Abstract
Sea anemones produce a myriad of toxic peptides and proteins of which a large group acts on voltage-gated Na+ channels. However, in comparison to other organisms, their venoms and toxins are poorly studied. Most of the known voltage-gated Na+ channel toxins isolated from sea anemone venoms act on neurotoxin receptor site 3 and inhibit the inactivation of these channels. Furthermore, it seems that most of these toxins have a distinct preference for crustaceans. Given the close evolutionary relationship between crustaceans and insects, it is not surprising that sea anemone toxins also profoundly affect insect voltage-gated Na+ channels, which constitutes the scope of this review. For this reason, these peptides can be considered as insecticidal lead compounds in the development of insecticides.
1. Introduction
Sea anemones are ocean dwelling, solitary members of the phylum Cnidaria and the class Anthozoa invertebrates. The name Cnidaria (with a silent “c”) refers to the cnidae, or nematocysts, which all Cnidarians possess. The phylum Cnidaria includes anemones, corals, jellyfish, and hydras. Sea anemones, named after a terrestrial flower, have a basic radial symmetry with tentacles that surround a central mouth opening (see Figure 1). The tentacles are used to catch food and transfer it to their mouth. Each stinging capsule (nematocyst) in the tentacles, and other parts of the sea anemone, contains a coiled hollow filament, usually barbed, containing venom. This is used to immobilize smaller organisms, for defense against predators, and to fight territorial disputes. When triggered by mechanical or chemical stimulation, the capsule everts and drives the filament into its prey, discharging its venom.
Figure 1.
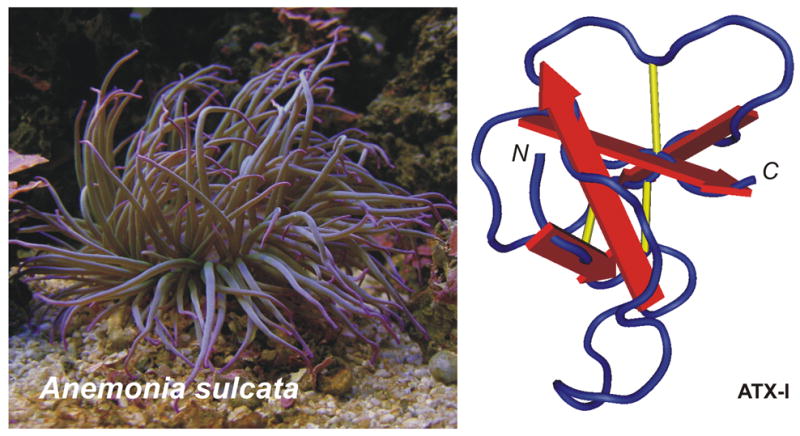
Left panel: Anemonia sulcata; right panel: NMR structure of ATX-I (PDB accession code 1ATX).
This venom contains a variety of active compounds, including potent toxins affecting voltage-gated Na+ and K+ channels (including the hERG channel), acid-sensing ion channels, pore-forming toxins (actinoporins) and protease inhibitors (Belmonte et al., 1994; Béress et al., 1975; Bruhn and Béress, 1978; Diochot et al., 2004; Diochot et al., 2003; Diochot et al., 1998). The fact that sea anemone toxins affect voltage-gated ion channels seems logical since these targets are an important component of the action potential in the signal transduction process of both vertebrates and invertebrates. In fact, toxins from some species (e.g. Anemonia sulcata, Phyllodiscus semoni) can even be dangerous to humans. But, in most cases, a sting by the nematocysts will cause local inflammations, pain and sometimes edema. Actinoporins have been reported as highly toxic to fish and crustaceans, which may be the natural prey of sea anemones. In addition to their role in predation, it has been suggested that actinoporins could act, when released in water, as efficient repellents against potential predators.
From an evolutionary point of view, the existence of crustacean-selective toxins in sea anemones is very interesting. It may help us to understand why we can encounter insect-selective toxins in sea anemones, even when insects and sea anemones in an everyday life will never “encounter one another”. Indeed, Zrzavy and Stys have proposed a taxon, the so-called ‘Pancrustacea’, comprising all crustaceans and hexapods (Zrzavy and Stys, 1997). It should hereby be explained that Hexapoda is a subphylum of the phylum Arthropoda and comprises the class Insecta, in addition to some wingless arthropods such as Collembola, Protura and Diplura. It is known that the taxonomy of Insecta is very extensive with the majority of invertebrates being classified as insects (approximately 1 million extant species) and 95% of the earth’s creatures being invertebrates. Furthermore, a monophyletic Pancrustacea taxon has been supported by several molecular studies (Giribet and Ribera, 2000; Nardi et al., 2003; Shultz and Regier, 2000), in which most of the subphylum Crustacea is paraphyletic with respect to insects. This means that insects are derived from crustacean ancestors and that by definition crustacean-selective toxins found in sea anemones may be considered as ‘lead compounds’ for molecules with an anti-insect profile, i.e. insecticides. On the basis of this peculiar evolutionary and pharmacological feature, this review will focus on sea anemone venom toxins as a source of insecticidal peptides acting on voltage-gated Na+ (Nav) channels (for a description of Nav channels, see section 3).
