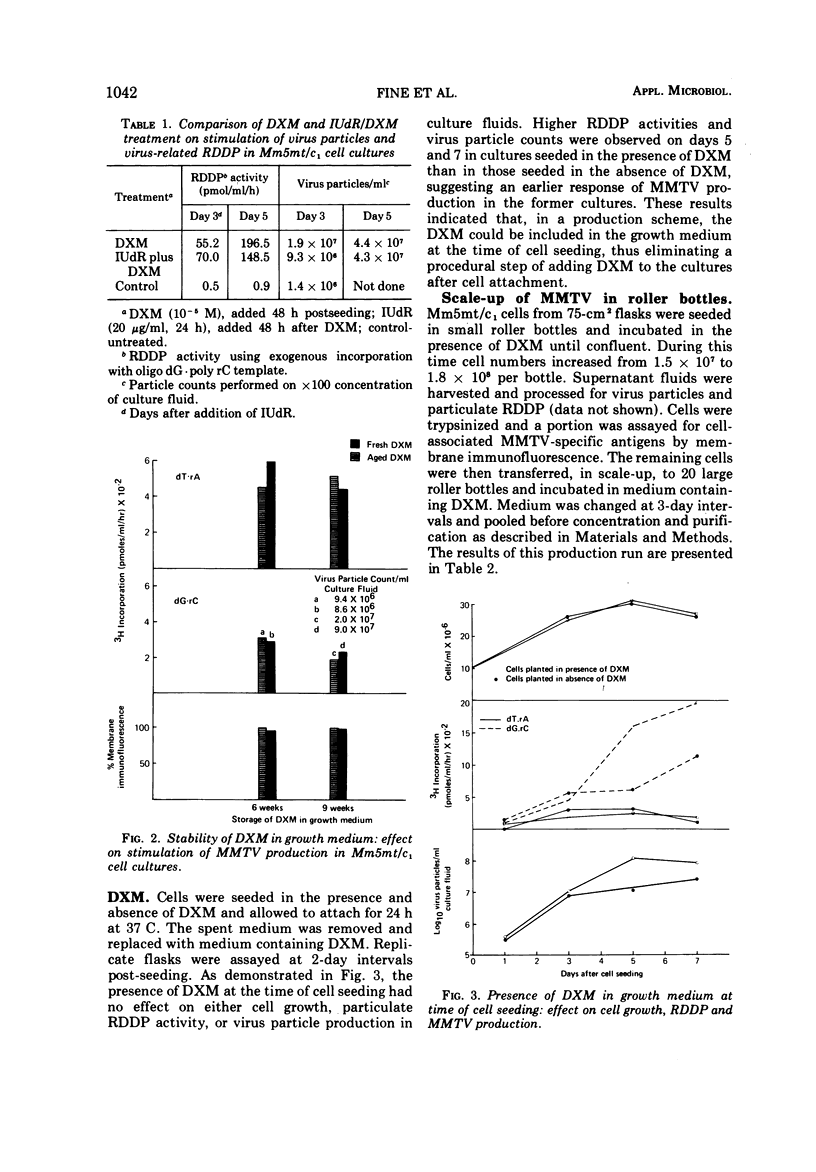
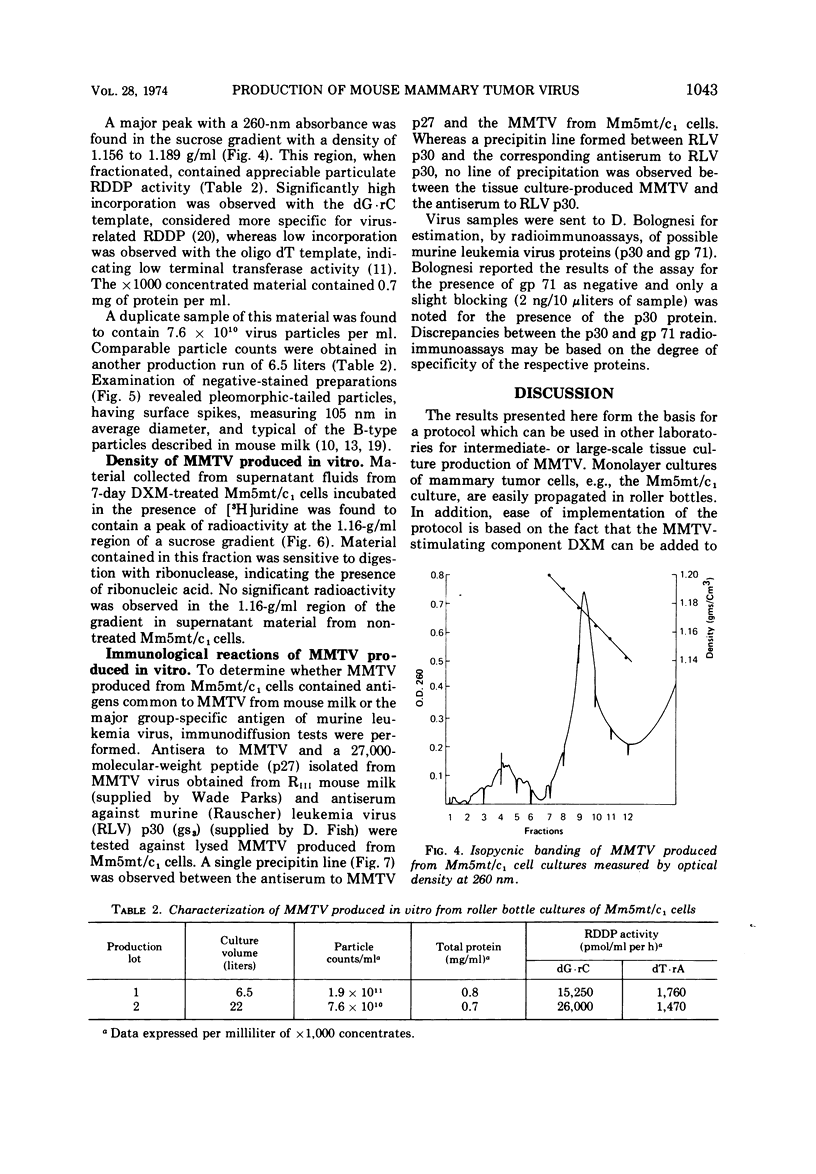
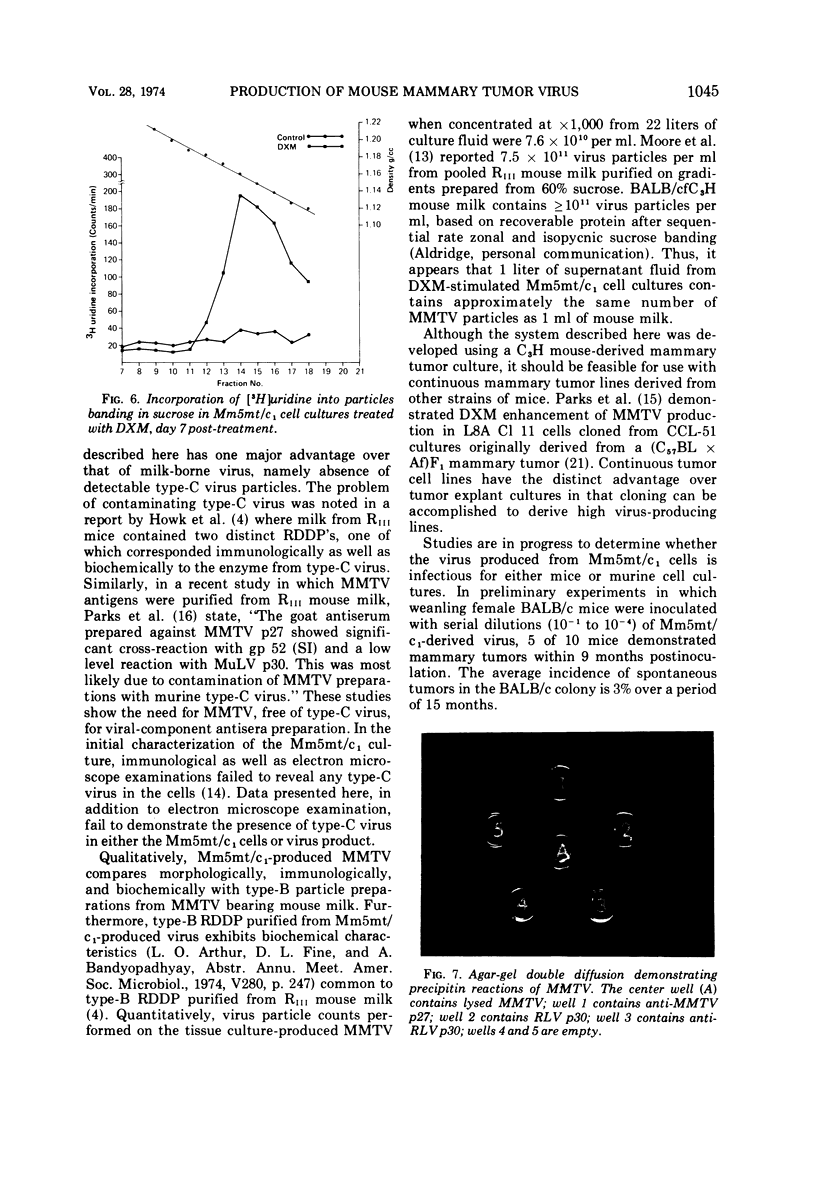
Abstract
An in vitro system for production, purification, and concentration of mouse mammary tumor virus is described. Monolayer cultures of C3H mouse mammary tumor cells propagated at 34 C in roller bottles in the presence of dexamethasone, a glucocorticoid hormone, release B-type particles which possess ribonucleic acid and a ribonucleic acid-dependent deoxyribonucleic acid polymerase. One thousandfold concentration by ultracentrifugation with subsequent gradient fractionation yielded > 7 × 1010 particles per ml in the 1.16- to 1.18-g/ml region. Mouse mammary tumor virus produced in this system was free of detectable C-type virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentvelzen P. The biology of the mouse mammary tumor virus. Int Rev Exp Pathol. 1972;11:259–297. [PubMed] [Google Scholar]
- Cardiff R. D., Blair P. R., DeOme K. B. In vitro cultivation of the mouse mammary tumor virus: replication of MTV in tissue culture. Virology. 1968 Oct;36(2):313–317. doi: 10.1016/0042-6822(68)90152-9. [DOI] [PubMed] [Google Scholar]
- Fine D. L., Plowman J. K., Kelley S. P., Arthur L. O., Hillman E. A. Enhanced production of mouse mammary tumor virus in dexamethasone-treated, 5-iododeoxyuridine-stimulated mammary tumor cell cultures. J Natl Cancer Inst. 1974 Jun;52(6):1881–1886. doi: 10.1093/jnci/52.6.1881. [DOI] [PubMed] [Google Scholar]
