Abstract
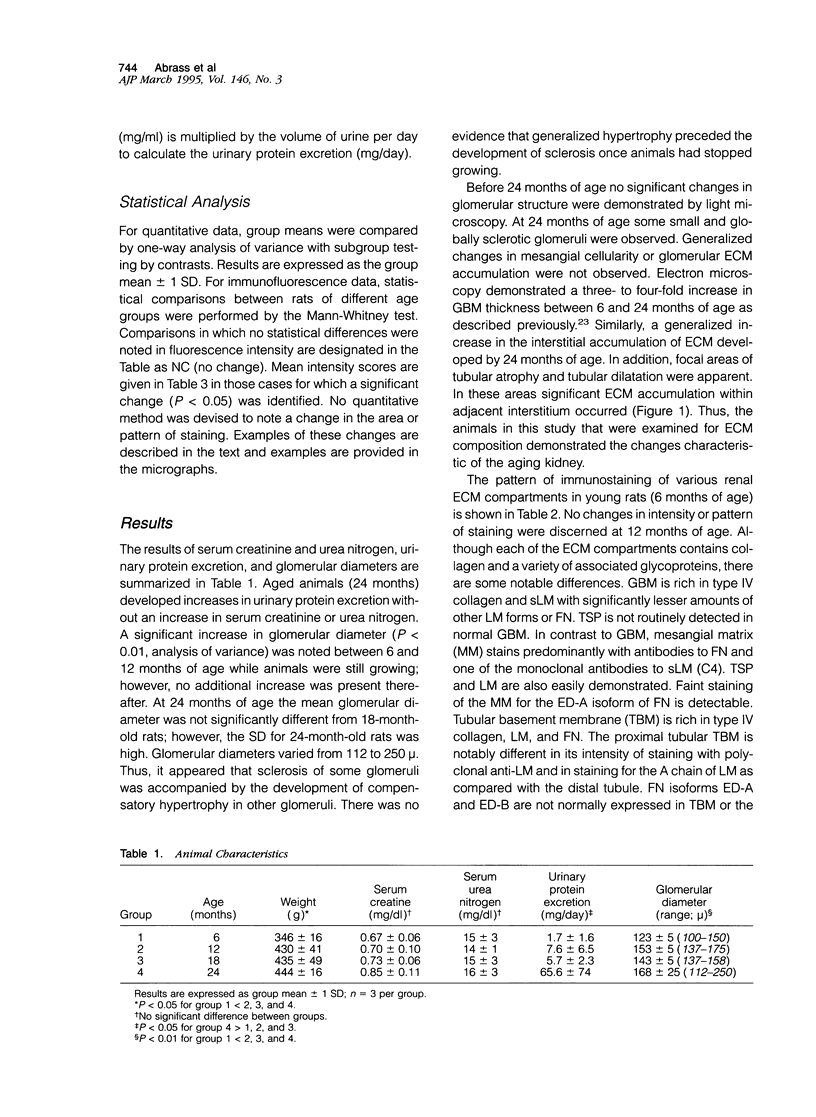
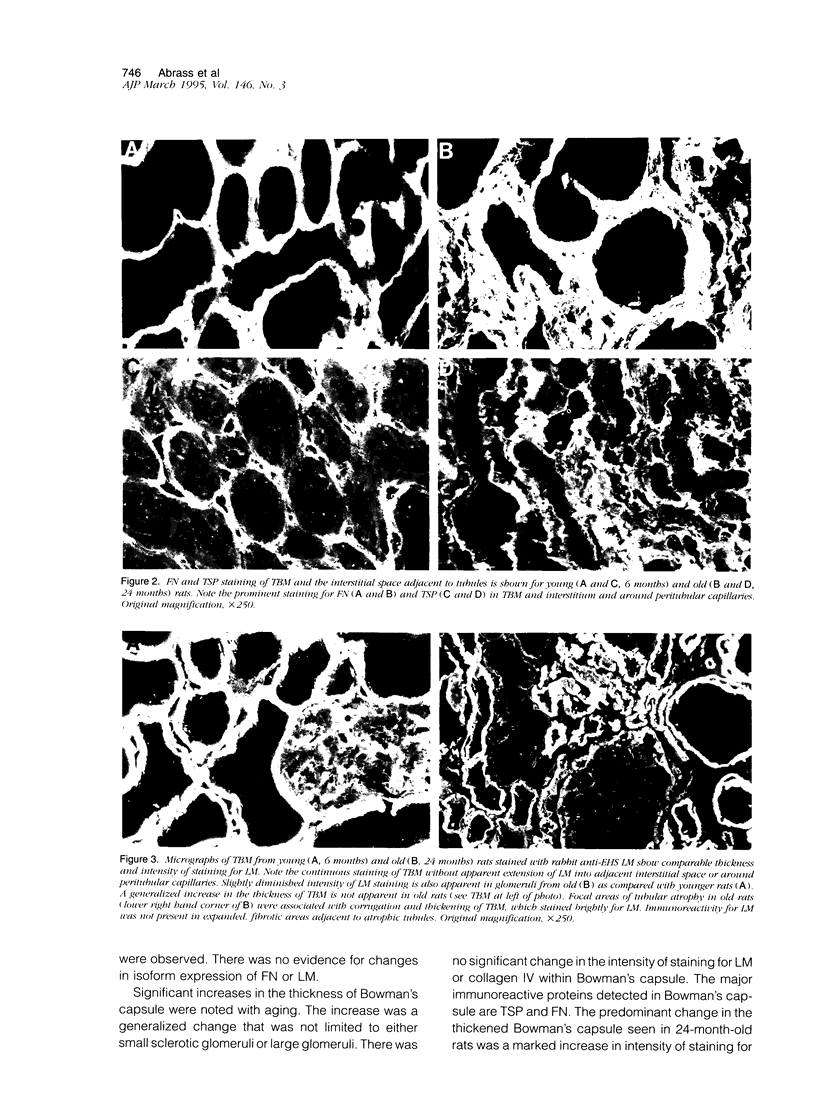
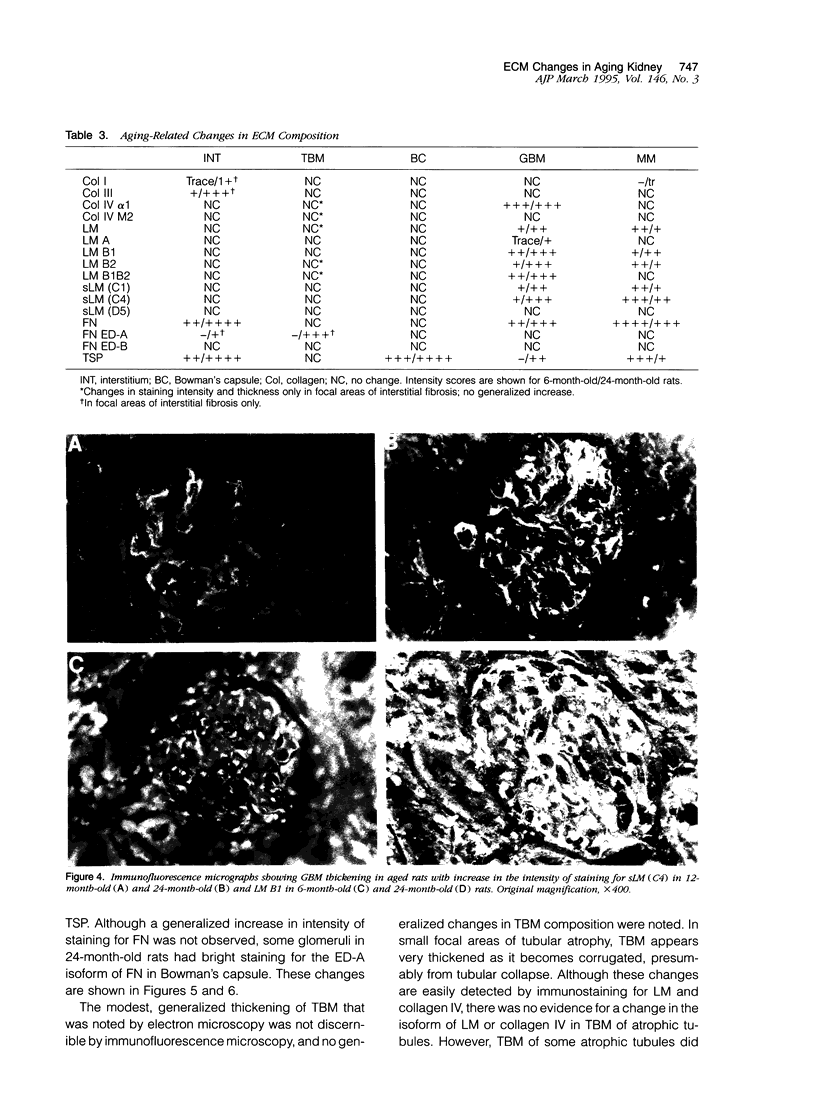
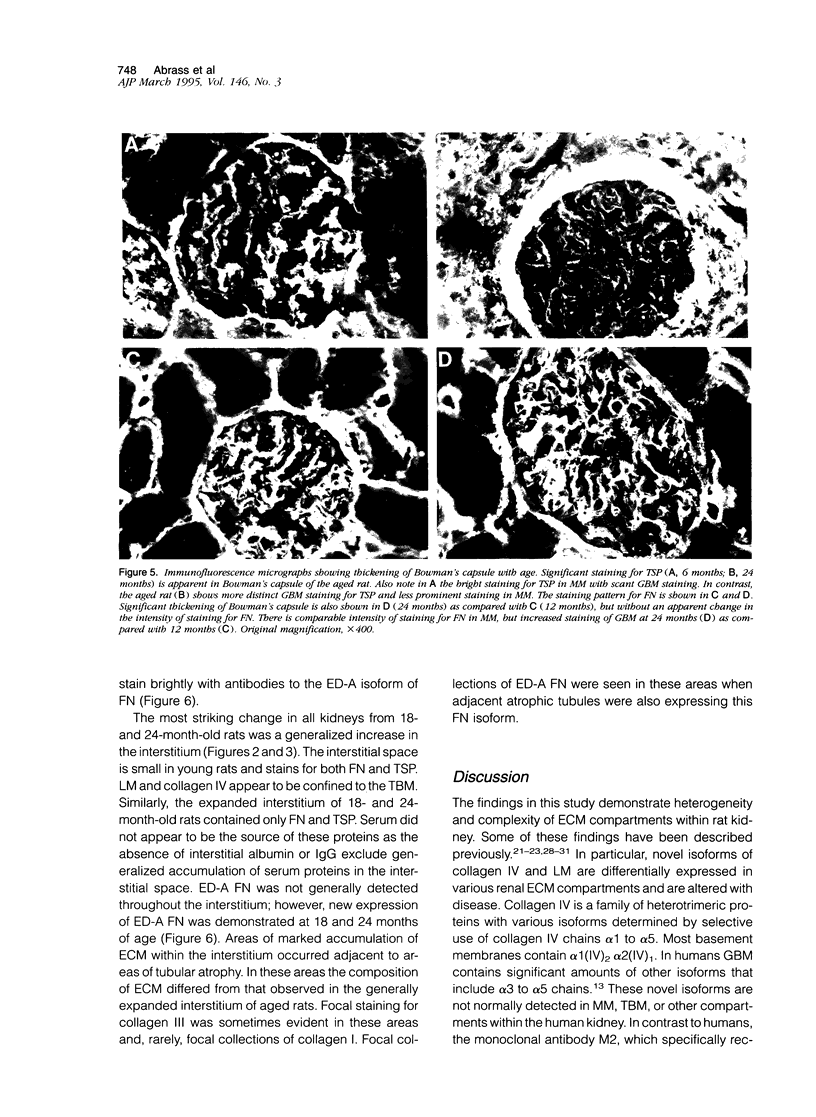
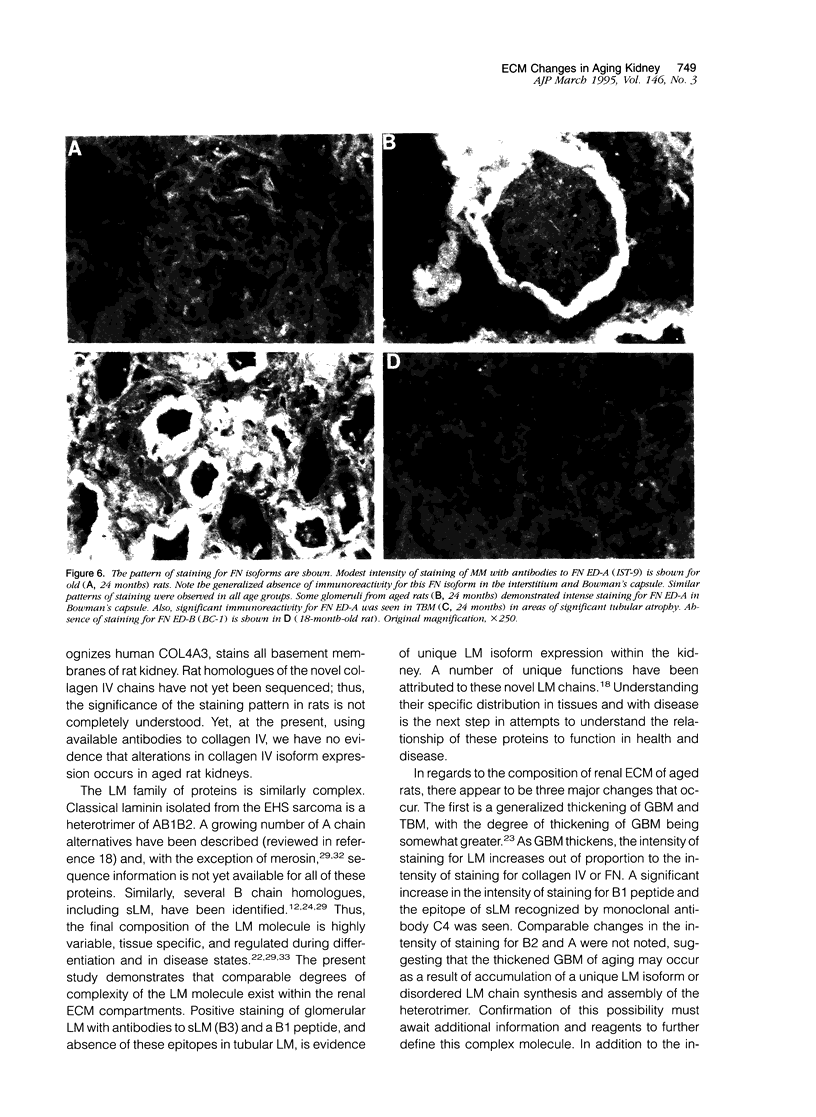
The composition of renal extracellular matrices was examined in 6-, 12-, 18-, and 24-month-old rats by immunofluorescence microscopy. No change in composition of tubular basement membrane was detected. Increased immunostaining for laminin chains B1 and s-laminin and thrombospondin characterized the thickened glomerular basement membrane. Interstitial collagens I and III were not detected in globally sclerotic glomeruli. The major change noted in the aged rat kidney at 24 months was generalized expansion of the interstitium by thrombospondin and fibronectin. In areas of tubular atrophy there was new expression of extra domain A (EDA)+ fibronectin. Collagens I and III were detected focally in the interstitium adjacent to areas of tubular atrophy, but otherwise collagens I, III, and IV and laminin did not contribute to the interstitial fibrosis. Interstitial fibrosis was detectable at 18 months of age and preceded the development of sclerotic glomeruli, tubular atrophy, or accumulations of interstitial collagen. These changes in extracellular matrix composition distinguish the aging kidney from other sclerotic forms of renal disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Irwin M. H., St John P. L., Perry E. W., Accavitti M. A., Heck L. W., Couchman J. R. