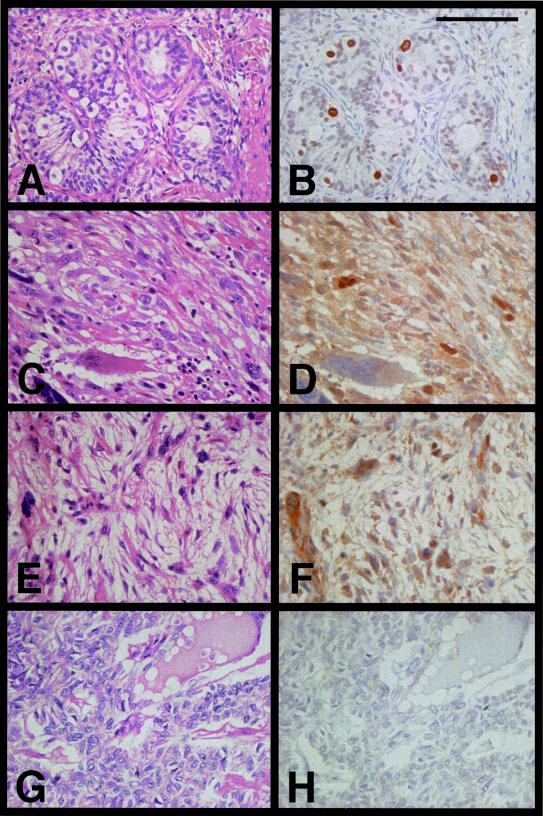
To test whether the JOY-1 polyclonal antibody could detect SSX proteins in paraffin-embedded tissues, we first analyzed a human testicular sample as a positive control by immunohistochemistry. SSX protein expression in human testis was localized in the nuclei of spermatogenic cells, mainly in spermatogonia and occasionally in primary spermatocytes close to the basement membrane (Figure 6, A and B). We could not find JOY-1 staining in either interstitial cells or Sertoli cells. These findings were consistent with the previous study using monoclonal antibody (E3AS) against SSX.7,13
Figure 6.
H&E and immunohistochemical staining using the anti-SSX JOY-1 polyclonal antibody. A and B: Immature human testis from a 2-year-old boy with congenital heart disease. Seminiferous tubule exhibited intratubular staining of early spermatogenic cells, mainly spermatogonia. Neither Sertoli cells, interstitial cells, nor maturated spermatogenic cells were stained with JOY-1. C and D: Metastatic osteosarcoma of lung. E and F: MPNST. G and H: Synovial sarcoma containing a SYT-SSX2 fusion transcript. Heterogeneous SSX nuclear staining was found in osteosarcoma and MPNST, whereas no staining was observed in synovial sarcoma. Scale bar, 100 μm.
We next analyzed more than 30 samples of bone and soft tissue tumors by immunohistochemistry using JOY-1 polyclonal antibody. Each of 15 samples showing high expression of SSX mRNA (NASBA value >4.5) or low (NASBA value <3.0) was studied. The results of immunohistochemical analysis are summarized in Table 3. In the former samples, heterogeneous nuclear staining of tumor cells was observed in three of four specimens of osteosarcoma (Figure 6, C and D), two of four specimens of MPNST (Figure 6, E and F), both of two specimens of malignant fibrous histiocytoma, and a specimen of myxoid liposarcoma, whereas no staining was found in all four specimens of synovial sarcoma (Figure 6, G and H). By contrast, all 15 specimens in the latter samples did not show any SSX nuclear positivity (data not shown).
Table 3.