- Howk R. S., Rye L. A., Killeen L. A., Scolnick E. M., Parks W. P. Characterization and separation of viral DNA polymerase in mouse milk. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2117–2121. doi: 10.1073/pnas.70.7.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keydar J., Gilead Z., Hartman J. R., Ben-Shaul Y. In vitro production of mouse mammary tumor virus in a mouse mammary tumor ascites line. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2983–2987. doi: 10.1073/pnas.70.10.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASFARGUES E. Y., MOORE D. H., MURRAY M. R., HAAGENSEN C. D., POLLARD E. C. Production of the milk agent in cultures of mouse mammary carcinoma. J Biophys Biochem Cytol. 1959 Jan 25;5(1):93–96. doi: 10.1083/jcb.5.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lasfargues E. Y., Kramarsky B., Sarkar N. H., Lasfargues J. C., Moore D. H. An established R3 mouse mammary tumor cell line; kinetics of mammary tumor virus (MTV) production. Proc Soc Exp Biol Med. 1972 Jan;139(1):242–247. doi: 10.3181/00379727-139-36119. [DOI] [PubMed] [Google Scholar]
- Lasfargues E. Y., Kramarsky B., Sarkar N. H., Lasfargues J. C., Pillsbury N., Moore D. H. Stimulation of mammary tumor virus production in a mouse mammary tumor cell line. Cancer Res. 1970 Apr;30(4):1109–1117. [PubMed] [Google Scholar]