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989 Dec;109(6 Pt 2):3477–3491. doi: 10.1083/jcb.109.6.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrass C. K., Peterson C. V., Raugi G. J. Phenotypic expression of collagen types in mesangial matrix of diabetic and nondiabetic rats. Diabetes. 1988 Dec;37(12):1695–1702. doi: 10.2337/diab.37.12.1695. [DOI] [PubMed] [Google Scholar]
- Ainsworth S. K., Hirsch H. Z., Brackett N. C., Jr, Brissie R. M., Williams A. V., Jr, Hennigar G. R. Diabetic glomerulonephropathy: histopathologic, immunofluorescent, and ultrastructural studies of 16 cases. Hum Pathol. 1982 May;13(5):470–478. doi: 10.1016/s0046-8177(82)80030-0. [DOI] [PubMed] [Google Scholar]
- Brandis A., Bianchi G., Reale E., Helmchen U., Kühn K. Age-dependent glomerulosclerosis and proteinuria occurring in rats of the Milan normotensive strain and not in rats of the Milan hypertensive strain. Lab Invest. 1986 Aug;55(2):234–243. [PubMed] [Google Scholar]
- Bruneval P., Foidart J. M., Nochy D., Camilleri J. P., Bariety J. Glomerular matrix proteins in nodular glomerulosclerosis in association with light chain deposition disease and diabetes mellitus. Hum Pathol. 1985 May;16(5):477–484. doi: 10.1016/s0046-8177(85)80086-1. [DOI] [PubMed] [Google Scholar]
- Butkowski R. J., Wieslander J., Kleppel M., Michael A. F., Fish A. J. Basement membrane collagen in the kidney: regional localization of novel chains related to collagen IV. Kidney Int. 1989 May;35(5):1195–1202. doi: 10.1038/ki.1989.110. [DOI] [PubMed] [Google Scholar]