SSX Immunostaining Using JOY-1 Antibody
| Pathologic diagnosis | Positive | Negative | Total |
|---|---|---|---|
| NASBA value < 3.0 | |||
| Lipoma | 0 | 3 | 3 |
| Schwannoma | 0 | 2 | 2 |
| Hemangioma | 0 | 2 | 2 |
| Enchondroma | 0 | 2 | 2 |
| Desmoid | 0 | 2 | 2 |
| Leiomyosarcoma | 0 | 2 | 2 |
| Myxoid liposarcoma | 0 | 1 | 1 |
| Chondrosarcoma | 0 | 1 | 1 |
| 15 | |||
| NASBA value > 4.5 | |||
| Osteosarcoma | 3 | 1 | 4 |
| MPNST | 2 | 2 | 4 |
| MFH | 2 | 0 | 2 |
| Myxoid liposarcoma | 1 | 0 | 1 |
| Synovial sarcoma | 0 | 4 | 4 |
| 15 |
Discussion
Cancer/testis (CT) genes are a growing class of genes that encode tumor-associated proteins with physiological expression restricted to male germ cells in the testis but not in other somatic tissues. In malignancy, the regulation of CT genes is disrupted, resulting in CT antigen expression in various types of tumors. Since their initial identification by T-cell epitope cloning, the list of CT antigens has been expanded through serological expression cloning (SEREX) and differential mRNA expression analysis. In the ∼20 CT antigens or antigen families identified to date,9,22,23,24,25,26,27,28,29,30,31,32,33,34 both humoral and cellular immune responses have been shown for only few CT antigens, including MAGE-A,22,35 NY-ESO-1,36,37 and SSX. Although SSX genes were first identified through cloning of the synovial sarcoma translocation, the SSX2 gene was also independently isolated by the SEREX method as melanoma tumor antigen HOM-MEL-40.9 Most recently, a combined proteasome-assisted epitope prediction approach with the screening of CD8+ T cells derived from a tumor lesion, the SSX2-derived CD8+ T cell epitope was identified, and a spontaneous CD8+ T-cell response against this epitope was found in melanoma patients.10 Based on their immunogenicity in cancer patients and restricted expression to tumors, CT antigens could be the ideal molecular targets for cancer vaccine. Since clinical trials with MAGE-A38 and NY-ESO-139 have already been in progress, an additional trial targeted to SSX could be anticipated.
Tureci and colleagues8 described the RT-PCR analysis of the expression of the well-known five SSX genes in 325 specimens of human neoplasms of various types. SSX1, −2, and −4 were shown to be expressed in 8%, 15%, and 15% of all tumors, respectively, with a low frequency of SSX5 expression and no SSX3 expression. At least one SSX family member was expressed in 57% of head and neck cancers, in 50% of ovarian cancers, in 43% of melanomas, in 27% of colorectal cancers, and in 23% of breast cancers, whereas leukemias, leiomyosarcomas, seminomas, and thyroid cancers examined did not express any SSX genes. We have previously reported that 16 of 17 human osteosarcomas expressed at least one of the five SSX genes and four of them expressed all five SSX genes tested.13 In the following study, we also found more frequent SSX expression in the bone and soft tissue tumors compared to epithelial and hematopoietic malignancies by the RT-PCR analysis: expression of at least one of the SSX family members was observed in giant cell tumor (11 of 12, 92%), followed by malignant fibrous histiocytoma (9 of 11, 82%), MPNST (8 of 11, 73%), carcinoma (12 of 23, 52%: kidney cancer, 3 of 9; lung cancer, 3 of 5; liver cancer, 2 of 2; breast cancer, 0 of 2; colon cancer, 1 of 1; thyroid cancer, 1 of 1; vagina cancer, 1 of 1; head and neck cancer, 1 of 1; bladder cancer, 0 of 1), myeloma (1 of 4, 25%), and lymphoma (1 of 7, 15%) (unpublished data). These data, together with those of Ayyoub and colleagues,40 prompt us to develop cancer immunotherapy targeted to SSX for patients with the bone and soft tissue sarcomas. However, these expression rates were based on the RT-PCR method and the precise level of expression in each sample was still unclear. Therefore, it would be of great significance to evaluate more accurately the status of SSX expression for the development of immunogenic cancer vaccines.
In the present study, we have established a competitive NASBA assay for SSX mRNA quantification using a chemiluminescence-based Hybrigene detection system. In this assay, isothermal nucleic acid amplification of target RNAs is accomplished by the simultaneous enzymatic activities of avian myeloblastosis virus-reverse transcriptase (AMV-RT), T7 RNA polymerase, and RNase H. Quantification of SSX mRNA is achieved through the competitive co-amplification of in vitro-generated RNA, which acts as an internal control for estimating the effect of inhibitors in clinical samples. Because the number of copies for SSX mRNA is calculated using the detection signal ratio of the products, the amplification inhibitors do not directly affect assay results.29
The expression levels of SSX mRNA in human bone and soft tissue tumors were widely distributed (Figure 3). Although the lowest detection for SSX transcripts was 0.6 (log10 4) in a sample of chondrosarcoma, the highest detection was 6.6 (log10 3.6 × 106) in a sample of Askin tumor. Thus, the actual dynamic range for the quantification of SSX mRNA by NASBA was more than 5 logarithmic orders in 211 samples of bone and soft tissue tumors. The amount of mRNA transcribed from genes producing antigenic peptides has to be greater than a threshold to allow recognition of the tumor cells by cytotoxic T lymphocytes. For MAGE-A1, this threshold was found to correspond to three mRNA molecules per cell in cultured cell lines, whereas it could be reduced to 3 molecules per 10 cells in surgical samples because those contained considerable amounts of normal interstitial tissues.41,42 In this NASBA assay, the values of SSX mRNA expression levels were calculated as log10 (number of copies/1 μg of total RNA) in tissues. Because 10 μg of total RNA corresponds to 1 million cells in general, those values are considered to measure the number of mRNA molecules per 105 cells in logarithmic orders. If the threshold was similar between MAGE-A1 and SSX, the amount of SSX mRNA expression in the tumors showing the NASBA value greater than 4.5 (log10 3 × 104) reached a level sufficient to allow recognition by cytotoxic T lymphocytes. Taken together, these findings suggested that this NASBA assay system might allow us to select the appropriate patients suitable for cancer immunotherapy targeted to SSX.