- Manning J. S., Hackett A. J., Cardiff R. D., Mel H. C., Blair P. B. Isopycnic zonal centrifugation and characterization of the mouse mammary tumor virus (MTV) in different gradient solutions. Virology. 1970 Apr;40(4):912–919. doi: 10.1016/0042-6822(70)90137-6. [DOI] [PubMed] [Google Scholar]
- McCaffrey R., Smoler D. F., Baltimore D. Terminal deoxynucleotidyl transferase in a case of childhood acute lymphoblastic leukemia. Proc Natl Acad Sci U S A. 1973 Feb;70(2):521–525. doi: 10.1073/pnas.70.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe J. H., Brandt P. M. Rapid semiquantitative method for screening large numbers of virus samples by negative staining electron microscopy. Appl Microbiol. 1970 Aug;20(2):259–262. doi: 10.1128/am.20.2.259-262.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Sarkar N. H., Charney J., Kramarsky B. Some physical and biological characteristics of the mouse mammary tumor virus. Cancer Res. 1973 Jan;33(1):5–10. [PubMed] [Google Scholar]
- Owens R. B., Hackett A. J. Tissue culture studies of mouse mammary tumor cells and associated viruses. J Natl Cancer Inst. 1972 Nov;49(5):1321–1332. [PubMed] [Google Scholar]
- PIKOVSKI M. A. The survival of the mammary tumor milk agent in cultures of heterologous cells. J Natl Cancer Inst. 1953 Apr;13(5):1275–1282. [PubMed] [Google Scholar]