- Carnemolla B., Balza E., Siri A., Zardi L., Nicotra M. R., Bigotti A., Natali P. G. A tumor-associated fibronectin isoform generated by alternative splicing of messenger RNA precursors. J Cell Biol. 1989 Mar;108(3):1139–1148. doi: 10.1083/jcb.108.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtoy P. J., Timpl R., Farquhar M. G. Comparative distribution of laminin, type IV collagen, and fibronectin in the rat glomerulus. J Histochem Cytochem. 1982 Sep;30(9):874–886. doi: 10.1177/30.9.7130672. [DOI] [PubMed] [Google Scholar]
- Couser W. G., Stilmant M. M. Mesangial lesions and focal glomerular sclerosis in the aging rat. Lab Invest. 1975 Nov;33(5):491–501. [PubMed] [Google Scholar]
- DeSimone D. W., Norton P. A., Hynes R. O. Identification and characterization of alternatively spliced fibronectin mRNAs expressed in early Xenopus embryos. Dev Biol. 1992 Feb;149(2):357–369. doi: 10.1016/0012-1606(92)90291-n. [DOI] [PubMed] [Google Scholar]
- Ekblom M., Klein G., Mugrauer G., Fecker L., Deutzmann R., Timpl R., Ekblom P. Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell. 1990 Jan 26;60(2):337–346. doi: 10.1016/0092-8674(90)90748-4. [DOI] [PubMed] [Google Scholar]
- Engvall E., Earwicker D., Haaparanta T., Ruoslahti E., Sanes J. R. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990 Sep;1(10):731–740. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furcht L. T. Critical factors controlling angiogenesis: cell products, cell matrix, and growth factors. Lab Invest. 1986 Nov;55(5):505–509. [PubMed] [Google Scholar]
