Abstract
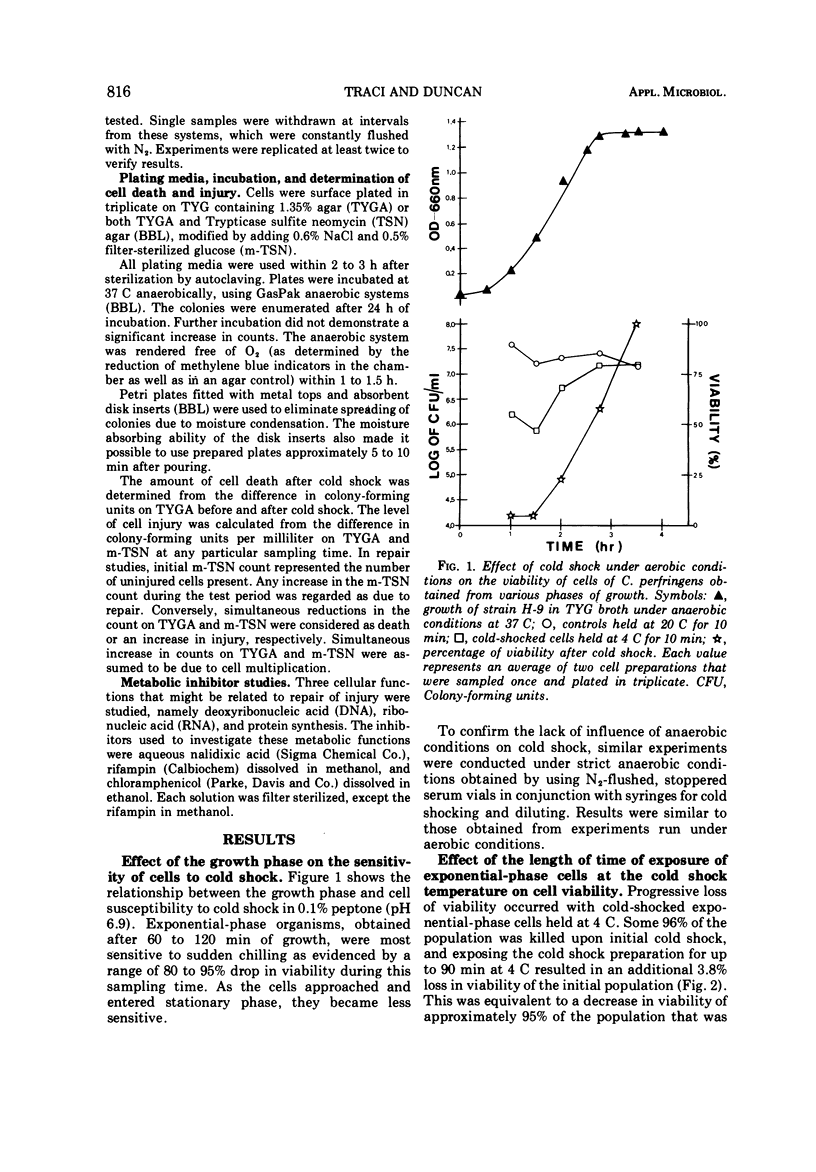
Several observations have been made in regard to cold shock lethality of Clostridium perfringens: (i) loss of viability was not consequence of exposure of the cells to air; (ii) stationary-phase cells were much more resistant to cold shock at 4 C than exponential-phase cells; (iii) at 4 C 96% of an initial population of exponential-phase cells was killed upon cold shock and 95% of the remaining population was killed within 90 min of continued exposure at 4 C; (iv) the minimal temperature differential for detectable cold shock lethality was between 17 and 23 C, and the maximum beyond which lethality was not appreciably increased was between 28 and 33 C. Up to 75% of viable cold-shocked cells were injured, as demonstrated by cold shocking late exponential-phase cells at 10 C and using differential plating procedure for recovery. Repair of injury was temperature dependent, and occurred in a complex medium and 0.1% peptone but not water. Nalidixic acid, chloramphenicol, and rifampin did not inhibit repair of injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Farrell J., Rose A. H. Cold shock in a mesophilic and a psychrophilic pseudomonad. J Gen Microbiol. 1968 Mar;50(3):429–439. doi: 10.1099/00221287-50-3-429. [DOI] [PubMed] [Google Scholar]
- GORRILL R. H., McNEIL E. M. The effect of cold diluent on the viable count of Pseudomonas pyocyanea. J Gen Microbiol. 1960 Apr;22:437–442. doi: 10.1099/00221287-22-2-437. [DOI] [PubMed] [Google Scholar]
- Iandolo J. J., Ordal Z. J. Repair of thermal injury of Staphylococcus aureus. J Bacteriol. 1966 Jan;91(1):134–142. doi: 10.1128/jb.91.1.134-142.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder I. G. Interrelated effects of cold shock and osmotic pressure on the permeability of the Escherichia coli membrane to permease accumulated substrates. J Bacteriol. 1972 Jul;111(1):211–219. doi: 10.1128/jb.111.1.211-219.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEYNELL G. G. The effect of sudden chilling on Escherichia coli. J Gen Microbiol. 1958 Oct;19(2):380–389. doi: 10.1099/00221287-19-2-380. [DOI] [PubMed] [Google Scholar]