- Parks W. P., Howk R. S., Scolnick E. M., Oroszlan S., Gilden R. V. Immunochemical characterization of two major polypeptides from murine mammary tumor virus. J Virol. 1974 Jun;13(6):1200–1210. doi: 10.1128/jvi.13.6.1200-1210.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks W. P., Scolnick E. M., Kozikowski E. H. Dexamethasone stimulation of murine mammary tumor virus expression: a tissue culture source of virus. Science. 1974 Apr 12;184(4133):158–160. doi: 10.1126/science.184.4133.158. [DOI] [PubMed] [Google Scholar]
- SANFORD K. K., ADERVONT H. B., HOBBS G. L., EARLE W. R. Maintenance of the mammary-tumor agent in long-term cultures of mouse mammary carcinoma. J Natl Cancer Inst. 1961 May;26:1185–1191. [PubMed] [Google Scholar]
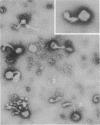
- Sarkar N. H., Moore D. H. Electron microscopy in mammary cancer research. J Natl Cancer Inst. 1972 Apr;48(4):1051–1058. [PubMed] [Google Scholar]
- Scolnick E. M., Parks W. P., Todaro G. J., Aaronson S. A. Immunological characterization of primate C-type virus reverse transcriptases. Nat New Biol. 1972 Jan 12;235(54):35–40. doi: 10.1038/newbio235035a0. [DOI] [PubMed] [Google Scholar]
- Sykes J. A., Whitescarver J., Briggs L. Observations on a cell line producing mammary tumor virus. J Natl Cancer Inst. 1968 Dec;41(6):1315–1327. [PubMed] [Google Scholar]
- Todaro G. J., Aaronson S. A., Rands E. Rapid detection of mycoplasma-infected cell cultures. Exp Cell Res. 1971 Mar;65(1):256–257. doi: 10.1016/s0014-4827(71)80077-0. [DOI] [PubMed] [Google Scholar]