- Glukhova M. A., Frid M. G., Shekhonin B. V., Vasilevskaya T. D., Grunwald J., Saginati M., Koteliansky V. E. Expression of extra domain A fibronectin sequence in vascular smooth muscle cells is phenotype dependent. J Cell Biol. 1989 Jul;109(1):357–366. doi: 10.1083/jcb.109.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. S., Tarloff J. B., Hook J. B. Age-related nephropathy in laboratory rats. FASEB J. 1988 Apr;2(7):2241–2251. doi: 10.1096/fasebj.2.7.3280378. [DOI] [PubMed] [Google Scholar]
- Goyal M., Wiggins R. Fibronectin mRNA and protein accumulation, distribution, and breakdown in rabbit anti-glomerular basement membrane disease. J Am Soc Nephrol. 1991 Jun;1(12):1334–1342. doi: 10.1681/ASN.V1121334. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Weeks B. S., Schnaper H. W., Kibbey M. C., Yamamura K., Grant D. S. The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitam Horm. 1993;47:161–186. doi: 10.1016/s0083-6729(08)60446-x. [DOI] [PubMed] [Google Scholar]
- Laurie G. W., Horikoshi S., Killen P. D., Segui-Real B., Yamada Y. In situ hybridization reveals temporal and spatial changes in cellular expression of mRNA for a laminin receptor, laminin, and basement membrane (type IV) collagen in the developing kidney. J Cell Biol. 1989 Sep;109(3):1351–1362. doi: 10.1083/jcb.109.