2. Sequences and structures
The characterization of sea anemone toxins began as early as 1968 when Shapiro purified a toxin which he called Condylactis toxin from the sea anemone Condylactis gigantea (Shapiro, 1968). This toxin appeared to cause an increase in action potential duration in lobster giant axons. Several years later, in 1975, Béress and co-workers isolated three toxins from Anemonia sulcata venom (Béress et al., 1975) which became widely used tools to study Nav channels. Presently, some 50 toxins active on Nav channels have been identified. Most of these proteins were first characterized as cardiac stimulants and neurotoxins (Barhanin et al., 1981; Sanchez-Rodriguez and Cruz-Vazquez, 2006; Schweitz et al., 1985). In 1991, Norton proposed a classification of sea anemone polypeptides dividing this group into three classes: two made up of molecules containing 46–49 amino acid residues including Type 1 (genera Anthopleura and Anemonia belonging to family Actiniidae) and Type 2 (genera Radianthus and Stichodactyla belonging to family Stichodactylidae) and one comprising shorter polypeptides containing 27–32 residues (Type 3; e.g. PaTX from Entacmaea actinostoloides and Da I and II from Dofleinia armata) (Honma et al., 2003; Nishida et al., 1985; Norton, 1991). Subsequently, other genera have been added to the Type 1 and Type 2 classes (see Figure 2) and have therefore rendered this division based on family classification obsolete. These two classes of polypeptides are similar with respect to the locations of the six half-cystines as well as several other residues thought to play a role in biological activity. Their molecular weight ranges between 3000 and 5000 Da. Due to the fact that the toxin halcurin possesses structural features of both Type 1 and 2 toxins and is present in the primitive Halcurias sp., both Type 1 and 2 toxins are considered to have evolved from the same ancestral gene (Ishida et al., 1997). Nevertheless, they are immunologically distinguishable from each other because there is no antigenic cross-reactivity between both types of toxins (Schweitz et al., 1985; Norton, 1991). Recently, three peptide toxins (Am I–III) with acute toxicity in crabs were isolated from the sea anemone Anthopleura maculata (Honma et al., 2005). Am I was weakly lethal to crabs (LD50 830 μg/kg) and Am III was potently lethal (LD50 70 μg/kg), while Am II was only paralytic (ED50 420 μg/kg). The complete amino acid sequences of the three toxins were determined by cDNA cloning. Although Am III is an analogue of the Type 1 sea anemone Nav channel toxins, both Am I (27 residues) and II (46 residues) are structurally novel peptide toxins (Honma et al., 2005).
Figure 2.
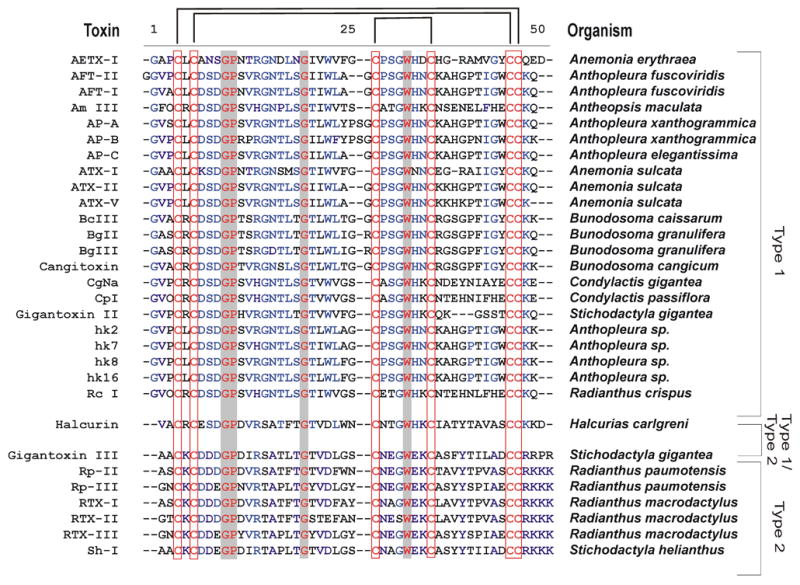
Sequence alignment of Type 1 and Type 2 sea anemone toxins. The conserved cysteine spacing and bonding pattern is indicated by red squares and black bars above the sequence. Conserved residues over all sequences are indicated in red text with grey background. Highly conserved residues per Type are indicated in blue. Hydroxylated proline residues are indicated with the letter O. References for the toxins can be found throughout the text.
The first three-dimensional structures of sea anemone toxins active on Nav channels, determined by NMR spectroscopy, were AP-A in 1988 (Torda et al., 1988) and ATX-I in 1989 (Widmer et al., 1989). Both molecules contain a core of four strands of anti parallel β-sheets connected by two loops (see Figure 1). Meanwhile, the structures of Sh-I (Wilcox et al., 1993), ATX-III (Manoleras and Norton, 1994) and AP-B (Monks et al., 1995) have also been published. AP-B seems to exist in multiple conformations in solution as a result of cis-trans isomerization about the Gly40-Pro41 peptide bond. Three loops connect the four β-sheets, the longest and least well defined being the first loop, extending from residues 8–17.
3. Nav channels and site 3 toxins
Nav channels are transmembrane protein complexes that form pores across the cell membrane through which specific ions can diffuse (Yu and Catterall, 2003). These channels are key elements in cellular function since they participate in the generation and propagation of action potentials in neurons and most electrically excitable cells present in different tissues from various organisms. Studies have indicated that these channels are composed of a pore-forming α subunit (approximately 260 kDa) which can be associated with up to four different β subunits (30–40 kDa) (Yu et al., 2003). The α-subunit is sufficient for functional expression, but the kinetics, expression level and voltage dependence of channel gating can be modified by the β subunits. The α subunits are organized in four homologous domains (I–IV), each containing six transmembrane α helices (S1–S6) and a pore lining loop located between the S5 and S6 segments. The S4 transmembrane segments in each domain contain positively charged amino acid residues at every third position and are thought to act as voltage sensors. The short intracellular loop connecting homologous domains III and IV, in particular a short sequence of hydrophobic residues (the IFM sequence), serves as the inactivation gate (Yu and Catterall, 2003). To date, nine mammalian channel isoforms have been identified (Goldin et al., 2000). The primary structure of insect Nav channels is similar to that of mammals (Zlotkin, 1999). The first insect Nav channel gene, para, was cloned from Drosophila melanogaster and heterologously expressed (Warmke et al., 1997). Since then, several other proteins, e.g. housefly Vssc1, cockroach ParaCSMA and cockroach BgNav1-1 have been functionally expressed in Xenopus laevis oocytes (Soderlund and Knipple, 2003; Tan et al., 2005). An auxiliary-regulatory β subunit for the insect Nav channels, tipE, has also been identified (Feng et al., 1995).
As crucial components of the development of action potentials, Nav channels are one of the foremost targets of venoms. Toxins from scorpion, sea anemone, cone snail, spider and insect venoms have been used to describe up to nine different receptor sites on the α-subunit of Nav channels (Wang and Wang, 2003). All of them are linked to specific effects on channel function but only sites 1–5 are molecularly defined (Leipold et al., 2005). Sea anemone venoms contain toxins which are known to prolong action potential kinetics via their ability to bind to the extracellular site 3 (loop between S3 and S4 in DIV) on the Nav channels in a membrane potential-dependent manner (see Figure 3) (Bosmans et al., 2002; Cestèle et al., 1997; Gordon and Zlotkin, 1993; Leipold et al., 2004; Pauron et al., 1985; Rogers et al., 1996). Other ligands that bind to this site, although structurally unrelated, are scorpion α-toxins and some spider toxins (δ-atracotoxins) (Gordon et al., 1996; Nicholson et al., 2004; Possani et al., 1999).
