Abstract
Cigarette smoking is significantly more prevalent in individuals with schizophrenia than in non-affected populations. Certain neurocognitive deficits and disruptions common in schizophrenia may be altered by smoking, leading to the hypothesis that schizophrenics engage in smoking behavior to alleviate specific neurocognitive symptoms of the disorder. Additionally, research suggests that individuals with schizophrenia have altered auditory event related potentials (ERPs) and abnormalities in evoked gamma oscillations which are both indices of sensory information processing. This study was conducted to examine the effect of acute administration of nicotine and the non-specific nicotinic antagonist mecamylamine on the P20 and N40 components of the auditory event related potential (ERP) and evoked gamma oscillations in mice. Acute nicotine (1 mg/kg) significantly increased P20 amplitude, an effect that was blocked by pretreatment with mecamylamine (2 mg/kg). Additionally, acute nicotine increased the normal burst of evoked gamma following an auditory stimulus. The increase in evoked gamma was also blocked by mecamylamine pretreatment. Although acute nicotine decreased amplitude of the N40 component, this decrease was not attenuated by mecamylamine. These results replicate findings that nicotine may enhance early sensory information processing through the nicotinic acetylcholinergic receptor system in an established model (ERPs) and extend these findings in an emerging, novel model (evoked gamma oscillations) of sensory information processing. The results also support the hypothesis that nicotine may be beneficial to individuals with deficits in neurocognitive functions, such as those suffering from schizophrenia.
Keywords: Schizophrenia, Animal model
INTRODUCTION
Researchers have long posited a role for nicotine or nicotine-like compounds in the treatment of schizophrenia. It is well-documented that cigarette smoking is significantly more prevalent in individuals suffering from schizophrenia than in non-affected populations (Hughes et al., 1986, Ziedonis et al., 1994, Diwan et al., 1998). Several theories have been offered to explain this relationship. Prominent among these theories is the self-medication hypothesis that suggests schizophrenics smoke to alleviate symptoms or deficits associated with the disorder (Kumari and Postma, 2005). Recently, researchers have focused specifically on the possibility that the nicotine in cigarettes may attenuate or eliminate certain neurocognitive deficits associated with schizophrenia (Freedman et al., 1994, Levin and Rezvani, 2000, Kumari and Postma, 2005).
Individuals with schizophrenia exhibit a well-documented deficit in gating of the P50 component of auditory event-related potentials (ERPs) (Adler et al., 1982, Freedman et al., 1983, Boutros et al., 1991, Boutros et al., 1993). In a normal population the amplitude of the P50 response to the second auditory stimulus (S2) of an identical pair is reduced relative to the P50 amplitude in response to the first stimulus (S1). In schizophrenics, the amplitudes of both responses are approximately equal and this gating deficit has typically been described as a failure to inhibit the S2 in a paired-stimulus task and has been conceptualized as a loss of sensory gating abilities (Adler et al 1998). However, there is evidence, historical and more recent, that this gating deficit in unmedicated schizophrenia may be a result of decreased S1 amplitude without further reduction of the S2 (Freedman et al., 1983, Jin and Potkin, 1996, Stevens and Wear, 1997, Clementz and Blumenfeld, 2001, Moxon et al., 2003). The P50 gating deficits are reportedly attenuated by nicotine in people with schizophrenia (Adler et al., 1982, Adler et al., 1993, Griffith et al., 1998) and their first degree relatives (Adler et al., 1982, Adler et al., 1993, Griffith et al., 1998), supporting a possible role for nicotine in symptom management and treatment in schizophrenia. Some studies have attributed this effect to a reduction in S2, whereas others indicate that nicotine acts primarily to increase S1 amplitude, thus restoring the normal gating profile (Adler et al., 1993, Crawford et al., 2002, Metzger et al., 2006).
In addition to changes in P50 amplitude and deficits in P50 gating, individuals with schizophrenia also exhibit alterations in gamma activity. Gamma activity is oscillatory electroencephalographic (EEG) activity that falls in the 30 to 70 Hz range (Herrmann and Demiralp, 2005) and has been associated with a range of perceptual and cognitive processes in humans (Pantev et al., 1991, Basar-Eroglu et al., 1996, Demiralp et al., 1996, Basar et al., 2001, Demiralp et al., 2006). A recent study found that negative symptoms in schizophrenia are associated with a reduction of gamma synchrony, while positive symptoms are correlated with gamma synchrony increases relative to a non-affected group (Lee et al., 2003). Individuals with schizophrenia also exhibit a reduction in induced and evoked gamma (Clementz et al., 1997, Haig et al., 2000, Spencer et al., 2003, Gallinat et al., 2004). There has been some speculation that the P50 component of the ERP may be an element of the gamma band response, suggesting that changes in P50 amplitude and gating may be rooted in changes to the underlying gamma activity (Clementz et al., 1997, Johannesen et al., 2005). There is very limited information regarding the effects of nicotine on gamma oscillations in normal or disordered populations. A study in rat hippocampul slices reported that the infusion of highly concentrated nicotine (100 μg) promoted hippocampul gamma activity (Song et al., 2005). Although there are no studies of the direct effects of nicotine on gamma activity in humans, a study in smokers found that chronic smokers exhibited larger gamma band oscillations than never smokers (Crawford et al., 2002). There are no studies in the literature examining the effects of nicotine on gamma in vivo, either in humans or in animals.
Animal models of ERPs and ERP deficits are frequently used to assess pharmacological and behavioral manipulations as well as neuroanatomical substrates of a variety of human conditions. Models of the ERP changes associated with schizophrenia have been used to explore potential mechanisms for the observed ERP deficits and as a tool for the development of new therapeutic agents (Stevens and Wear, 1997, Stevens et al., 1998, Connolly et al., 2003, Simosky et al., 2003, Connolly et al., 2004, Maxwell et al., 2004a, Maxwell et al., 2004b, Umbricht et al., 2004). Although many studies propose the P20/N40 waveform difference in mice to be analogous to the human P50 (Stevens and Wear, 1997, Simosky et al., 2003), recently several studies have indicated that the P20 and N40 waveforms respond independently to pharmacological and behavioral manipulations (Connolly et al., 2004, Maxwell et al., 2004a, Maxwell et al., 2004b, Umbricht et al., 2004, Metzger et al., 2006). In fact, a recent study on the effects of nicotine on ERP components demonstrated opposite effects of nicotine on the P20 and N40 amplitudes in mice with nicotine increasing P20 amplitude and decreasing N40 amplitude (Metzger et al., 2006). We believe the mouse P20 and N40 to be analogous to the human P50 and N100 respectively based on previous research in our lab and others (Connolly et al., 2003, Siegel et al., 2003, Connolly et al., 2004, Maxwell et al., 2004a, Maxwell et al., 2004b, Umbricht et al., 2004, Siegel et al., 2005, Metzger et al., 2006). Thus, we have chosen to analyze the components independently in this study.