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurie G. W., Leblond C. P., Inoue S., Martin G. R., Chung A. Fine structure of the glomerular basement membrane and immunolocalization of five basement membrane components to the lamina densa (basal lamina) and its extensions in both glomeruli and tubules of the rat kidney. Am J Anat. 1984 Apr;169(4):463–481. doi: 10.1002/aja.1001690408. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Hernandez A., Chung A. E. The ultrastructural localization of two basement membrane components: entactin and laminin in rat tissues. J Histochem Cytochem. 1984 Mar;32(3):289–298. doi: 10.1177/32.3.6198358. [DOI] [PubMed] [Google Scholar]
- Morel-Maroger Striker L., Killen P. D., Chi E., Striker G. E. The composition of glomerulosclerosis. I. Studies in focal sclerosis, crescentic glomerulonephritis, and membranoproliferative glomerulonephritis. Lab Invest. 1984 Aug;51(2):181–192. [PubMed] [Google Scholar]
- Nast C. C., Adler S. G., Artishevsky A., Kresser C. T., Ahmed K., Anderson P. S. Cyclosporine induces elevated procollagen alpha 1 (I) mRNA levels in the rat renal cortex. Kidney Int. 1991 Apr;39(4):631–638. doi: 10.1038/ki.1991.75. [DOI] [PubMed] [Google Scholar]
- Oyama F., Hirohashi S., Shimosato Y., Titani K., Sekiguchi K. Deregulation of alternative splicing of fibronectin pre-mRNA in malignant human liver tumors. J Biol Chem. 1989 Jun 25;264(18):10331–10334. [PubMed] [Google Scholar]
- Paulsson M., Saladin K. Mouse heart laminin. Purification of the native protein and structural comparison with Engelbreth-Holm-Swarm tumor laminin. J Biol Chem. 1989 Nov 5;264(31):18726–18732. [PubMed] [Google Scholar]