- Miller L. L., Ordal Z. J. Thermal injury and recovery of Bacillus subtilis. Appl Microbiol. 1972 Dec;24(6):878–884. doi: 10.1128/am.24.6.878-884.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss C. W., Speck M. L. Release of biologically active peptides from Escherichia coli at subzero temperatures. J Bacteriol. 1966 Mar;91(3):1105–1111. doi: 10.1128/jb.91.3.1105-1111.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson M. D., Tomlins R. I., Ordal Z. J. Biosynthesis during recovery of heat-injured Salmonella typhimurium. J Bacteriol. 1971 Mar;105(3):1234–1236. doi: 10.1128/jb.105.3.1234-1236.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Janssen D. W., Busta F. F. Characterization of the repair of injury induced by freezing Salmonella anatum. Appl Microbiol. 1972 Apr;23(4):803–809. doi: 10.1128/am.23.4.803-809.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Jezeski J. J., Busta F. F. Repair of injury in freeze-dried Salmonella anatum. Appl Microbiol. 1971 Sep;22(3):401–407. doi: 10.1128/am.22.3.401-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Speck M. L. Metabolic process during the repair of freeze-injury in Escherichia coli. Appl Microbiol. 1972 Oct;24(4):585–590. doi: 10.1128/am.24.4.585-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray B., Speck M. L. Repair of injury induced by freezing Escherichia coli as influenced by recovery medium. Appl Microbiol. 1972 Aug;24(2):258–263. doi: 10.1128/am.24.2.258-263.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring K. The effect of low temperatures of permeability in Streptomyces hydrogenans. Biochem Biophys Res Commun. 1965 May 18;19(5):576–581. doi: 10.1016/0006-291x(65)90377-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal L. J., Martin S. E., Pariza M. W., Iandolo J. J. Ribosome synthesis in thermally shocked cells of Staphylococcus aureus. J Bacteriol. 1972 Jan;109(1):243–249. doi: 10.1128/jb.109.1.243-249.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., DARK F. A. Effect of chilling on Aerobacter aerogenes in aqueous suspension. J Gen Microbiol. 1962 Dec;29:719–730. doi: 10.1099/00221287-29-4-719. [DOI] [PubMed] [Google Scholar]
- Smeaton J. R., Elliott W. H. Selective release of ribonuclease-inhibitor from Bacillus subtilis cells by cold shock treatment. Biochem Biophys Res Commun. 1967 Jan 10;26(1):75–81. doi: 10.1016/0006-291x(67)90255-0. [DOI] [PubMed] [Google Scholar]
- Tomlins R. I., Pierson M. D., Ordal Z. J. Effect of thermal injury on the TCA cycle enzymes of Staphylococcus aureus MF 31 and Salmonella typhimurium 7136. Can J Microbiol. 1971 Jun;17(6):759–765. doi: 10.1139/m71-121. [DOI] [PubMed] [Google Scholar]


