Short abstract
Microarray analysis reveals hundreds of hitherto unsuspected Hox gene targets.
Abstract
Genetic studies of the targets of the Hox genes have revealed only the tip of the iceberg. Recent microarray studies that have identified hundreds more transcriptional responses to Hox genes in Drosophila will help elucidate the role of Hox genes in development and evolution.
Hox genes are well known for their role in specifying segmental identities [1], a role highlighted by homeotic mutant flies with a leg in place of an antenna or four wings instead of two. Present in all bilaterian animals, Hox genes encode homeodomain transcription factors that operate in many different tissues and cell types, and modulate a wide range of cell responses by controlling the expression of subordinate target genes [2]. The complexity of the regulatory networks controlled by Hox genes, together with the short and degenerate DNA sites at which Hox proteins bind, have hampered the identification of their target genes [3]. Nevertheless, the identification of Hox-regulated gene networks is fundamental if we are to understand the developmental processes of morphogenesis and cell differentiation in animals, and in particular the evolution and functional diversification of serially homologous structures.
Many groups have started to use microarray profiling to systematically detect genes differentially expressed as the result of the activity of Hox genes. The sensitivity of this technique for identifying biologically relevant targets of Hox genes has been questioned, however [4], as the effects of Hox gene function can be elicited locally, affecting only a small subset of the Hox-expressing cells at a given time [5]. Such responses might be undetectable because of their small contribution to the total transcript population. Furthermore, the interpretation of any experimental set-up involving misexpression of Hox genes is complicated by two factors: their extensive cross-regulation [6] and their concentration-dependent activity [7].
Two recent papers by Hueber et al. [8] and Hersh et al. [9] exemplify this whole-genome quest for downstream targets of Hox gene function in Drosophila (Figure 1). The first group searched for Hox-regulated genes in the embryo by ubiquitously overexpressing each one of the Hox genes Deformed (Dfd), Sex combs reduced (Scr), Antennapedia (Antp), Ultrabithorax (Ubx), abdominal A (abd-A) or Abdominal B (Abd-B), and comparing the transcriptomes in these embryos with those of control embryos overexpressing a lacZ reporter construct. The second group focused on the transcriptional targets of Ubx in developing wing and haltere imaginal discs. These two serially homologous appendages develop from initially equivalent fields of cells; Ubx is the primary genetic switch that controls the unique characteristics of the halteres (hindwings), which develop a dramatically different morphology from that of the (fore)wings [10].
Figure 1.
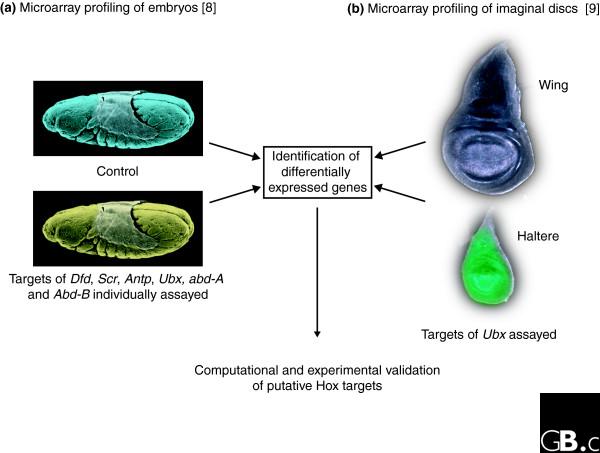
Microarray expression profiling for identification of Hox downstream targets. (a) Hueber et al. [8] compared Drosophila embryos overexpressing a control lacZ gene (blue) with embryos individually overexpressing various Hox genes (yellow). (b) Hersh et al. [9] searched for targets of Ultrabithorax (green) in haltere imaginal discs by comparing their transcriptome with that of wing imaginal discs (gray).
Studying completely different developmental stages, both groups reach the same key conclusion: each Hox gene regulates hundreds of downstream genes, and these genes belong to many different functional classes, ranging from other regulatory genes like transcription factors and signaling components to terminal differentiation genes (realizators) that execute a mixed repertoire of cell behaviors and enzymatic reactions. This finding is a firm demonstration by genomic means of a view previously established by conventional genetics - homeotic proteins are versatile transcription factors that interact with developmental regulatory networks at multiple levels and many developmental stages, modulating the transcription of numerous target genes [10-12].
For a sample of the putative targets, the accuracy of these genomic approaches has been tested by in situ hybridization and genetic manipulation. These tests show a low false-positive rate [8], providing some reassurance as to the accuracy of the genomic approaches. The sensitivity of the microarray method is evident from the fact that among the targets there are genes that, in normal development, show localized responses to Hox expression in cells that make only a minor contribution to the overall RNA pool, especially in the heterogeneous embryonic tissue [8]. Ubiquitous over-expression of the Hox genes in many segments amplifies the response of these targets, allowing their identification.