Figure 3.
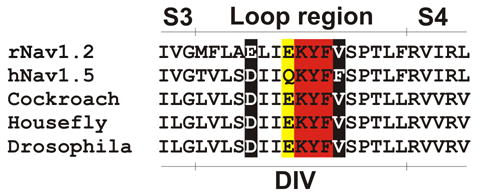
Sequence comparison of the loop region between S3 and S4 in DIV in mammalian and insect Nav channels. Residues indicated in red are reported to be important for scorpion α-toxin binding. Residues in yellow are reported to participate in sea anemone toxin binding. Residues in white text on a black background are reported to be crucial for binding of both groups.
In 1996, Rogers and co-workers converted extracellular acidic amino acids in domains I and IV of Nav1.2 to neutral or basic amino acids using site-directed mutagenesis (Rogers et al., 1996). Conversion of individual residues in the DIV S3–S4 loop identified seven residues whose mutation caused significant effects on binding of scorpion α-toxin (LqTx) or sea anemone toxin (ATX-II). Moreover, chimeric Nav channels in which amino acid residues at the extracellular end of S3 in DIV of Nav1.5 were substituted into the Nav1.2 sequence had reduced affinity for LqTx. Electrophysiological analysis showed that E1613R had 62- and 82-fold lower affinities for LqTx and ATX-II, respectively. These results indicated that non-identical amino acids of the DIV S3–S4 loop participate in scorpion α-toxin and sea anemone toxin binding to overlapping sites and that neighboring amino acid residues in the DIV S3 segment contribute to the difference in scorpion α-toxin binding affinity between cardiac and neuronal Nav channels. Also, further experiments using AP-B on Nav1.2 or Na v1.5 mutants have suggested the involvement of this loop region and more specific Glu1613 and Glu1616 for Nav1.2 and Asp1612 for Nav1.5 (see Figure 3) (Benzinger et al., 1998; Blumenthal and Seibert, 2003; Honma and Shiomi, 2006). Other research groups, focusing on finding residues important for scorpion α-toxin binding, have also highlighted residues in this loop region in DIV (Leipold et al., 2004). Since all the aforementioned Nav channel residues are similar or identical in their invertebrate counterparts, it could be hypothesized that changes in the selectivity of sea anemone toxins towards vertebrates or invertebrates could be caused by other channel residues in this vicinity. In concordance, Oliveira et al. suggested that nearby residues could also contribute to the interaction with sea anemone toxins (Oliveira et al., 2004).
4. Structure-function relationship of sea anemone toxins
Mutagenesis of AP-B resulted in the identification of a flexible loop in the region of residues 8–17 (Arg14 loop) as being important for binding to Nav channels (Dias-Kadambi et al., 1996a; Dias-Kadambi et al., 1996b). Furthermore, researchers in the lab of Dr. Blumenthal have shown that Leu18 is an absolute requirement for the binding of AP-B to its target (Blumenthal and Seibert, 2003; Honma and Shiomi, 2006). Neighboring residues are either less sensitive, or their sensitivity is dependent on the nature of the mutation, especially the introduction of negative charges at these positions that is poorly tolerated. In addition, Arg12, Ser19 and Lys49 are reported to be important for toxin affinity and channel isoform specificity of AP-B (Gallagher and Blumenthal, 1994; Seibert et al., 2004). It should be noted that AP-B has no selectivity between neuronal and cardiac Nav channels, while AP-A is selective for the latter. Only two residues located outside the flexible loop region (Trp33 and Lys37) seem to be indispensable for pharmacological activity. It has been shown that Lys37 can interact directly with Asp1612 of rat Nav1.5 (Benzinger et al., 1998; Blumenthal and Seibert, 2003).
In comparison to scorpion α- and β-toxins (Cohen et al., 2005; Ye et al., 2005), not much work has been done on the insect- or mammalian-specificity of sea anemone toxins (Pelhate et al., 1984). Early work on ATX-I has indicated its preferential toxicity against crabs, rather than mice (see Table 1) (Norton, 1991; Schweitz et al., 1981). The proof of the link between insects and crustaceans has been described on a genetic level (Boore et al., 1998). Therefore, since both crabs (crustaceans) and insects belong to the same family (Arthropoda) one could hypothesize that ATX-I preferentially targets insects rather than mice. The same argument could be made for Sh-I and Rp-II. In fact, a study by Salgado and Kem in 1992 investigated the membrane actions of ShI, CgII and CpI on action potentials and voltage-clamp membrane currents of the giant axon from the crayfish Procambarus clarkii (Salgado and Kem, 1992). Sh-I and CgII were also tested on the cockroach (Periplaneta americana) giant axon. Both toxins were particularly lethal to crustaceans, moderately toxic to an insect (cockroach), and essentially non-toxic to a mammal (mouse). Both toxins prolonged crayfish giant axon action potentials by selectively slowing Nav channel inactivation without affecting activation. However, more experiments on cloned Nav channels of insects and mammals should be carried out. ATX-II is toxic to crabs but the activity on mice via i.c.v. injection is still pronounced (see Table 1) (Norton, 1991; Schweitz et al., 1981). However, ATX-II is also very effective on the para insect channel and binds with high affinity to cockroach neuronal membranes, while its binding affinity for rat brain synaptosomes is low (Gordon et al., 1996; Gordon and Zlotkin, 1993; Schweitz et al., 1981; Warmke et al., 1997). Mutagenesis of ATX-II has provided an insight into the pharmacologically important epitopes of this toxin: either acetylation or fluorescamine treatment of ATX-II that destroyed the positive charges of the three ε-amino groups of residues Lys35, Lys36 and Lys46, and of the α-amino function of Gly1, produced an almost complete loss of toxicity and a considerable decrease in binding activity (Barhanin et al., 1981). Furthermore, these authors showed that carbethoxylation of His32 and His37 provoked an important decrease of both toxicity and binding activity, and it was also found that modification of the guanidine side chain of Arg14 could destroy both toxicity and binding of the toxin to Nav channels. Last, but not least, Barhanin et al. concluded that modification of the carboxylate functions of Asp7, Asp9 and Gln47, with glycine ethyl ester in the presence of a soluble carbodimide completely abolished the toxicity but left the affinity for the sea anemone toxin receptor unchanged. Nevertheless, ATX-II displays a high potency when tested on the mammalian cardiac Nav1.5 channel (Oliveira et al., 2004). Two toxins from the Bunodosoma granulifera, BgII and BgIII, have been thoroughly tested on mice, cloned channels, dorsal root ganglia and rat brain synaptosomes (Bosmans et al., 2002; Goudet et al., 2001; Loret et al., 1994; Salceda et al., 2002). Both toxins seem to have a preference for insects. BgII in particular has a 100-fold higher potency on the insect channel, para, as compared to other mammalian channels when expressed in Xenopus laevis oocytes (see Table 1, Figure 4). However, BgII does affect rat brain synaptosomes, with a KD of 9 nM, but no experiments have yet been carried out on insect preparations. It should be stressed that BgII and BgIII only differ in one residue (N16D) which is situated in the aforementioned Arg14 loop. Yet, their potencies towards Nav channels are remarkably different. Another trademark of BgII and BgIII is that they have a particularly devastating effect on the inactivation of the insect Nav channel, para. This is in sharp contrast to vertebrate channels. The inactivation is extremely slowed such that the channel simply does not inactivate. This removal of inactivation is also seen when ATX-II is applied on para (Gordon et al., 1996; Gordon and Zlotkin, 1993; Schweitz et al., 1981; Warmke et al., 1997).