The current study examines the effects of nicotine on the P20 and N40 components of the ERP as well as evoked gamma oscillations. This study seeks to better characterize the effects of nicotine in the established ERP model and in the less-studied evoked gamma model of cognitive function. The relationship between the P20 ERP component and evoked gamma also will be examined. Based on previous reports in the literature, we hypothesize that nicotine will increase the amplitude of the P20 component while decreasing N40 amplitude (Siegel et al., 2005, Metzger et al., 2006). Given the possible relationship between the human P50 component and gamma oscillations (Clementz et al., 1997, Johannesen et al., 2005), we hypothesize that nicotine also will increase evoked gamma and that increases in P20 and gamma will be significantly correlated. Finally, to assess the mechanism by which nicotine is causing its effects, we will administer the non-specific nicotinic cholinergic receptor antagonist mecamylamine by itself and in combination with nicotine.
EXPERIMENTAL PROCEDURES
Animals
Eleven male C57BL/6J mice were obtained at 8 weeks of age from Jackson Laboratories (Bar Harbor, ME). All testing was conducted between 10 and 12 weeks of age. Mice were housed four to five per cage until electrode implant surgery and single-housed post-surgery in a light- and temperature-controlled Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility. Water and standard rodent chow were available ad libitum. Experiments were conducted at the University of Pennsylvania during the light phase between 10:00 AM and 2:00 PM. Mice were acclimated to the housing facility for at least one week before all procedures. All protocols were performed in accordance with University Laboratory Animal Resources guidelines and were approved by the Institutional Animal Care and Use Committee.
Drugs
Nicotine hydrogen tartrate salt (1.0 mg/kg) and mecamylamine hydrochloride (2.0 mg/kg) (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 0.09% saline and administered intraperitoneally at a volume of 0.1 ml. All drug doses are expressed as drug base and were selected based on previous research on nicotine’s effects on ERPs (Siegel et al., 2005, Metzger et al., 2006) and mecamylamine’s ability to block behavioral effects of nicotine (Damaj et al., 2003, Gould and Stephen Higgins, 2003). A minimum washout period of 24 hours was allowed between each drug testing session.
Surgery
Animals underwent stereotaxic implantation of electrode assemblies (Plastic Products, Roanoke, VA) for later nonanesthetized recording of auditory ERPs. Animals were anesthetized with isoflurane for the duration of the surgical procedure. Unipolar recording electrodes were placed unilaterally in the CA3 hippocampal region, (1.4 mm posterior, 2.65 mm lateral, and 2.75 mm deep relative to bregma) and referenced to the ipsilateral frontal sinus to reflect whole brain electrical activity. The electrode pedestal was secured to the skull using dental cement and Super Glue. All surgical procedures were consistent with previously published descriptions (Connolly et al., 2003, Siegel et al., 2003, Connolly et al., 2004, Maxwell et al., 2004a, Maxwell et al., 2004b, Siegel et al., 2005, Metzger et al., 2006).
Recording
ERP and gamma testing was conducted over fours days with two recording sessions per day. The first recording session was a baseline saline trial followed by a second session consisting of an i.p. injection of the test compound. On the first testing day, animals were acclimated to all handling and testing procedures. Mice received mecamylamine (2 mg/kg) on test day two, nicotine (1 mg/kg) on test day three, and a combination treatment of mecamylamine (2 mg/kg) followed 5 minutes later by nicotine (1 mg/kg) on the fourth testing day. Testing commenced five minutes after the last injection. Stimuli were generated by Micro1401 hardware and Spike 5 software (Cambridge Electronic Design, Cambridge, UK) and were delivered through speakers attached to the cage top. All recordings were performed in a home cage environment, which was placed in a Faraday cage 15 minutes before stimulus onset. A series of 50 white noise clicks (10 ms in duration) were presented with a 9-s interstimulus interval at 85 dB compared with background of 70 dB. Data for the ERP (P20 and N40 components) and evoked gamma analyses were treated differently. Waveforms for the ERP analyses were filtered between 1 and 500 Hz and baseline corrected at stimulus onset. Individual sweeps were rejected for movement artifact based on a criterion of 2 times the root mean squared amplitude per mouse. Average waves were created from 50-ms pre-stimulus to 200-ms post-stimulus. The P20 component was selected for each ERP by determining the maximum positive deflection between 10 and 30 ms. The N40 was selected by determining the maximum negative deflection between 25 to 60 ms (Figure 1A, B). A Fast Fourier Transform (FFT) was performed on the EEG data from the saline trial to identify the frequency band of the gamma band response. Data for evoked gamma analyses were digitally band pass filtered between 31–61 Hz with 3dB cutoff points of 26.6 and 65.8 Hz and artifact rejected for movement based on the same standards as ERP data. Gamma data were rectified by squaring to produce a positive value for gamma activity at each point and enable measurement of the area under the curve (AUC) for the time window between 0 and 70ms (evoked gamma period) (Figure 2). The evoked window contains the normal, time-delimited burst of evoked gamma following a stimulus as well as early and mid latency components. The preceding temporal window captures basal levels of gamma in the brain that are independent of stimulus presentation.
Figure 1.