SSX expression might be associated with tumor progression and with tumors of higher malignant potential. Previously we have reported that almost all samples of osteosarcoma expressed at least one of the five SSX genes, whereas osteoblast cell, benign osteoblastoma, and low-grade osteosarcoma did not express any SSX genes.13 Similarly, SSX transcripts and clinical stage were statistically correlated with a higher frequency of expression among stage 4 tumors in neuroblastoma examined by RT-PCR.43 On the contrary, an immunohistochemical analysis of SSX protein expression in melanoma showed that 14 of 35 (40%) primary melanoma specimens were positive for SSX expression, whereas 20 of 66 (30%) metastatic lesions express SSX.7 Thus, the correlation between SSX expression and disease progression has been still ambiguous. In the present study, we quantified the amounts of SSX mRNA in 136 benign and 75 malignant bone and soft tissue tumors of various histological types, and found that SSX mRNA expression in malignant tumors was statistically higher than in benign tumors, even if eight samples of synovial sarcoma, showing especially higher expression, were excluded. Furthermore, this study revealed that SSX mRNA expression levels examined by this NASBA assay were associated with clinical stage. These results demonstrated that SSX expression could be strongly correlated with tumor progression and increased aggressiveness.
In this study, we also developed anti-SSX-specific polyclonal antibody (JOY-1) and evaluated the levels of SSX protein expression in several samples using immunohistochemistry to examine whether those were in agreement with the levels of SSX mRNA measured by the NASBA assay. Apart from four specimens of synovial sarcoma, within the samples showing high NASBA value (>4.5), we observed SSX nuclear staining in 8 of 11 (73%) specimens of bone and soft tissue tumors (Table 3). There were several reasons necessary to consider for this discrepancy, including sampling bias, the sensitivity of antibody for the clinical samples, antigen exposure, protein stability, and posttranslational modification. By contrast, all 15 specimens of low NASBA value sample (<3.0) and all four specimens of synovial sarcoma were negative for immunostaining of SSX. From these results, we speculated that the JOY-1 antibodies mainly picked up the N-terminal part of SSX, which was not included in the SYT-SSX fusion protein expressed in synovial sarcoma. Collectively, these findings suggested that this NASBA method was more sensitive and quantitative to analyze the expression of SSX for the clinical samples compared to the immunohistochemical examination.
In all of 211 bone and soft tissue tumors analyzed in this study, we found that 8 samples of synovial sarcoma showed markedly high and quite narrow range of expression level (5.7 to 6.5; median, 6.1) (Figure 4). We speculated that those results were due to high expression of the SYT-SSX fusion gene, but not that of the native SSX genes by the following two reasons. First, the NASBA forward and reverse primers were designed to anneal to the portion of SSX retained in the SYT-SSX fusion gene, and the sequence of NASBA products of synovial sarcoma samples was identical to that of the fusion gene expressed in the respective tumors. Second, despite the high expression level, all of the specimens of synovial sarcoma did not show immunoreactivity with JOY-1 antibody against SSX (Figure 6, G and H). Although human synovial sarcoma was shown to exclusively harbor the chromosomal translocation t(X:18) that produced the chimeric gene SYT-SSX,1,2,3 precise transcriptional level of SYT-SSX has never been reported to date. Here, we could deduce from this NASBA analysis that synovial sarcoma expressed ∼100 SYT-SSX mRNA molecules per cell, leading to the malignant transforming activity of SYT-SSX fusion protein in synovial sarcoma.