Table 1.
Primary structures of sea anemone toxins (based on Norton, 1991).
| LD50 (μ/kg)
|
Rat brain synaptosomes (KD in nM) | Cockroach central nervous system (ICM in nM) | EC50 (nM) on rat heart muscle (Na, 1.5)) | EC50 (nM) on insect channel (para) | |||
|---|---|---|---|---|---|---|---|
| Toxin | Crab | Mice i.p. | Mice i.c. | ||||
| AFT-I | 100–150 | 98 | −2 | ||||
| AFT-II | 100–150 | 450 | 2.62* | ||||
| ATX-I | 2 | >4000 | 236 | 7000 | |||
| ATX-II | 2 | 160 | 2.5 | 90–150 | 0.5–1.5AA | 15–49* | − 10* |
| ATX-V | 5.2 | 19 | 1.6 | 50 | 2 | ||
| AP-A | 11 | 66 | 5.3 | 120 | 3 | ||
| AP-B | 39 | 8 | 0.2 | 35 | 2 | ||
| BgII | 0.4A | 9 | 500* | 5.5* | |||
| BgIII | 21A | 72 | 5100* | 1300* | |||
| Rp-I | 36 | 145 | 1.5 | 900 | 3000 | ||
| Rp-II | 15 | 4200 | 12 | >100000 | 5000 | ||
| Sh-I | 0.5–3** | >15000 | 116 | 40000*** | >8000 | ||
Results obtained from cloned channels expressed in ooytes and/or mammalian cells
LD50 varies with crab species
KD in crab is 14 nM.
i.c.v. injections
EC50 in locus central nervous system in 6 nM, LD50 in mice (S.C.) is 22 pmole/g and 2.5 pmole/g in B. Germanica.
Figure 4.
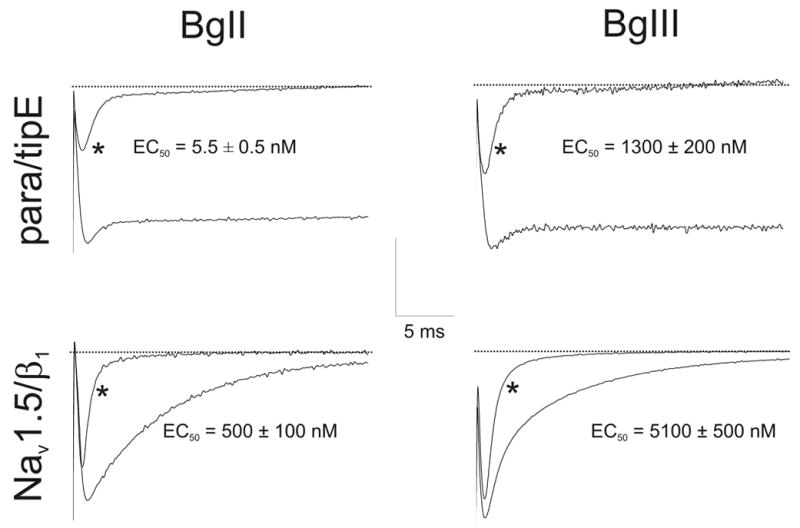
Effects of sea anemone toxins on Nav channels. The left column represents the effects of BgII, right column the effects of BgIII on the cloned Nav channels expressed in Xenopus laevis oocytes. * represents control conditions where no toxin was added. Traces shown are before, and after, addition of: Para/BgII (20 nM); Para/BgIII (5 μM); Nav1.5/BgII (1 μM); Nav1.5/BgIII (60 μM). Current traces were evoked by depolarisations ranging from −20 to 10 mV depending on the Nav channel, from a holding potential of −90 mV. Scale bar: y-axis scale for para/tipE is 0.5 μA; Nav1.5/β1 is 1 μA. EC50 values are shown on the figure.
Wang and co-workers (2004) have studied four ‘naturally occurring mutant or isoform’ toxins from an Anthopleura sp. that were expressed in E. coli and tested in contractile force studies. They suggest that residues at positions 14, 22, 25 and 37 (with an emphasis on Arg14) are important to explain the isoform-specific features of the toxins towards their pharmacological effects (Wang et al., 2004).
More recently, a new Type 1 peptide toxin with a strong paralytic activity on crustacea (LD50 approx. 1 μg/kg) was isolated from the sea anemone Condylactis gigantea (Standker et al., 2006). This new toxin, CgNa, increased action potential duration in dorsal root ganglia neurons under current clamp conditions. CgNa also prolonged the cardiac action potential duration and enhanced contractile force albeit at 100-fold higher concentrations than ATX-II. The action on Nav channel inactivation and cardiac excitation-contraction coupling resembles previous results with compounds obtained from this and other sea anemones (see previous section).