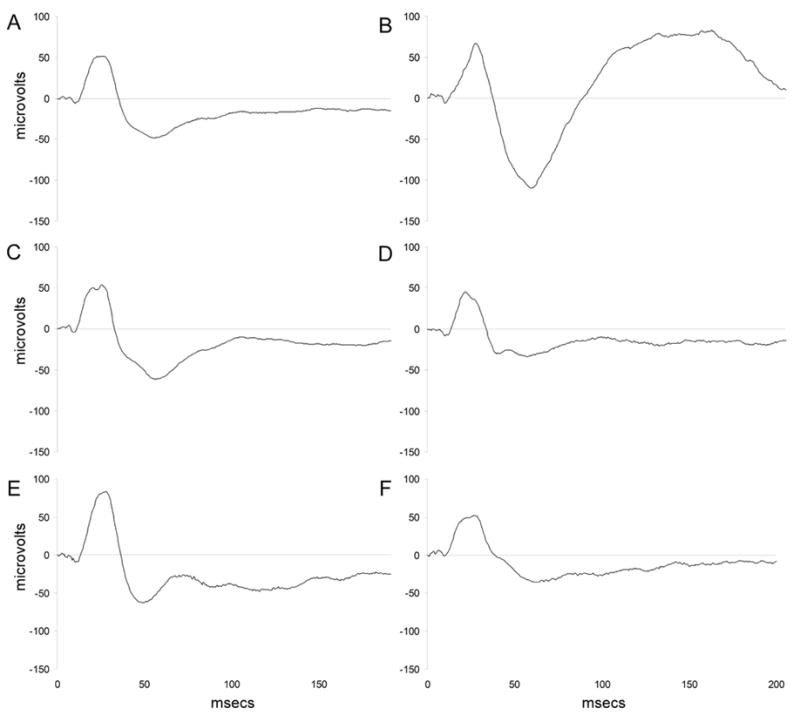
Grand average event related potential tracings and average ERPs for each drug condition. (A) Grand average ERP tracing. (B) ERP tracing from a single, saline-treated mouse. (C) Average ERP trace for mice following administration of saline. (D) Average ERP trace for mice following administration of 2 mg/kg mecamylamine. (E) Average ERP trace for mice following administration of 1 mg/kg nicotine. (F) Average ERP trace for mice following administration of 2 mg/kg mecamylamine followed by 1 mg/kg nicotine. ERP amplitude in microvolts is shown on the ordinate and time in milliseconds in displayed on the abscissa.
Figure 2.
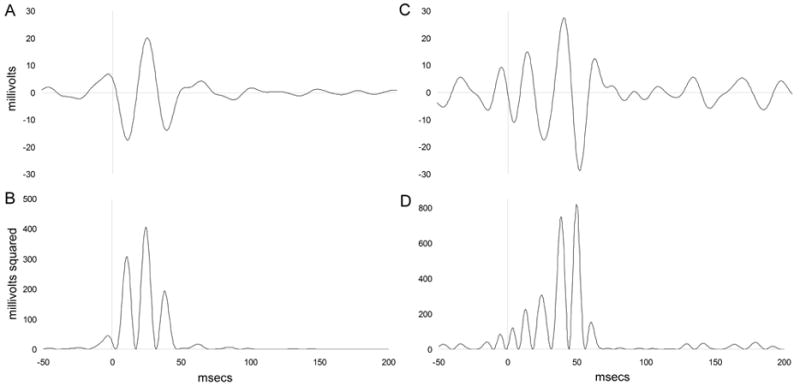
Grand average gamma oscillation tracings and gamma tracings from a single saline treated mouse. An 85 dB white noise stimulus was presented at 0 ms. (A) Grand average gamma filtered data; data were digitally band pass filtered between 31–61 Hz with 3dB cutoff points of 26.6 and 65.8 Hz and artifact rejected for movement. Inset depicts raw EEG data from a single mouse. (B) Grand average rectified gamma data; gamma data were rectified by squaring to produce a positive value for gamma activity at each point and enable measurement of the area under the curve (AUC). (C) Gamma filtered data from an individual mouse in the saline condition; data were digitally band pass filtered between 31–61 Hz with 3dB cutoff points of 26.6 and 65.8 Hz and artifact rejected for movement. (D) Rectified gamma data from a single mouse in the saline condition; gamma data were rectified by squaring to produce a positive value for gamma activity at each point and enable measurement of the area under the curve (AUC). The evoked window (0 – 70 ms) contains the normal, time-delimited burst of evoked gamma following a stimulus.
Analysis
Repeated measures analyses of variance (ANOVAs) were performed on baseline data for each of the dependent variables to assess possible effects of time and repeated testing. ERP component data were analyzed using separate general linear model, repeated measures ANOVAs to determine the effects of nicotine and mecamylamine on the P20 and N40 ERP components. Area under the curve was calculated for the evoked gamma period (0 to 70 ms) within the frequency window identified by the FFT. Gamma data were analyzed using a general linear model, repeated measures ANOVA to determine the effects of nicotine and mecamylamine on AUC. For all analyses, Fisher’s LSD post-hoc tests were used to determine differences among drug groups. Pearson’s r correlations were performed for the grand averages of the P20 and N40 amplitudes and AUC for evoked gamma to explore potential relationships between the ERP components and gamma.
RESULTS
P20
A repeated measures ANOVA on baseline data for the P20 component revealed no significant differences over time. There was a significant overall effect of drug treatments on P20 amplitude [F(3,30) = 3.82, p = 0.02]. Post hoc testing revealed that mecamylamine alone had no significant effect on P20 amplitude while treatment with nicotine alone significantly increased P20 amplitude. Nicotine’s effect to increase P20 amplitude was blocked by pre-treatment with mecamylamine (Figure 3).
Figure 3.
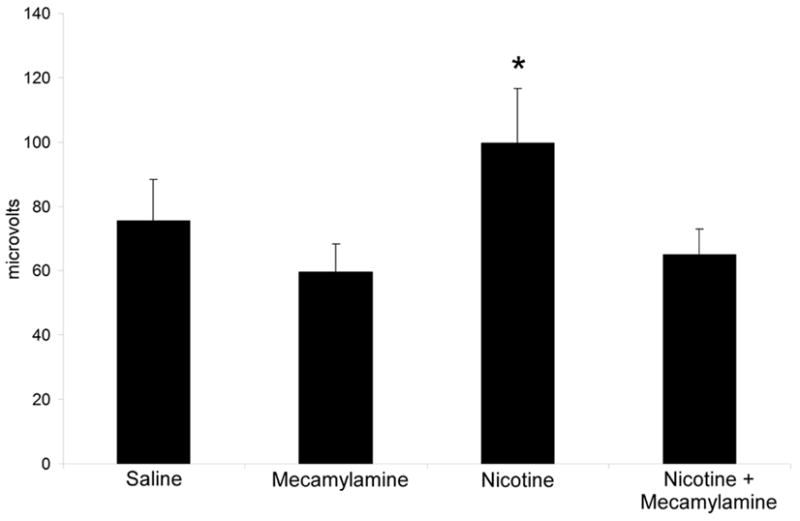
Mean amplitude of the P20 in C57BL/6J mice in each drug condition. Nicotine causes an amplification of the P20 ERP that is reversed by the non-specific nicotinic antagonist mecamylamine. Error bars represent standard error of the mean. Asterisk indicates a significant difference between the drug condition and the saline condition (p<0.05).