Previous genetic studies have preferentially identified genes encoding transcription factors and signaling proteins among candidate Hox direct targets [3], but this bias is not evident in the whole-genome studies. Indeed, many housekeeping genes are identified among the downstream targets [8]. It seems plausible that the complexity of morphogenetic processes requires the coordinated control of housekeeping genes in a subtle fashion in many cells, rather than the abrupt on/off regulation of a limited set of targets. The observation that many of the realizator genes have general, and often partially redundant, roles is likely to have hindered their discovery by classic genetic approaches. It emphasizes the value of microarray expression profiling in tackling this largely unexplored aspect of Hox gene function.
There has been some discussion as to just how many targets there may be for a given Hox gene. These two studies provide no definitive answer. With microarray methods, the number of target genes revealed in a given tissue and developmental stage will depend heavily on the parameters set during statistical analysis of the expression data. Interestingly, a comparison of the two studies shows that rather similar numbers of targets for the Ubx Hox gene are reported in the heterogeneous tissue of whole embryos [8] and in the more homogeneous tissue of the developing wing and haltere discs [9]. This seems biologically implausible. We note also that the sets of target genes identified by Hueber et al. [8] at two consecutive embryonic stages are quite distinct, showing only 22% of common targets. Even combined, these sets are unlikely to represent a comprehensive listing of Hox targets.
In both studies, only a fraction of the genes identified as targets will be directly regulated by Hox proteins. Others will be responding indirectly as secondary effects of the direct targets. It is noteworthy that in the study by Hueber et al. [8], the older embryos, which have been exposed to ectopic Hox expression for longer, consistently show more Hox-responsive targets than the younger embryos, suggesting that the proportion of secondary targets may be greater in the older embryos. Similarly, it should be remembered that in the study of wing and haltere development, Hersh et al. [9] are studying the cumulative effects of Ubx throughout embryonic and larval development, and so will also see responses that lie a long way downstream from the direct actions of Ubx.
The safest way to identify direct targets is to characterize the cis-regulatory elements that mediate their Hox response. The availability of several sequenced Drosophila genomes allows the use of sequence conservation in non-coding sequences to spot candidate cis-regulatory blocks. These can then be scanned for motifs corresponding to putative binding sites for Hox proteins and other transcription factors. Using this approach, Hueber et al. [8] suggest that about 20-30% of the Dfd-regulated genes in the embryo are direct targets. Six of these putative direct target sequences were tested experimentally; all were shown to bind Deformed protein in vitro. The authors conclude, perhaps somewhat optimistically, that "the combination of microarray analysis with bioinformatics approaches will allow us in the future to not only identify direct Hox target genes, but also to construct complete Hox regulatory networks" (our italics). We suspect that the hard grind of experimental work will still be required to validate the microarray data. It will certainly be required to turn phenomenology into a detailed understanding of mechanism.
A key aspect of mechanism that is still not fully understood is how the different Hox proteins in a single species mediate such distinct biological activities, particularly in view of their similar DNA-binding specificities in vitro [13]. The authors of the comparative survey in Drosophila embryos conclude that Hox genes achieve their functional specificity by regulating largely unique sets of downstream genes [8], implying that in vivo they have distinct target selectivities. While their data clearly provide support for this idea, there is still substantial overlap in the sets of Hox targets. For those Hox genes that were studied under strictly comparable conditions, about half the targets were found to be regulated by two or more Hox genes, and the other half were uniquely regulated by a single gene. One gene, abd-A, does show an exceptional number of unique targets in this study [8], but this result runs counter to genetic observations that suggest that abd-A and Ubx share many biological functions [14]. This exceptional behavior may perhaps be attributed to the distinct experimental conditions under which the abd-A assay was carried out [8]. By contrast, we might expect that Abd-B would show more unique targets, given both the divergent sequence of the Abd-B homeodomain, and the highly modified morphology of the posterior segments that it controls [15,16]. There is some suggestion of this in the data [8].
In the same study, Hueber et al. [8] checked whether targets held in common by more than one Hox gene were regulated in a similar manner or not. They note that there is a trend for Hox genes functioning in the same body part (head, trunk, posterior end) to regulate common targets similarly [8]. It should be noted, however, that the disparity observed between Hox genes specifying different parts is not extensive and cannot alone account for the morphological diversification of body parts. It will be interesting to see how this functional convergence or divergence is mediated by the structure of the Hox proteins. We suspect that, more than 20 years after the discovery of the homeoproteins, there is still much to be learnt about the functional domains of Hox proteins.