In a very recent paper by Moran et al. (Moran et al., 2006), the authors try to resolve the bioactive surface of ATX-II. To this end, they established an efficient expression system for this toxin and mutagenized it throughout. Six residues were found to constitute the anti-insect bioactive surface of ATX-II (Val2, Leu5, Asn16, Leu18, and Ile41). Further analysis of nine ATX-II mutants on Nav1.5 indicated that the bioactive surfaces interacting with insect and mammal channels practically coincide but differ from the bioactive surface AP-B. All residues important for activity excluding Arg12 and Lys49 appear in both ATX-II and AP-B. Yet, Ser19 seems to be important for the anti-mammalian activity but only has a small effect on the activity of ATX-II toward insects, suggesting that this residue is not a major contributor to the insecticidal activity of this toxin (as opposed to AP-B). The authors also investigated a major variation between the bioactive surfaces of AP-B and ATX-II that consists of a Trp33 and Lys37 in AP-B as compared to a Trp31 and a Lys35 in ATX-II. Substitution of these residues in AP-B using their ATX-II equivalents had no effect on the insecticidal activity and only a slight effect on Nav1.5. These conspicuous disparities in bioactive surfaces imply that despite similarities in the Arg14 loop, the interaction site of ATX-II with site 3 is different from that of AP-B. Support for this conclusion can be found in the report that the binding sites of AP-A and AP-B on Nav1.4 and Nav1.5 are slightly different (Benzinger et al., 1997).
5. Sea anemone toxins as insecticides?
Abundant use of one of the most commonly used insecticides in crop protection, pyrethroids, has led to the development of resistance in many insect species. One of the most important mechanisms is that of knockdown resistance (or kdr), caused by several mutations in the para gene (L1014F and M918T) which confers cross resistance to the entire class of pyrethroids (Soderlund and Knipple, 2003; Zlotkin, 1999). Another problem is that most insecticides cause toxicity in organisms other than insects because of the general conservation of Nav channel structure throughout the animal kingdom. Nevertheless, reports of toxins which show selectivity towards insects or mammals are being published (Bosmans et al., 2002). In fact, projects to replace classical chemical insecticides have already been undertaken (Zlotkin et al., 2000). Already in 1988, Bloomquist and Soderlund studied the effects of saturating concentrations of DDT (1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane) and the pyrethroid insecticides cismethrin and deltamethrin on veratridine-dependent activation of Nav channels using measurements of 22Na+ uptake into mouse brain synaptosomes. They also conducted additional experiments to assess the interactions of insecticides and ATX-II as modifiers of alkaloid-dependent uptake. DDT and ATX-II acted synergistically to increase uptake stimulated by veratridine. Moreover, DDT shifted the potency of ATX-II for enhancing veratridine-dependent uptake to 5-fold lower concentrations. In contrast, DDT and sub-saturating concentrations of ATX-II acted independently in their enhancement of Nav channels activation by batrachotoxin. Combining several insecticidal synergetic peptides like insect-selective sea anemone and scorpion toxins (e.g. Regev et al., 2003) in a baculovirus could also prove to be valuable. By itself this virus is already insect-selective (for a general overview on baculovirus biology, see Zlotkin et al., 2000 and Inceoglu et al., 2001). However, its natural kill-rate is too slow and its host range is limited (which could also be interpreted as advantageous). In 1993, Hammock and co-workers developed recombinant baculovirus insecticides using two approaches (Hammock et al., 1993). In one approach an insect-specific scorpion neurotoxin (AaHIT1) was expressed by the virus leading to a dramatic reduction in time to death (as compared to the native baculovirus). In the second approach an insect juvenile hormone esterase was expressed which lead to a significant reduction in feeding time indicating that an increase in lethality is not always necessary.
Despite this promising application, research on characterizing the differences in binding sites of insect- and mammalian-specific toxins towards Nav channels is progressing slowly. The identification and comparison of the components that are involved in the interaction of sea anemone toxins with their targets is an absolute requirement to design novel insecticides. Beyond this, insect-selective toxins could be truncated (engineered) in order to become more stable when administering them. Importantly recent reports suggest that some insect-selective peptide toxins, such as ω-atracotoxin-Hv1a might be orally active in certain species (Mukherjee et al., 2006). Surprisingly a ω-atracotoxin-Hv1a fusion protein was also topically effective (Khan et al., 2006).
Not only do arthropods destroy about 20–30% of the world’s food supply (Oerke, 1994) but they are also responsible for the transmission of many human diseases. This should be an incentive to explore the sea anemone toxin world more carefully and by doing so it could mean the beginning of a new era in insect pest control.