N40
A repeated measures ANOVA on baseline data for the N40 component revealed no significant differences over time. There was a significant overall effect of drug treatment on N40 amplitude [F(3,30) = 7.40, p = 0.0008]. Post hoc testing revealed that all treatments, mecamylamine alone, nicotine alone, and the combined mecamylamine and nicotine treatment significantly decreased N40 amplitude (Figure 4).
Figure 4.
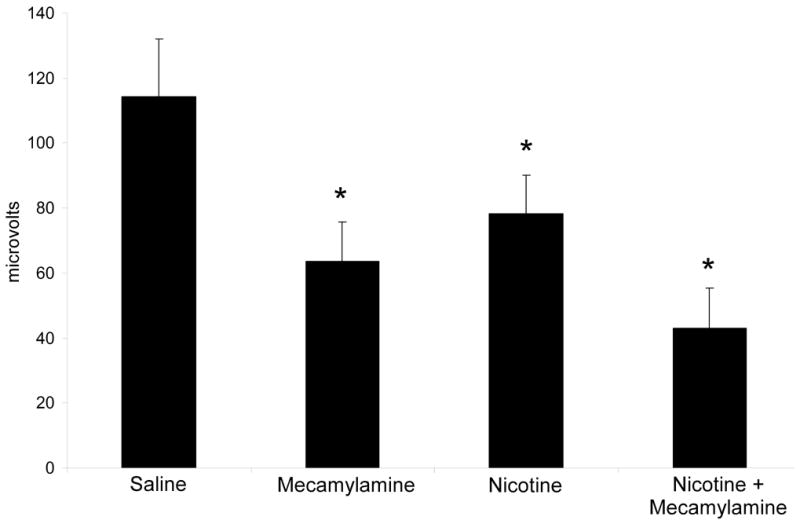
Mean amplitude of the N40 in C57BL/6J mice in each drug condition. Both nicotine and mecamylamine result in a reduction in the N40 ERP. Error bars represent standard error of the mean. Asterisk indicates a significant difference between the drug condition and the saline condition (p<0.05).
Evoked Gamma
A repeated measures ANOVA on baseline data for evoked gamma revealed no significant differences over time. There was a significant overall effect of drug treatment on evoked gamma [F(3,30) = 6.66, p = 0.001]. Post hoc testing revealed that mecamylamine alone had no significant effect on evoked gamma while treatment with nicotine alone significantly increased evoked gamma. The nicotine-induced increases were blocked by pre-treatment with mecamylamine (Figure 5).
Figure 5.
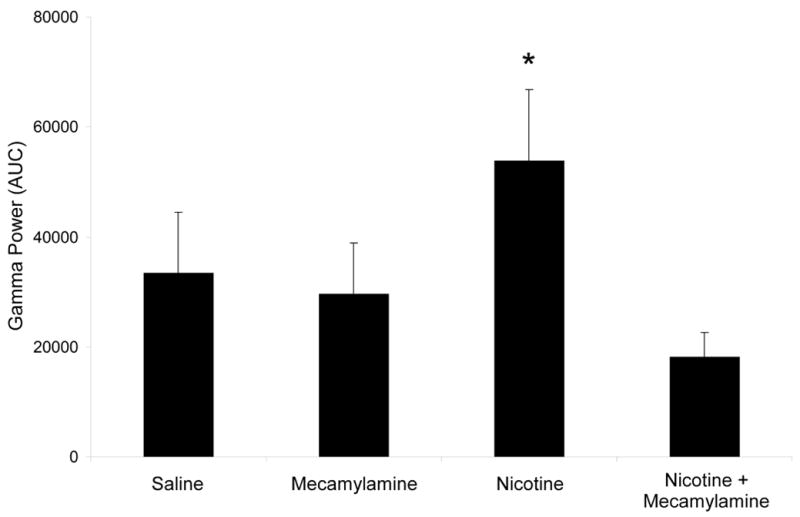
Mean area under the curve for rectified evoked gamma oscillations in C57BL/6J mice in each drug condition. Nicotine causes an increase in evoked gamma oscillations that is reversed by the non-specific nicotinic antagonist mecamylamine. Error bars represent standard error of the mean. Asterisk indicates a significant difference between the drug condition and the saline condition (p<0.05).
P20 and N40 Component/Gamma Correlations
Individual data points for all animals in all four drug conditions were used to correlate P20 and N40 amplitudes with AUC for evoked gamma. P20 amplitude was significantly positively correlated (r = 0.84, p ≤ 0.001) with the AUC for evoked gamma. The amplitude of the N40 component also was significantly positively correlated with AUC for evoked gamma (r = 0.62, p ≤ 0.001) (Figure 6).
Figure 6.