To understand how Hox proteins achieve their biological activity, we shall probably need a detailed understanding of Hox-targeted enhancers. Several studies have shown that the activity of Hox genes is highly context dependent, in the sense that the landscape of transcription factors and signaling molecules in a given cell at a given time guides specific Hox effects [2]. The few exhaustively studied cases of embryonic enhancers channeling Hox inputs have confirmed that several transcriptional regulators collaborate to generate the appropriate output [17-20]. Similarly, Hersh et al. have used genetic tests, in vitro binding assays and in vivo activity assays with reporter constructs to show that one direct target of Ubx protein in the haltere is activated [9] by Ubx binding, whereas others are repressed [9,21,22]. Hox proteins confer the positional information along the anterior-posterior body axis, but other factors provide the cell/tissue-type information, and information about the precise position within a segment. The effect of Hox expression depends on all of these parameters. In this context, the remarkable aspects of Hox proteins as transcription factors are their versatility to act in so many distinct contexts, and the durability of their axially restricted expression domains, which are maintained by complex epigenetic mechanisms long after the information that specified these domains has decayed [23].
The nature of Hox-responsive enhancers, and the architecture of entire Hox-regulated networks, has important implications for the evolution of morphological traits. We are still some way from understanding the molecular changes that bring new batteries of genes under Hox regulation to generate novel morphologies. Sean Carroll's group has been using the Ubx-controlled haltere network as a paradigm to gain some insight into this question. Some of the cases they have studied point to the flexible "unsystematic, undesigned assembly of regulatory elements during evolution" [22], whereas others suggest the evolution of a "single [Ubx] core binding sequence within the context of previously existing cis-regulatory elements" [9]. General principles, apart from the fact that Ubx regulation in the haltere occurs through monomer binding sites, are not yet clear.
The studies reviewed here focus on what happens downstream of Hox gene expression. We should not forget though, that while the distinct sets of targets associated with each Hox gene in each organism are likely to have a major role in the diversification of segments, subtleties in the regulation of the Hox genes themselves have also been shown to play a part in the detailed patterning of individual segments [24], and changes in this regulation are important for the generation of diversity between different lineages of animals [25].
Delving into the molecular aspects of Hox gene function, there is also a danger that we will focus disproportionately on the role of this one gene family in developmental control and morphological evolution. It is perhaps worth stressing that the Hox genes do not provide the full instruction set to make a particular structure. The wing, for example, develops just fine without Hox gene input. By and large, and certainly for much of adult development, the Hox genes are modulating a generic set of instructions, which, in the absence of Hox gene expression, are still capable of patterning segments and making segment appendages.
The same applies to their role in evolution: Hox genes are not the be-all and end-all of morphological evolution that some textbook accounts would have us believe. Natural selection has long been viewed as a tinkerer, exploiting whatever comes to hand to generate novel structures or functions, so long as they are of adaptive value. Hox-mediated regionalization is only one of the levels at which this tinkering can act. It may be a particularly opportune level to drive the diversification of serial homologs, particularly in view of the large number and diverse set of targets that the Hox genes regulate, but we must expect selection to exploit many other aspects of the developmental process as well. The Hox genes are a good test case to study how gene networks change as animals evolve, but they are only one part of a story that will prove yet more complicated.
Acknowledgements
We are indebted to Pawel Herzyk for the analysis of microarray data. Our work on Drosophila Hox genes is supported by the Wellcome Trust, and by a Marie Curie Intra-European fellowship to AP.