In conclusion, the competitive NASBA technique enables rapid, reproducible, sensitive, and accurate quantification of SSX mRNA expression. The level of SSX mRNA quantified by this technique might serve in clinical samples as a useful index to select the patients with bone and soft tissue tumors suitable for immunotherapy targeted to SSX, and to distinguish malignant musculoskeletal tumors from benign tumors, although establishment of a proper cutoff value will be indispensable in future studies.
Acknowledgments
We thank Yumiko Koyanagi, Kikuichi Nakagawa, Reiko Kitazume, Yoshimi Yamaguchi, and Naomi Yamato for invaluable technical assistance.
Footnotes
Supported by grants from the Haraguchi Memorial Fund (to N.N.); the Ministry of Education, Science, Sports, and Culture of Japan (to K.I. and K.Y.); and the Takeda Science Foundation (to K.I.).
N.N., S.J., and Y.T. contributed equally to this study.
References
- Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AML, Gusterson BA, Cooper CS. Identification of novel genes, SYT and SSX, involved in the t(X;18)(p112;q112) translocation found in human synovial sarcoma. Nat Genet. 1994;7:502–508. doi: 10.1038/ng0894-502. [DOI] [PubMed] [Google Scholar]
- Crew AJ, Clark J, Fisher C, Gill S, Grimer R, Chand A, Shipley J, Gusterson BA, Cooper CS. Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to Krüppel-associated box in human synovial sarcoma. EMBO J. 1995;14:2333–2340. doi: 10.1002/j.1460-2075.1995.tb07228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw B, Balemans M, Olde Weghuis D, Geurts van Kessel A. Identification of two alternative fusion genes, SYT-SSX1 and SYT-SSX2, in t(X;18)(p112;q112)-positive synovial sarcomas. Hum Mol Genet. 1995;4:1097–1099. doi: 10.1093/hmg/4.6.1097. [DOI] [PubMed] [Google Scholar]
- Gure AO, Tureci O, Sahin U, Tsang S, Scanlan MJ, Jager E, Knuth A, Pfreundschuh M, Old LJ, Chen YT. SSX: a multigene family with several members transcribed in normal testis and human cancer. Int J Cancer. 1997;72:965–971. doi: 10.1002/(sici)1097-0215(19970917)72:6<965::aid-ijc8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Gure AO, Wei IJ, Old LJ, Chen YT. The SSX gene family: characterization of 9 complete genes. Int J Cancer. 2002;101:448–453. doi: 10.1002/ijc.10634. [DOI] [PubMed] [Google Scholar]
- Lim FL, Soulez M, Koczan D, Thiesen HJ, Knight JC. A KRAB-related domain and a novel transcription repression domain in proteins encoded by SSX genes that are disrupted in human sarcomas. Oncogene. 1998;17:2013–2018. doi: 10.1038/sj.onc.1202122. [DOI] [PubMed] [Google Scholar]
- dos Santos NR, Torensma R, de Vries TJ, Schreurs MW, de Bruijn DR, Kater-Baats E, Ruiter DJ, Adema GJ, van Muijen GN, Geurts van Kessel A. Heterogeneous expression of the SSX cancer/testis antigens in human melanoma lesions and cell lines. Cancer Res. 2000;60:1654–1662. [PubMed] [Google Scholar]
- Tureci O, Chen YT, Sahin U, Gure AO, Zwick C, Villena C, Tsang S, Seitz G, Old LJ, Pfreundschuh M. Expression of SSX genes in human tumors. Int J Cancer. 1998;77:19–23. doi: 10.1002/(sici)1097-0215(19980703)77:1<19::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Tureci O, Sahin U, Schobert I, Koslowski M, Scmitt H, Schild HJ, Stenner F, Seitz G, Rammensee HG, Pfreundschuh M. The SSX-2 gene, which is involved in the t(X;18) translocation of synovial sarcomas, codes for the human tumor antigen HOM-MEL-40. Cancer Res. 1996;56:4766–4772. [PubMed] [Google Scholar]
- Ayyoub M, Stevanovic S, Sahin U, Guillaume P, Servis C, Rimoldi D, Valmori D, Romero P, Cerottini JC, Rammensee HG, Pfreundschuh M, Speiser D, Levy F. Proteasome-assisted identification of a SSX-2-derived epitope recognized by tumor-reactive CTL infiltrating metastatic melanoma. J Immunol. 2002;168:1717–1722. doi: 10.4049/jimmunol.168.4.1717. [DOI] [PubMed] [Google Scholar]