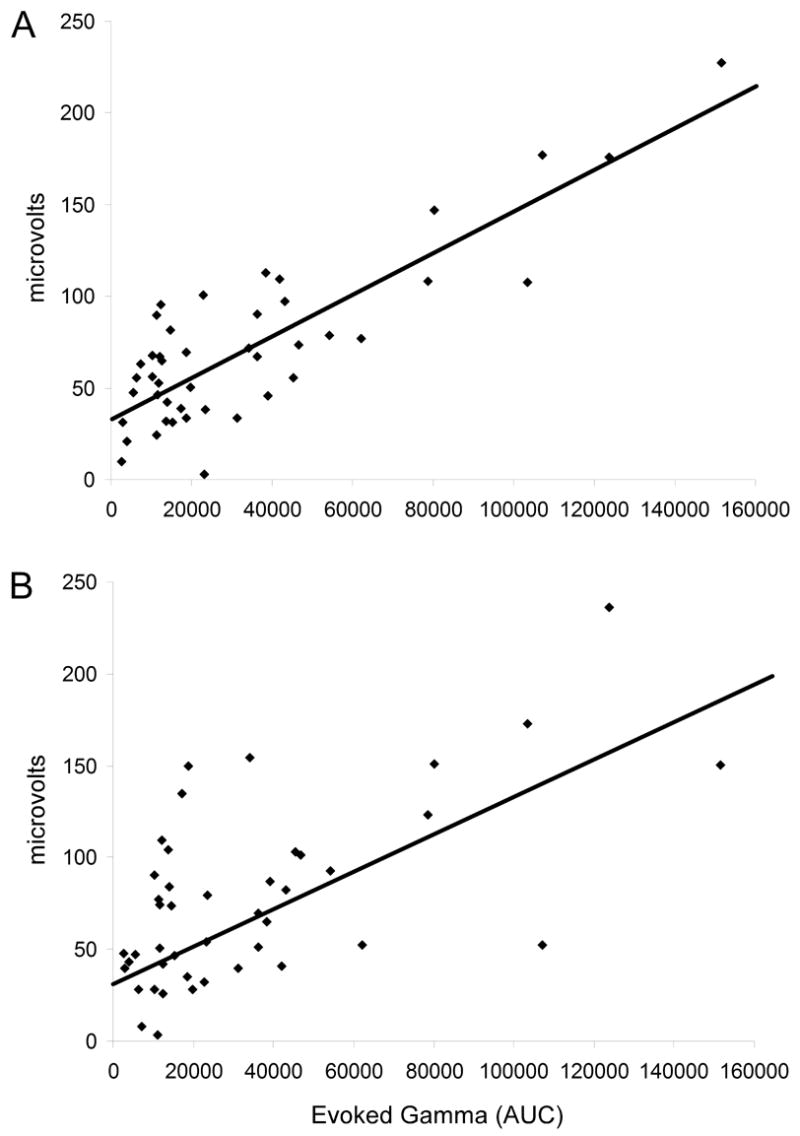
Pearson’s r correlations were performed for P20 and N40 amplitudes and AUC for evoked gamma across all four drug conditions. (A) P20 amplitude was significantly positively correlated (r = 0.84, p ≤ 0.001) with AUC for evoked gamma. (B) N40 amplitude was significantly positively correlated (r = 0.62, p ≤ 0.001) with AUC for evoked gamma.
DISCUSSION
A thorough review of the literature suggests that nicotine may enhance cognitive performance in humans with particular gains in disordered populations such as individuals with schizophrenia (Sacco et al., 2004). Additionally, nicotine reportedly attenuates gating deficits of the P50 component of the ERPs in individuals with schizophrenia (Adler et al., 1982, Adler et al., 1993, Griffith et al., 1998). There also is evidence that heavy smoking may enhance gamma oscillations, electrical activity in the brain that are associated with a variety of cognitive functions including object representation and memory maintenance (Pantev et al., 1991, Basar-Eroglu et al., 1996, Demiralp et al., 1996, Tallon-Baudry and Bertrand, 1999, Bertrand and Tallon-Baudry, 2000, Basar et al., 2001, Demiralp et al., 2006). Gamma oscillations also are reportedly compromised in individuals with schizophrenia (Clementz et al., 1997, Haig et al., 2000, Spencer et al., 2003, Gallinat et al., 2004). The present study examined the effects of nicotine and the non-specific nicotinic antagonist mecamylamine on the P20 and N40 components of the ERP as well as evoked gamma oscillations in C57BL/6J male mice.
Although deficits in gating of the P50 component are sometimes modeled by P20/N40 gating deficits in the mouse and are frequently used to assess specific aspects of information-processing deficits in humans, we chose to examine the individual amplitudes of the P20 and N40 components of the ERP in the present study. This decision was based on both historical and recent reports that suggest that the gating deficits observed in unmedicated and unmedicated schizophrenia patients are due to a deficit in responding to the initial stimulus (S1) rather than decreased responding to the second stimulus (S2) (Freedman et al., 1983, Jin et al., 1997, Clementz and Blumenfeld, 2001, Moxon et al., 2003). The finding that nicotine has opposite effects on the amplitudes of the P20 and N40 components supports reports that the P20 and N40 are distinctly separate components of the ERP. Our finding that nicotine increases the amplitude of the P20 component is consistent with a recent report that nicotine increases P20 amplitude to the S1 but has no effects on P20 amplitude to the S2 (Metzger et al., 2006). Our findings lend support to the hypothesis that nicotine’s ability to attenuate P50 gating deficits is likely due to its increase of P50 amplitude to the S1.
The finding that nicotine decreases amplitude of the N40 component replicate previous reports of nicotine-induced N40 decreases from our laboratory (Siegel et al., 2005, Metzger et al., 2006), but is inconsistent with a recent report that acute nicotine administration increases N40 amplitude (Radek et al., 2006). This difference is likely accounted for by a methodological distinction between the two studies. Radek and colleagues used a P20/N40 difference waveform to evaluate the effects of nicotine on N40 amplitude while we evaluated the amplitude of the P20 and N40 components separately (Connolly et al., 2004, Maxwell et al., 2004a, Maxwell et al., 2004b, Umbricht et al., 2004, Metzger et al., 2006). It is possible that nicotine-induced increases in P20 amplitude may have masked any decreases in the amplitude of the N40 when the P20/N40 waveform was analyzed as a single rather than as separate components. Additionally, our study was performed on C57BL/6J mice while Radek and colleagues used DBA/2 mice. A recent study reported strain differences in the effects of nicotine on P20 amplitude in these two strains, with DBA/2 mice demonstrating greater P20 increases in response to acute nicotine administration (Metzger et al., 2006). The greater P20 effects in the DBA/2 mice may have further masked any N40 differences in the P20/N40 waveform. The opposing effects of nicotine on the amplitudes of the P20 and N40 components observed in our study and other reports in the literature suggest that these components may be affected differentially by certain pharmacological manipulations. These findings highlight the importance of examining these ERP components separately.