Acknowledgments
This work was support in part by grants G.0330.06 (F.W.O.-Vlaanderen) and OT-05-64 (K.U.Leuven). F.B. is a postdoctoral fellow in the National Institutes of Health (NIH) – Research Foundation – Flanders (FWO) Research Career Transition Award Program and is an honorary Fellow of the Belgian American Educational Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barhanin J, Hugues M, Schweitz H, Vincent JP, Lazdunski M. Structure-function relationships of sea anemone toxin II from Anemonia sulcata. J Biol Chem. 1981;256:5764–5769. [PubMed] [Google Scholar]
- Belmonte G, Menestrina G, Pederzolli C, Krizaj I, Gubensek F, Turk T, Macek P. Primary and secondary structure of a pore-forming toxin from the sea anemone, Actinia equina L., and its association with lipid vesicles. Biochim Biophys Acta. 1994;1192:197–204. doi: 10.1016/0005-2736(94)90119-8. [DOI] [PubMed] [Google Scholar]
- Benzinger GR, Kyle JW, Blumenthal KM, Hanck DA. A specific interaction between the cardiac sodium channel and site-3 toxin anthopleurin B. J Biol Chem. 1998;273:80–84. doi: 10.1074/jbc.273.1.80. [DOI] [PubMed] [Google Scholar]
- Benzinger GR, Drum CL, Chen LQ, Kallen RG, Hanck DA. Differences in the binding sites of two site-3 sodium channel toxins. Pflugers Arch. 1997;424:742–749. doi: 10.1007/s004240050460. [DOI] [PubMed] [Google Scholar]
- Béress L, Béress R, Wunderer G. Isolation and characterisation of three polypeptides with neurotoxic activity from Anemonia sulcata. FEBS Lett. 1975;50:311–314. doi: 10.1016/0014-5793(75)80517-5. [DOI] [PubMed] [Google Scholar]
- Blumenthal KM, Seibert AL. Voltage-gated sodium channel toxins: poisons, probes, and future promise. Cell Biochem Biophys. 2003;38:215–238. doi: 10.1385/CBB:38:2:215. [DOI] [PubMed] [Google Scholar]
- Boore JL, Lavrov DV, Brown WM. Gene translocation links insects and crustaceans. Nature. 1998;392:667–668. doi: 10.1038/33577. [DOI] [PubMed] [Google Scholar]
- Bosmans F, Aneiros A, Tytgat J. The sea anemone Bunodosoma granulifera contains surprisingly efficacious and potent insect-selective toxins. FEBS Lett. 2002;532:131–134. doi: 10.1016/s0014-5793(02)03653-0. [DOI] [PubMed] [Google Scholar]
- Bruhn HD, Béress L. Polyvalent proteinase inhibitor from the sea anemone Anemonia sulcata: effect on coagulation and fibrinolysis. Thromb Haemost. 1978;39:552–554. [PubMed] [Google Scholar]
- Cestèle S, Gordon D, Kopeyan C, Rochat H. Toxin III from Leiurus quinquestriatus quinquestriatus: a specific probe for receptor site 3 on insect sodium channels. Insect Biochem Mol Biol. 1997;27:523–528. doi: 10.1016/s0965-1748(97)00027-1. [DOI] [PubMed] [Google Scholar]
- Cohen L, Karbat I, Gilles N, Ilan N, Benveniste M, Gordon D, Gurevitz M. Common features in the functional surface of scorpion beta-toxins and elements that confer specificity for insect and mammalian voltage-gated sodium channels. J Biol Chem. 2005;280:5045–5053. doi: 10.1074/jbc.M408427200. [DOI] [PubMed] [Google Scholar]
- Dias-Kadambi BL, Combs KA, Drum CL, Hanck DA, Blumenthal KM. The role of exposed tryptophan residues in the activity of the cardiotonic polypeptide anthopleurin B. J Biol Chem. 1996a;271:23828–23835. doi: 10.1074/jbc.271.39.23828. [DOI] [PubMed] [Google Scholar]
- Dias-Kadambi BL, Drum CL, Hanck DA, Blumenthal KM. Leucine 18, a hydrophobic residue essential for high affinity binding of anthopleurin B to the voltage-sensitive sodium channel. J Biol Chem. 1996b;271:9422–9428. doi: 10.1074/jbc.271.16.9422. [DOI] [PubMed] [Google Scholar]
- Diochot S, Baron A, Rash LD, Deval E, Escoubas P, Scarzello S, Salinas M, Lazdunski M. A new sea anemone peptide, APETx2, inhibits ASIC3, a major acid-sensitive channel in sensory neurons. Embo J. 2004;23:1516–1525. doi: 10.1038/sj.emboj.7600177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diochot S, Loret E, Bruhn T, Béress L, Lazdunski M. APETx1, a new toxin from the sea anemone Anthopleura elegantissima, blocks voltage-gated human ether-a-go-go-related gene potassium channels. Mol Pharmacol. 2003;64:59–69. doi: 10.1124/mol.64.1.59. [DOI] [PubMed] [Google Scholar]
- Diochot S, Schweitz H, Béress L, Lazdunski M. Sea anemone peptides with a specific blocking activity against the fast inactivating potassium channel Kv3.4. J Biol Chem. 1998;273:6744–6749. doi: 10.1074/jbc.273.12.6744. [DOI] [PubMed] [Google Scholar]
- Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–1011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- Gallagher MJ, Blumenthal KM. Importance of the unique cationic residues arginine 12 and lysine 49 in the activity of the cardiotonic polypeptide anthopleurin B. J Biol Chem. 1994;269:254–259. [PubMed] [Google Scholar]
- Giribet G, Ribera C. A review of arthropod phylogeny: new data based on ribosomal DNA sequences and direct character optimization. Cladistics. 2000;16:204–231. doi: 10.1111/j.1096-0031.2000.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, et al. Nomenclature of voltage-gated sodium channels. Neuron. 2000;28:365–368. doi: 10.1016/s0896-6273(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Gordon D, Martin-Eauclaire MF, Cestèle S, Kopeyan C, Carlier E, Khalifa RB, Pelhate M, Rochat H. Scorpion toxins affecting sodium current inactivation bind to distinct homologous receptor sites on rat brain and insect sodium channels. J Biol Chem. 1996;271:8034–8045. doi: 10.1074/jbc.271.14.8034. [DOI] [PubMed] [Google Scholar]
- Gordon D, Zlotkin E. Binding of an alpha scorpion toxin to insect sodium channels is not dependent on membrane potential. FEBS Lett. 1993;315:125–128. doi: 10.1016/0014-5793(93)81147-r. [DOI] [PubMed] [Google Scholar]