- Brasseur F, Rimoldi D, Lienard D, Lethe B, Carrel S, Arienti F, Suter L, Vanwijck R, Bourlond A, Humblet Y, Vacca A, Conese M, Lahaye T, Degiovanni G, Deraemaecker R, Beauduin M, Sastre X, Salamon E, Dreno B, Jager E, Knuth A, Chevreau C, Suciu S, Lachapelle JM, Pouillart P, Parmiani G, Lejeune F, Cerottini JC, Boon T, Marchand M. Expression of MAGE genes in primary and metastatic cutaneous melanoma. Int J Cancer. 1995;63:375–380. doi: 10.1002/ijc.2910630313. [DOI] [PubMed] [Google Scholar]
- Goydos JS, Patel M, Shih W. NY-ESO-1 and CTp11 expression may correlate with stage of progression in melanoma. J Surg Res. 2001;98:76–80. doi: 10.1006/jsre.2001.6148. [DOI] [PubMed] [Google Scholar]
- Naka N, Araki N, Nakanishi H, Itoh K, Mano M, Ishiguro S, de Bruijn DR, Myoui A, Ueda T, Yoshikawa H. Expression of SSX genes in human osteosarcomas. Int J Cancer. 2002;98:640–642. doi: 10.1002/ijc.10277. [DOI] [PubMed] [Google Scholar]
- Compton J. Nucleic acid sequence-based amplification. Nature. 1991;350:91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- Heim A, Grumbach IM, Zeuke S, Top B. Highly sensitive detection of gene expression of an intronless gene: amplification of mRNA, but not genomic DNA by nucleic acid sequence based amplification (NASBA). Nucleic Acids Res. 1998;26:2250–2251. doi: 10.1093/nar/26.9.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpkins SA, Chan AB, Hays J, Popping B, Cook N. An RNA transcription-based amplification technique (NASBA) for the detection of viable Salmonella enterica. Lett Appl Microbiol. 2000;30:75–79. doi: 10.1046/j.1472-765x.2000.00670.x. [DOI] [PubMed] [Google Scholar]
- Enneking WF, Spanier SS, Goodman MA. A system for surgical staging of musculoskeletal sarcoma. Clin Orthop. 1980;153:106–120. [PubMed] [Google Scholar]
- Peabody TD, Gibbs CP, Jr, Simon MA. Evaluation and staging of musculoskeletal neoplasms. J Bone Joint Surg Am. 1998;80:1204–1218. doi: 10.2106/00004623-199808000-00016. [DOI] [PubMed] [Google Scholar]
- Fletcher CDM, Unni KK, Mertens F. Pathology and genetics of tumours of soft tissue and bone. Fletcher CDM, Unni KK, Mertens F, editors. Lyon; World Health Organization Classification of Tumours. 2002:9–367. [Google Scholar]
- Hayashi T, Kobayashi H, Miyachi H, Ohshima T, Ujiiye T, Kawase M, Hotta T, Takemura Y. A competitive nucleic acid sequence-based amplification assay for the quantification of human MDR1 transcript in leukemia cells. Clin Chim Acta. 2004;342:115–126. doi: 10.1016/j.cccn.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- Boel P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, Coulie P, Boon T, van der Bruggen P. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/s1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1995;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureci O, Sahin U, Zwick C, Koslowski M, Seitz G, Pfreundschuh M. Identification of a meiosis-specific protein as a member of the class of cancer/testis antigens. Proc Natl Acad Sci USA. 1998;95:5211–5216. doi: 10.1073/pnas.95.9.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YT, Gure AO, Tsang S, Stockert E, Jager E, Knuth A, Old LJ. Identification of multiple cancer/testis antigens by allogeneic antibody screening of a melanoma cell line library. Proc Natl Acad Sci USA. 1998;95:6919–6923. doi: 10.1073/pnas.95.12.6919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U, Koslowski M, Tureci O, Eberle T, Zwick C, Romeike B, Moringlane JR, Schwechheimer K, Feiden W, Pfreundschuh M. Expression of cancer testis genes in human brain tumors. Clin Cancer Res. 2000;6:3916–3922. [PubMed] [Google Scholar]