Acute nicotine administration increased evoked gamma oscillations in the present study. Although there are no studies in the literature on the effects of nicotine on evoked gamma, Crawford and colleagues reported that chronic smokers displayed significantly greater evoked gamma oscillations than never smokers in response to an auditory stimulus (Crawford et al., 2002). The finding that nicotine increases evoked gamma in our mouse study suggests that the increased gamma in chronic smokers is potentially due to smokers’ sustained use of nicotine and may represent long-term changes in the nicotinic acetylcholinergic receptor (nAChR) system. This is further supported by the finding that mecamylamine, a non-specific nicotinic antagonist, blocked nicotine-induced increases in evoked gamma. It has been suggested that gamma oscillations and synchronization play a role in sensory perception (Engel and Singer, 2001) and that smoking may facilitate this process by increasing gamma activity (Crawford et al., 2002). Additionally, Kahana and colleagues have reported that gamma activity increases with working memory load (Howard et al., 2003). Based on our finding that nicotine increases the gamma band response to auditory stimuli, we postulate that the mechanisms by which nicotine enhances the ability of external stimuli to generate gamma oscillations may also contribute to its reported ability to improve working memory (Rezvani and Levin, 2001, Levin, 2002, Levin et al., 2006).
To address the speculation that the P50 component of the human ERP may be an element of the gamma band response (Clementz et al., 1997, Johannesen et al., 2005), we correlated P20 amplitudes and area under the curve for evoked gamma. P20 amplitudes were significantly positively correlated with evoked gamma across all conditions, supporting a potential relationship between the generators of the two measures. Additionally, N40 amplitudes were positively correlated with evoked gamma across all conditions. This latter finding suggests that gamma activity may also contribute to human N100, which is similar in a variety of psychometric and pharmacological response properties with the mouse N40 (Connolly et al., 2003, Siegel et al., 2003, Connolly et al., 2004, Maxwell et al., 2004a, Maxwell et al., 2004b, Umbricht et al., 2004). However, the observation that nicotine reduced the N40 while increasing gamma oscillations suggests that the effects of nicotine on other frequency domain contributions to the cortically generated N40 component overshadow those of increased gamma.
Animal models of auditory ERPs have been used to better understand the mechanisms of multiple disorders (Light and Braff, 1999) and also have served as screening devices for novel treatments for these disorders (Simosky et al., 2001, O’Neill et al., 2003, Maxwell et al., 2004a, Maxwell et al., 2004b) The findings from the current study suggest that animal models of ERPs, as well as models of evoked gamma, may be useful as tools in the study of the mechanisms of reinforcement and motivation for smoking in both disordered and non-disordered populations. These models also may serve as a screening device to identify novel compounds that would aid in smoking cessation. As we have reported, nicotine increases the amplitude of the P20 component and evoked gamma while decreasing the N40 amplitude. Animal models could be used to identify drugs that block nicotine’s effects on the ERP and gamma as a means to reduce the reinforcing effects of nicotine. Alternatively, ERP models could serve to identify drugs that replicate nicotine’s cognitive-enhancing effects without its addictive liability or the damaging aspects of smoking.
The non-specific nicotinic-acetylcholinergic receptor antagonist mecamylamine blocked nicotine-induced changes in the ERP and evoked gamma, supporting a role for the nAChR system in nicotine’s effects. One limitation of the study is mecamylamine’s non-specific profile. Although we can conclude from the findings that the nAChR system is involved, we cannot identify activation of which specific nicotinic acetylcholinergic receptor subtype, or subtypes, are contributing to nicotine’s effects on ERPs and evoked gamma. Multiple studies have supported involvement of the low affinity alpha-7 nicotinic acetylcholine receptor in gating deficits in schizophrenia (Leonard et al., 1996, Freedman et al., 1997, Adler et al., 1999) while a recent study in mice suggests a partial role for alpha4beta2 subtype receptors in nicotine’s effects on ERPs (Radek et al., 2006). Future studies should address questions remaining about the role of the nAChR subtypes in nicotine’s effects on ERP components as well as evoked gamma. Identifying the specific nAChR systems involved would allow for development of treatments or medications targeted to block or mimic specific effects of nicotine by interacting with only the receptor subtypes responsible for a specific response (eg increase P20 amplitude without decreasing N40 amplitude).
One limitation of this study was the within-subject design which required each animal to be tested multiple times and receive all of the drug treatments. The half-life of mecamylamine in rats is 1.2 hours (Debruyne et al., 2003) while the half –life of nicotine in mice is 6–7 minutes (Petersen et al., 1984). We used 24 hours washout periods to allow for complete clearance of the drugs between testing. In addition, to address the potential effects of time and repeated drug exposure, we performed a repeated measures ANOVA on the baseline, pre-treatment data for each testing day for each of the dependent variables. As reported, there were no significant effects of repeated testing for any of the measured variables, suggesting that neither time nor exposure to multiple drugs affected baseline responses in this study.
In summary, the present study demonstrates that acute administration of nicotine increases P20 amplitude and evoked gamma oscillations while decreasing N40 amplitude. These data further support previous reports that the P20 and N40 are independent components with unique psychometric and pharmacologic response properties. Changes in P20 amplitudes were positively correlated with increases in evoked gamma, consistent with the proposal that P20 and gamma are related. Furthermore, nicotine-induced changes in P20 and gamma oscillations were blocked by pretreatment with mecamylamine implicating the nAChR system in nicotine’s effects on these components. Determination of specific nAChR receptor subtype involvement will require further study. Based on these findings, we propose that acute nicotine or nicotine agonists would increase P50 amplitude and gamma oscillations in conditions, such as schizophrenia, where these electrophysiological measures are reduced.
ABBREVIATIONS
- EEG
Electroencephalogram
- ERP
Event related potential
- S1
First auditory stimulus
- S2
Second auditory stimulus
- FFT
Fast Fourier Transform
- AUC
Area under the curve
- ANOVA
Analysis of variance
- nAChR
Nicotinic acetylcholinergic receptor
Footnotes
This work was supported by the National Cancer Institute/National Institute on Drug Abuse Transdisciplinary Tobacco Use Research Center Grant P5084718 and P50 MH064045-065134.