- Goudet C, Ferrer T, Galan L, Artiles A, Batista CF, Possani LD, Alvarez J, Aneiros A, Tytgat J. Characterization of two Bunodosoma granulifera toxins active on cardiac sodium channels. Br J Pharmacol. 2001;134:1195–1206. doi: 10.1038/sj.bjp.0704361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammock BD, McCutchen BF, Beetham J, Choudary PV, Fowler E, Ichinose R, Ward VK, Vickers JM, Bonning BC, Harshman LG, Grant D, Uematsu T, Maeda S. Development of recombinant viral insecticides by expression of an insect-specific toxin and insect-specific enzyme in Nuclear Polyhedrosis Viruses. Arch Insect Biochem Physiol. 1993;22:315–344. doi: 10.1002/arch.940220303. [DOI] [PubMed] [Google Scholar]
- Honma T, Hasegawa Y, Ishida M, Nagai H, Nagashima Y, Shiomi K. Isolation and molecular cloning of novel peptide toxins from the sea anemone Antheopsis maculata. Toxicon. 2005;45:33–41. doi: 10.1016/j.toxicon.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Honma T, Iso T, Ishida M, Nagashima Y, Shiomi K. Occurrence of type 3 sodium channel peptide toxins in two species of sea anemones (Dofleinia armata and Entacmaea ramsayi) Toxicon. 2003;41:637–639. doi: 10.1016/s0041-0101(02)00368-9. [DOI] [PubMed] [Google Scholar]
- Honma T, Shiomi K. Peptide toxins in sea anemones: structural and functional aspects. Mar Biotechnol (NY) 2006;8:1–10. doi: 10.1007/s10126-005-5093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inceoglu AB, Kamita SG, Hinton AC, Huang Q, Severson TF, Kang K, Hammock BD. Recombinant baculoviruses for insect control. Pest Management Sci. 2001;57:981–987. doi: 10.1002/ps.393. [DOI] [PubMed] [Google Scholar]
- Ishida M, Yokoyama A, Shimakura K, Nagashima Y, Shiomi K. Halcurin, a polypeptide toxin from the sea anemone Halcurias sp., with a structural resemblance to type 1 and 2 toxins. Toxicon. 1997;35:537–544. doi: 10.1016/s0041-0101(96)00143-2. [DOI] [PubMed] [Google Scholar]
- Khan SA, Zafar Y, Briddon RW, Malik KA, Mukhtar Z. Spider venom toxin protects plants from insect attack. Transgenic Res. 2006;15:349–357. doi: 10.1007/s11248-006-0007-2. [DOI] [PubMed] [Google Scholar]
- Leipold E, Hansel A, Olivera BM, Terlau H, Heinemann SH. Molecular interaction of delta-conotoxins with voltage-gated sodium channels. FEBS Lett. 2005;579:3881–3884. doi: 10.1016/j.febslet.2005.05.077. [DOI] [PubMed] [Google Scholar]
- Leipold E, Lu S, Gordon D, Hansel A, Heinemann SH. Combinatorial interaction of scorpion toxins Lqh-2, Lqh-3, and LqhalphaIT with sodium channel receptor sites-3. Mol Pharmacol. 2004;65:685–691. doi: 10.1124/mol.65.3.685. [DOI] [PubMed] [Google Scholar]
- Loret EP, del Valle RM, Mansuelle P, Sampieri F, Rochat H. Positively charged amino acid residues located similarly in sea anemone and scorpion toxins. J Biol Chem. 1994;269:16785–16788. [PubMed] [Google Scholar]
- Manoleras N, Norton RS. Three-dimensional structure in solution of neurotoxin III from the sea anemone Anemonia sulcata. Biochemistry. 1994;33:11051–11061. doi: 10.1021/bi00203a001. [DOI] [PubMed] [Google Scholar]
- Monks SA, Pallaghy PK, Scanlon MJ, Norton RS. Solution structure of the cardiostimulant polypeptide anthopleurin-B and comparison with anthopleurin-A. Structure. 1995;3:791–803. doi: 10.1016/s0969-2126(01)00214-3. [DOI] [PubMed] [Google Scholar]
- Moran Y, Cohen L, Kahn R, Karbat I, Gordon D, Gurevitz M. Expression and mutagenesis of the sea anemone toxin Av2 reveals key amino acid residues important for activity on voltage-gated sodium channels. Biochemistry. 2006;45:8864–8873. doi: 10.1021/bi060386b. [DOI] [PubMed] [Google Scholar]
- Mukherjee AK, Sollod BL, Wikel SK, King GF. Orally active acaricidal peptide toxins from spider venom. Toxicon. 2006;47:182–187. doi: 10.1016/j.toxicon.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Nardi F, Spinsanti G, Boore JL, Carapelli A, Dallai R, Frati F. Hexapod origins: monophyletic or paraphyletic? Science. 2003;299:1887–1889. doi: 10.1126/science.1078607. [DOI] [PubMed] [Google Scholar]
- Nicholson GM, Little MJ, Birinyi-Strachan LC. Structure and function of delta-atracotoxins: lethal neurotoxins targeting the voltage-gated sodium channel. Toxicon. 2004;43:587–599. doi: 10.1016/j.toxicon.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Nishida S, Fujita S, Warashina A, Satake M, Tamiya N. Amino acid sequence of a sea anemone toxin from Parasicyonis actinostoloides. Eur J Biochem. 1985;150:171–173. doi: 10.1111/j.1432-1033.1985.tb09003.x. [DOI] [PubMed] [Google Scholar]
- Norton RS. Structure and structure-function relationships of sea anemone proteins that interact with the sodium channel. Toxicon. 1991;29:1051–1084. doi: 10.1016/0041-0101(91)90205-6. [DOI] [PubMed] [Google Scholar]
- Oerke E-C. Crop production and crop protection—estimated losses in major food and cash crops. Elsevier; Amsterdam: 1994. Estimated crop losses due to pathogens, animal pests and weeds; pp. 72–78. [Google Scholar]
- Oliveira JS, Redaelli E, Zaharenko AJ, Cassulini RR, Konno K, Pimenta DC, Freitas JC, Clare JJ, Wanke E. Binding specificity of sea anemone toxins to Nav 1.1–1.6 sodium channels: unexpected contributions from differences in the IV/S3–S4 outer loop. J Biol Chem. 2004;279:33323–33335. doi: 10.1074/jbc.M404344200. [DOI] [PubMed] [Google Scholar]
- Pauron D, Barhanin J, Lazdunski M. The voltage-dependent Na+ channel of insect nervous system identified by receptor sites for tetrodotoxin, and scorpion and sea anemone toxins. Biochem Biophys Res Commun. 1985;131:1226–1233. doi: 10.1016/0006-291x(85)90222-0. [DOI] [PubMed] [Google Scholar]
- Pelhate M, Laufer J, Pichon Y, Zlotkin E. Effects of several sea anemone and scorpion toxins on excitability and ionic currents in the giant axon of the cockroach. J Physiol (Paris) 1984;79:309–317. [PubMed] [Google Scholar]