- Eichmuller S, Usener D, Dummer R, Stein A, Thiel D, Schadendorf D. Serological detection of cutaneous T-cell lymphoma-associated antigens. Proc Natl Acad Sci USA. 2001;98:629–634. doi: 10.1073/pnas.021386498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Kurashige T, Harada N, Noguchi Y, Saika T, Niikawa N, Aoe M, Nakamura S, Higashi T, Hiraki A, Wada H, Kumon H, Old LJ, Nakayama E. Identification of proacrosin binding protein sp32 precursor as a human cancer/testis antigen. Proc Natl Acad Sci USA. 2001;98:3282–3287. doi: 10.1073/pnas.041625098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B, Lim Y, Lee DY, Park SY, Lee H, Kim WH, Yang H, Bang YJ, Jeoung DI. Identification and characterization of a novel cancer/testis antigen gene CAGE. Biochem Biophys Res Commun. 2002;292:715–726. doi: 10.1006/bbrc.2002.6701. [DOI] [PubMed] [Google Scholar]
- Gure AO, Stockert E, Arden KC, Boyer AD, Viars CS, Scanlan MJ, Old LJ, Chen YT. CT10: a new cancer-testis (CT) antigen homologous to CT7 and the MAGE family, identified by representational-difference analysis. Int J Cancer. 2000;85:726–732. doi: 10.1002/(sici)1097-0215(20000301)85:5<726::aid-ijc21>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Zendman AJ, Cornelissen IM, Weidle UH, Ruiter DJ, van Muijen GN. CTp11, a novel member of the family of human cancer/testis antigens. Cancer Res. 1999;59:6223–6229. [PubMed] [Google Scholar]
- Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- Sahin U, Tureci O, Schmitt H, Cochlovius B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I, Pfreundschuh M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci USA. 1995;92:11810–11813. doi: 10.1073/pnas.92.25.11810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager E, Chen YT, Drijfhout JW, Karbach J, Ringhoffer M, Jager D, Arand M, Wada H, Noguchi Y, Stockert E, Old LJ, Knuth A. Simultaneous humoral and cellular immune response against cancer-testis antigen NY-ESO-1: definition of human histocompatibility leukocyte antigen (HLA)-A2-binding peptide epitopes. J Exp Med. 1998;187:265–270. doi: 10.1084/jem.187.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmori D, Dutoit V, Lienard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F, Cerottini JC, Romero P. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Res. 2000;60:4499–4506. [PubMed] [Google Scholar]
- Marchand M, van Baren N, Weynants P, Brichard V, Dreno B, Tessier MH, Rankin E, Parmiani G, Arienti F, Humblet Y, Bourlond A, Vanwijck R, Lienard D, Beauduin M, Dietrich PY, Russo V, Kerger J, Masucci G, Jager E, De Greve J, Atzpodien J, Brasseur F, Coulie PG, van der Bruggen P, Boon T. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Jager E, Gnjatic S, Nagata Y, Stockert E, Jager D, Karbach J, Neumann A, Rieckenberg J, Chen YT, Ritter G, Hoffman E, Arand M, Old LJ, Knuth A. Induction of primary NY-ESO-1 immunity: CD8+ T lymphocyte and antibody responses in peptide-vaccinated patients with NY-ESO-1+ cancers. Proc Natl Acad Sci USA. 2000;97:12198–12203. doi: 10.1073/pnas.220413497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyoub M, Brehm M, Metthez G, Talbot S, Dutoit V, Taub RN, Keohan ML, Gure AO, Chen YT, Williamson B, Jungbluth AA, Old LJ, Hesdorffer CS, Valmori D. SSX antigens as tumor vaccine targets in human sarcoma. Cancer Immun. 2003;3:13. [PubMed] [Google Scholar]
- Lethe B, van der Bruggen P, Brasseur F, Boon T. MAGE-1 expression threshold for the lysis of melanoma cell lines by a specific cytotoxic T lymphocyte. Melanoma Res. 1997;7:S83–S88. [PubMed] [Google Scholar]
- Serrano A, Lethe B, Delroisse JM, Lurquin C, De Plaen E, Brasseur F, Rimoldi D, Boon T. Quantitative evaluation of the expression of MAGE genes in tumors by limiting dilution of cDNA libraries. Int J Cancer. 1999;83:664–669. doi: 10.1002/(sici)1097-0215(19991126)83:5<664::aid-ijc16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Chi SN, Cheung NK, Cheung IY. Expression of SSX-2 and SSX-4 genes in neuroblastoma. Int J Biol Markers. 2002;17:219–223. doi: 10.1177/172460080201700401. [DOI] [PubMed] [Google Scholar]