Section Editor: Dr. David A. Lewis, Department of Psychiatry, University of Pittsburgh, W1652 BST, 3811 O Hara Street, Pittsburgh, PA 15213-2593
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler LE, Freedman R, Ross RG, Olincy A, Waldo MC. Elementary phenotypes in the neurobiological and genetic study of schizophrenia. Biol Psychiatry. 1999;46:8–18. doi: 10.1016/s0006-3223(99)00085-2. [DOI] [PubMed] [Google Scholar]
- Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry. 1982;17:639–654. [PubMed] [Google Scholar]
- Basar E, Basar-Eroglu C, Karakas S, Schurmann M. Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol. 2001;39:241–248. doi: 10.1016/s0167-8760(00)00145-8. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E. Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int J Psychophysiol. 1996;24:101–112. doi: 10.1016/s0167-8760(96)00051-7. [DOI] [PubMed] [Google Scholar]
- Bertrand O, Tallon-Baudry C. Oscillatory gamma activity in humans: a possible role for object representation. Int J Psychophysiol. 2000;38:211–223. doi: 10.1016/s0167-8760(00)00166-5. [DOI] [PubMed] [Google Scholar]
- Boutros N, Zouridakis G, Rustin T, Peabody C, Warner D. The P50 component of the auditory evoked potential and subtypes of schizophrenia. Psychiatry Res. 1993;47:243–254. doi: 10.1016/0165-1781(93)90082-r. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Zouridakis G, Overall J. Replication and extension of P50 findings in schizophrenia. Clin Electroencephalogr. 1991;22:40–45. doi: 10.1177/155005949102200109. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD. Multichannel electroencephalographic assessment of auditory evoked response suppression in schizophrenia. Exp Brain Res. 2001;139:377–390. doi: 10.1007/s002210100744. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Blumenfeld LD, Cobb S. The gamma band response may account for poor P50 suppression in schizophrenia. Neuroreport. 1997;8:3889–3893. doi: 10.1097/00001756-199712220-00010. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell C, Liang Y, Kahn JB, Kanes SJ, Abel T, Gur RE, Turetsky BI, Siegel SJ. The effects of ketamine vary among inbred mouse strains and mimic schizophrenia for the P80, but not P20 or N40 auditory ERP components. Neurochem Res. 2004;29:1179–1188. doi: 10.1023/b:nere.0000023605.68408.fb. [DOI] [PubMed] [Google Scholar]
- Connolly PM, Maxwell CR, Kanes SJ, Abel T, Liang Y, Tokarczyk J, Bilker WB, Turetsky BI, Gur RE, Siegel SJ. Inhibition of auditory evoked potentials and prepulse inhibition of startle in DBA/2J and DBA/2Hsd inbred mouse substrains. Brain Res. 2003;992:85–95. doi: 10.1016/j.brainres.2003.08.035. [DOI] [PubMed] [Google Scholar]
- Crawford HJ, McClain-Furmanski D, Castagnoli N, Jr, Castagnoli K. Enhancement of auditory sensory gating and stimulus-bound gamma band (40 Hz) oscillations in heavy tobacco smokers. Neurosci Lett. 2002;317:151–155. doi: 10.1016/s0304-3940(01)02454-5. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Kao W, Martin BR. Characterization of spontaneous and precipitated nicotine withdrawal in the mouse. J Pharmacol Exp Ther. 2003;307:526–534. doi: 10.1124/jpet.103.054908. [DOI] [PubMed] [Google Scholar]
- Debruyne D, Sobrio F, Hinschberger A, Camsonne R, Coquerel A, Barre L. Short-term pharmacokinetics and brain distribution of mecamylamine as a preliminary to carbon-11 labeling for nicotinic receptor investigation. J Pharm Sci. 2003;92:1051–1057. doi: 10.1002/jps.10302. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Basar-Eroglu C, Basar E. Distributed gamma band responses in the brain studied in cortex, reticular formation, hippocampus and cerebellum. Int J Neurosci. 1996;84:1–13. doi: 10.3109/00207459608987246. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Herrmann CS, Erdal ME, Ergenoglu T, Keskin YH, Ergen M, Beydagi H. DRD4 and DAT1 Polymorphisms Modulate Human Gamma Band Responses. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl011. [DOI] [PubMed] [Google Scholar]
- Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW. Differential prevalence of cigarette smoking in patients with schizophrenic vs mood disorders. Schizophr Res. 1998;33:113–118. doi: 10.1016/s0920-9964(98)00045-0. [DOI] [PubMed] [Google Scholar]
- Engel AK, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Bickford P, Byerley W, Coon H, Cullum CM, Griffith JM, Harris JG, Leonard S, Miller C, et al. Schizophrenia and nicotinic receptors. Harv Rev Psychiatry. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- Freedman R, Coon H, Myles-Worsley M, Orr-Urtreger A, Olincy A, Davis A, Polymeropoulos M, Holik J, Hopkins J, Hoff M, Rosenthal J, Waldo MC, Reimherr F, Wender P, Yaw J, Young DA, Breese CR, Adams C, Patterson D, Adler LE, Kruglyak L, Leonard S, Byerley W. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Natl Acad Sci U S A. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Gould TJ, Stephen Higgins J. Nicotine enhances contextual fear conditioning in C57BL/6J mice at 1 and 7 days post-training. Neurobiol Learn Mem. 2003;80:147–157. doi: 10.1016/s1074-7427(03)00057-1. [DOI] [PubMed] [Google Scholar]
- Griffith JM, O’Neill JE, Petty F, Garver D, Young D, Freedman R. Nicotinic receptor desensitization and sensory gating deficits in schizophrenia. Biol Psychiatry. 1998;44:98–106. doi: 10.1016/s0006-3223(97)00362-4. [DOI] [PubMed] [Google Scholar]
- Haig AR, Gordon E, De Pascalis V, Meares RA, Bahramali H, Harris A. Gamma activity in schizophrenia: evidence of impaired network binding? Clin Neurophysiol. 2000;111:1461–1468. doi: 10.1016/s1388-2457(00)00347-3. [DOI] [PubMed] [Google Scholar]
- Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schulze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK, Mitchell JE, Dahlgren LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG. P50 changes with visual interference in normal subjects: a sensory distraction model for schizophrenia. Clin Electroencephalogr. 1996;27:151–154. doi: 10.1177/155005949602700308. [DOI] [PubMed] [Google Scholar]