- Possani LD, Becerril B, Delepierre M, Tytgat J. Scorpion toxins specific for Na+-channels. Eur J Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Regev A, Rivkin H, Inceoglu B, Gershburg E, Hammock BD, Gurevitz M, Chejanovsky N. Further enhancement of baculovirus insecticidal efficacy with scorpion toxins that interact cooperatively. FEBS Lett. 2003;537:106–110. doi: 10.1016/s0014-5793(03)00104-2. [DOI] [PubMed] [Google Scholar]
- Rogers JC, Qu Y, Tanada TN, Scheuer T, Catterall WA. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3–S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J Biol Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- Salceda E, Garateix A, Soto E. The sea anemone toxins BgII and BgIII prolong the inactivation time course of the tetrodotoxin-sensitive sodium current in rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2002;303:1067–1074. doi: 10.1124/jpet.102.038570. [DOI] [PubMed] [Google Scholar]
- Salgado VL, Kem WR. Actions of three structurally distinct sea anemone toxins on crustacean and insect sodium channels. Toxicon. 1992;30:1365–1381. doi: 10.1016/0041-0101(92)90512-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Rodriguez J, Cruz-Vazquez K. Isolation and biological characterization of neurotoxic compounds from the sea anemone Lebrunia danae (Duchassaing and Michelotti, 1860) Arch Toxicol. 2006;80:436–441. doi: 10.1007/s00204-006-0059-3. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Bidard JN, Frelin C, Pauron D, Vijverberg HP, Mahasneh DM, Lazdunski M, Vilbois F, Tsugita A. Purification, sequence, and pharmacological properties of sea anemone toxins from Radianthus paumotensis. A new class of sea anemone toxins acting on the sodium channel. Biochemistry. 1985;24:3554–3561. doi: 10.1021/bi00335a025. [DOI] [PubMed] [Google Scholar]
- Schweitz H, Vincent JP, Barhanin J, Frelin C, Linden G, Hugues M, Lazdunski M. Purification and pharmacological properties of eight sea anemone toxins from Anemonia sulcata, Anthopleura xanthogrammica, Stoichactis giganteus, and Actinodendron plumosum. Biochemistry. 1981;20:5245–5252. doi: 10.1021/bi00521a023. [DOI] [PubMed] [Google Scholar]
- Seibert AL, Liu J, Hanck DA, Blumenthal KM. Role of Asn-16 and Ser-19 in anthopleurin B binding. Implications for the electrostatic nature of Na(V) site 3. Biochemistry. 2004;43:7082–7089. doi: 10.1021/bi0496135. [DOI] [PubMed] [Google Scholar]
- Shapiro BI. Purification of a toxin from tentacles of the anemone Condylactis gigantea. Toxicon. 1968;5:253–259. doi: 10.1016/0041-0101(68)90115-3. [DOI] [PubMed] [Google Scholar]
- Shultz JW, Regier JC. Phylogenetic analysis of arthropods using two nuclear protein-encoding genes supports a crustacean + hexapod clade. Proc Biol Sci. 2000;267:1011–1019. doi: 10.1098/rspb.2000.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderlund DM, Knipple DC. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem Mol Biol. 2003;33:563–577. doi: 10.1016/s0965-1748(03)00023-7. [DOI] [PubMed] [Google Scholar]
- Standker L, Béress L, Garateix A, Christ T, Ravens U, Salceda E, Soto E, John H, Forssmann WG, Aneiros A. A new toxin from the sea anemone Condylactis gigantea with effect on sodium channel inactivation. Toxicon. 2006 doi: 10.1016/j.toxicon.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Tan J, Liu Z, Wang R, Huang ZY, Chen AC, Gurevitz M, Dong K. Identification of amino acid residues in the insect sodium channel critical for pyrethroid binding. Mol Pharmacol. 2005;67:513–522. doi: 10.1124/mol.104.006205. [DOI] [PubMed] [Google Scholar]
- Torda AE, Mabbutt BC, van Gunsteren WF, Norton RS. Backbone folding of the polypeptide cardiac stimulant anthopleurin-A determined by nuclear magnetic resonance, distance geometry and molecular dynamics. FEBS Lett. 1988;239:266–270. doi: 10.1016/0014-5793(88)80931-1. [DOI] [PubMed] [Google Scholar]
- Wang L, Ou J, Peng L, Zhong X, Du J, Liu Y, Huang Y, Liu W, Zhang Y, Dong M, Xu AL. Functional expression and characterization of four novel neurotoxins from sea anemone Anthopleura sp. Biochem Biophys Res Commun. 2004;313:163–170. doi: 10.1016/j.bbrc.2003.11.102. [DOI] [PubMed] [Google Scholar]
- Wang SY, Wang GK. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- Warmke JW, Reenan RA, Wang P, Qian S, Arena JP, Wang J, Wunderler D, Liu K, Kaczorowski GJ, Van der Ploeg LH, et al. Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology. J Gen Physiol. 1997;110:119–133. doi: 10.1085/jgp.110.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer H, Billeter M, Wuthrich K. Three-dimensional structure of the neurotoxin ATX Ia from Anemonia sulcata in aqueous solution determined by nuclear magnetic resonance spectroscopy. Proteins. 1989;6:357–371. doi: 10.1002/prot.340060403. [DOI] [PubMed] [Google Scholar]
- Wilcox GR, Fogh RH, Norton RS. Refined structure in solution of the sea anemone neurotoxin ShI. J Biol Chem. 1993;268:24707–24719. [PubMed] [Google Scholar]
- Ye X, Bosmans F, Li C, Zhang Y, Wang DC, Tytgat J. Structural basis for the voltage-gated Na+ channel selectivity of the scorpion alpha-like toxin BmK M1. J Mol Biol. 2005;353:788–803. doi: 10.1016/j.jmb.2005.08.068. [DOI] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, et al. Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci. 2003;23:7577–7585. doi: 10.1523/JNEUROSCI.23-20-07577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotkin E. The insect voltage-gated sodium channel as target of insecticides. Annu Rev Entomol. 1999;44:429–455. doi: 10.1146/annurev.ento.44.1.429. [DOI] [PubMed] [Google Scholar]
- Zlotkin E, Fishman Y, Elazar M. AaIT: from neurotoxin to insecticide. Biochimie. 2000;82:869–881. doi: 10.1016/s0300-9084(00)01177-9. [DOI] [PubMed] [Google Scholar]
- Zrzavy J, Stys P. The basic body plan of arthropods: insights from evolutionary morphology and developmental biology. J Evol Biol. 1997;10:353–367. [Google Scholar]