References
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-N. [DOI] [PubMed] [Google Scholar]
- Hombria JC, Lovegrove B. Beyond homeosis - HOX function in morphogenesis and organogenesis. Differentiation. 2003;71:461–476. doi: 10.1046/j.1432-0436.2003.7108004.x. [DOI] [PubMed] [Google Scholar]
- Pearson JC, Lemons D, McGinnis W. Modulating Hox gene functions during animal body patterning. Nat Rev Genet. 2005;6:893–904. doi: 10.1038/nrg1689. [DOI] [PubMed] [Google Scholar]
- Lohmann I, McGinnis W. Hox genes: it's all a matter of context. Curr Biol. 2002;12:R514–R516. doi: 10.1016/S0960-9822(02)01025-4. [DOI] [PubMed] [Google Scholar]
- Rozowski M, Akam M. Hox gene control of segment-specific bristle patterns in Drosophila. Genes Dev. 2002;16:1150–1162. doi: 10.1101/gad.219302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DF, Rogers BT, Kalkbrenner A, Hamilton B, Holtzman SL, Kaufman T. Cross-regulation of Hox genes in the Drosophila melanogaster embryo. Mech Dev. 2001;102:3–16. doi: 10.1016/S0925-4773(01)00301-X. [DOI] [PubMed] [Google Scholar]
- Tour E, Hittinger CT, McGinnis W. Evolutionarily conserved domains required for activation and repression functions of the Drosophila Hox protein Ultrabithorax. Development. 2005;132:5271–5281. doi: 10.1242/dev.02138. [DOI] [PubMed] [Google Scholar]
- Hueber SD, Bezdan D, Henz SR, Blank M, Wu H, Lohmann I. Comparative analysis of Hox downstream genes in Drosophila. Development. 2007;134:381–392. doi: 10.1242/dev.02746. [DOI] [PubMed] [Google Scholar]
- Hersh BM, Nelson CE, Stoll SJ, Norton JE, Albert TJ, Carroll SB. The UBX-regulated network in the haltere imaginal disc of D. melanogaster. Dev Biol. 2007;302:717–727. doi: 10.1016/j.ydbio.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch F, Akam M. Ultrabithorax and the control of cell morphology in Drosophila halteres. Development. 2000;127:97–107. doi: 10.1242/dev.127.1.97. [DOI] [PubMed] [Google Scholar]
- Weatherbee SD, Halder G, Kim J, Hudson A, Carroll S. Ultrabithorax regulates genes at several levels of the wing-patterning hierarchy to shape the development of the Drosophila haltere. Genes Dev. 1998;12:1474–1482. doi: 10.1101/gad.12.10.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akam M. Hox genes: from master genes to micromanagers. Curr Biol. 1998;8:R676–R678. doi: 10.1016/S0960-9822(98)70433-6. [DOI] [PubMed] [Google Scholar]
- Ekker SC, Jackson DG, von Kessler DP, Sun BI, Young KE, Beachy PA. The degree of variation in DNA sequence recognition among four Drosophila homeotic proteins. EMBO J. 1994;13:3551–3560. doi: 10.1002/j.1460-2075.1994.tb06662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli-Gair J, Greig S, Micklem G, Akam M. Dissecting the temporal requirements for homeotic gene function. Development. 1994;120:1983–1995. doi: 10.1242/dev.120.7.1983. [DOI] [PubMed] [Google Scholar]
- Lovegrove B, Simoes S, Rivas ML, Sotillos S, Johnson K, Knust E, Jacinto A, Hombria JC. Coordinated control of cell adhesion, polarity, and cytoskeleton underlies Hox-induced organogenesis in Drosophila. Curr Biol. 2006;16:2206–2216. doi: 10.1016/j.cub.2006.09.029. [DOI] [PubMed] [Google Scholar]
- Foronda D, Estrada B, de Navas L, Sanchez-Herrero E. Requirement of Abdominal-A and Abdominal-B in the developing genitalia of Drosophila breaks the posterior downregulation rule. Development. 2006;133:117–127. doi: 10.1242/dev.02173. [DOI] [PubMed] [Google Scholar]
- Capovilla M, Brandt M, Botas J. Direct regulation of decapentaplegic by Ultrabithorax and its role in Drosophila midgut morphogenesis. Cell. 1994;76:461–475. doi: 10.1016/0092-8674(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Zhou B, Bagri A, Beckendorf SK. Salivary gland determination in Drosophila: a salivary-specific, fork head enhancer integrates spatial pattern and allows fork head autoregulation. Dev Biol. 2001;237:54–67. doi: 10.1006/dbio.2001.0367. [DOI] [PubMed] [Google Scholar]
- Grienenberger A, Merabet S, Manak J, Iltis I, Fabre A, Berenger H, Scott MP, Pradel J, Graba Y. Tgfbeta signaling acts on a Hox response element to confer specificity and diversity to Hox protein function. Development. 2003;130:5445–5455. doi: 10.1242/dev.00760. [DOI] [PubMed] [Google Scholar]
- Gebelein B, McKay DJ, Mann RS. Direct integration of Hox and segmentation gene inputs during Drosophila development. Nature. 2004;431:653–659. doi: 10.1038/nature02946. [DOI] [PubMed] [Google Scholar]
- Galant R, Walsh CM, Carroll SB. Hox repression of a target gene: extradenticle-independent, additive action through multiple monomer binding sites. Development. 2002;129:3115–3126. doi: 10.1242/dev.129.13.3115. [DOI] [PubMed] [Google Scholar]
- Hersh BM, Carroll SB. Direct regulation of knot gene expression by Ultrabithorax and the evolution of cis-regulatory elements in Drosophila. Development. 2005;132:1567–1577. doi: 10.1242/dev.01737. [DOI] [PubMed] [Google Scholar]
- Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- Stern DL. A role of Ultrabithorax in morphological differences between Drosophila species. Nature. 1998;396:463–466. doi: 10.1038/24863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averof M, Patel NH. Crustacean appendage evolution associated with changes in Hox gene expression. Nature. 1997;388:682–686. doi: 10.1038/41786. [DOI] [PubMed] [Google Scholar]