- Jin Y, Potkin SG, Patterson JV, Sandman CA, Hetrick WP, Bunney WE., Jr Effects of P50 temporal variability on sensory gating in schizophrenia. Psychiatry Res. 1997;70:71–81. doi: 10.1016/s0165-1781(97)03091-6. [DOI] [PubMed] [Google Scholar]
- Johannesen JK, Kieffaber PD, O’Donnell BF, Shekhar A, Evans JD, Hetrick WP. Contributions of subtype and spectral frequency analyses to the study of P50 ERP amplitude and suppression in schizophrenia. Schizophr Res. 2005;78:269–284. doi: 10.1016/j.schres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neurosci Biobehav Rev. 2005;29:1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lee KH, Williams LM, Haig A, Gordon E. “Gamma (40 Hz) phase synchronicity” and symptom dimensions in schizophrenia. Cognit Neuropsychiatry. 2003;8:57–71. doi: 10.1080/713752240. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adams C, Breese CR, Adler LE, Bickford P, Byerley W, Coon H, Griffith JM, Miller C, Myles-Worsley M, Nagamoto HT, Rollins Y, Stevens KE, Waldo M, Freedman R. Nicotinic receptor function in schizophrenia. Schizophr Bull. 1996;22:431–445. doi: 10.1093/schbul/22.3.431. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Development of nicotinic drug therapy for cognitive disorders. Eur J Pharmacol. 2000;393:141–146. doi: 10.1016/s0014-2999(99)00885-7. [DOI] [PubMed] [Google Scholar]
- Light GA, Braff DL. Human and animal studies of schizophrenia-related gating deficits. Curr Psychiatry Rep. 1999;1:31–40. doi: 10.1007/s11920-999-0008-y. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Kanes SJ, Abel T, Siegel SJ. Phosphodiesterase inhibitors: a novel mechanism for receptor-independent antipsychotic medications. Neuroscience. 2004a;129:101–107. doi: 10.1016/j.neuroscience.2004.07.038. [DOI] [PubMed] [Google Scholar]
- Maxwell CR, Liang Y, Weightman BD, Kanes SJ, Abel T, Gur RE, Turetsky BI, Bilker WB, Lenox RH, Siegel SJ. Effects of chronic olanzapine and haloperidol differ on the mouse N1 auditory evoked potential. Neuropsychopharmacology. 2004b;29:739–746. doi: 10.1038/sj.npp.1300376. [DOI] [PubMed] [Google Scholar]
- Metzger KL, Maxwell CR, Liang Y, Siegel SJ. Effects of Nicotine Vary Across Two Auditory Evoked Potentials in the Mouse. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Moxon KA, Gerhardt GA, Adler LE. Dopaminergic modulation of the P50 auditory-evoked potential in a computer model of the CA3 region of the hippocampus: its relationship to sensory gating in schizophrenia. Biol Cybern. 2003;88:265–275. doi: 10.1007/s00422-002-0372-8. [DOI] [PubMed] [Google Scholar]
- O’Neill HC, Rieger K, Kem WR, Stevens KE. DMXB, an alpha7 nicotinic agonist, normalizes auditory gating in isolation-reared rats. Psychopharmacology (Berl) 2003;169:332–339. doi: 10.1007/s00213-003-1482-2. [DOI] [PubMed] [Google Scholar]
- Pantev C, Makeig S, Hoke M, Galambos R, Hampson S, Gallen C. Human auditory evoked gamma-band magnetic fields. Proc Natl Acad Sci U S A. 1991;88:8996–9000. doi: 10.1073/pnas.88.20.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen DR, Norris KJ, Thompson JA. A comparative study of the disposition of nicotine and its metabolites in three inbred strains of mice. Drug Metab Dispos. 1984;12:725–731. [PubMed] [Google Scholar]
- Radek RJ, Miner HM, Bratcher NA, Decker MW, Gopalakrishnan M, Bitner RS. alpha(4)beta (2) Nicotinic receptor stimulation contributes to the effects of nicotine in the DBA/2 mouse model of sensory gating. Psychopharmacology (Berl) 2006;187:47–55. doi: 10.1007/s00213-006-0394-3. [DOI] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biol Psychiatry. 2001;49:258–267. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, Lenox RH, Gur RE, Bilker WB, Kanes SJ, Turetsky BI. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology. 2003;28:675–682. doi: 10.1038/sj.npp.1300087. [DOI] [PubMed] [Google Scholar]
- Siegel SJ, Maxwell CR, Majumdar S, Trief DF, Lerman C, Gur RE, Kanes SJ, Liang Y. Monoamine reuptake inhibition and nicotine receptor antagonism reduce amplitude and gating of auditory evoked potentials. Neuroscience. 2005;133:729–738. doi: 10.1016/j.neuroscience.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Adler LE, Freedman R. Clozapine improves deficient inhibitory auditory processing in DBA/2 mice, via a nicotinic cholinergic mechanism. Psychopharmacology (Berl) 2003;165:386–396. doi: 10.1007/s00213-002-1285-x. [DOI] [PubMed] [Google Scholar]
- Simosky JK, Stevens KE, Kem WR, Freedman R. Intragastric DMXB-A, an alpha7 nicotinic agonist, improves deficient sensory inhibition in DBA/2 mice. Biol Psychiatry. 2001;50:493–500. doi: 10.1016/s0006-3223(01)01093-9. [DOI] [PubMed] [Google Scholar]
- Song C, Murray TA, Kimura R, Wakui M, Ellsworth K, Javedan SP, Marxer-Miller S, Lukas RJ, Wu J. Role of alpha7-nicotinic acetylcholine receptors in tetanic stimulation-induced gamma oscillations in rat hippocampal slices. Neuropharmacology. 2005;48:869–880. doi: 10.1016/j.neuropharm.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens KE, Kem WR, Mahnir VM, Freedman R. Selective alpha7-nicotinic agonists normalize inhibition of auditory response in DBA mice. Psychopharmacology (Berl) 1998;136:320–327. doi: 10.1007/s002130050573. [DOI] [PubMed] [Google Scholar]
- Stevens KE, Wear KD. Normalizing effects of nicotine and a novel nicotinic agonist on hippocampal auditory gating in two animal models. Pharmacol Biochem Behav. 1997;57:869–874. doi: 10.1016/s0091-3057(96)00466-2. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Vyssotky D, Latanov A, Nitsch R, Brambilla R, D’Adamo P, Lipp HP. Midlatency auditory event-related potentials in mice: comparison to midlatency auditory ERPs in humans. Brain Res. 2004;1019:189–200. doi: 10.1016/j.brainres.2004.05.097. [DOI] [PubMed] [Google Scholar]
- Ziedonis DM, Kosten TR, Glazer WM, Frances RJ. Nicotine dependence and schizophrenia. Hosp Community Psychiatry. 1994;45:204–206. doi: 10.1176/ps.45.3.204. [DOI] [PubMed] [Google Scholar]