- Raugi G. J., Olerud J. E., Gown A. M. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987 Dec;89(6):551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Engvall E., Butkowski R., Hunter D. D. Molecular heterogeneity of basal laminae: isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990 Oct;111(4):1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes J. R., Hunter D. D., Green T. L., Merlie J. P. S-laminin. Cold Spring Harb Symp Quant Biol. 1990;55:419–430. doi: 10.1101/sqb.1990.055.01.042. [DOI] [PubMed] [Google Scholar]
- Saus J., Wieslander J., Langeveld J. P., Quinones S., Hudson B. G. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988 Sep 15;263(26):13374–13380. [PubMed] [Google Scholar]
- Takazakura E., Sawabu N., Handa A., Takada A., Shinoda A., Takeuchi J. Intrarenal vascular changes with age and disease. Kidney Int. 1972 Oct;2(4):224–230. doi: 10.1038/ki.1972.98. [DOI] [PubMed] [Google Scholar]
- Tolsma S. S., Volpert O. V., Good D. J., Frazier W. A., Polverini P. J., Bouck N. Peptides derived from two separate domains of the matrix protein thrombospondin-1 have anti-angiogenic activity. J Cell Biol. 1993 Jul;122(2):497–511. doi: 10.1083/jcb.122.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wewer U. M., Engvall E., Paulsson M., Yamada Y., Albrechtsen R. Laminin A, B1, B2, S and M subunits in the postnatal rat liver development and after partial hepatectomy. Lab Invest. 1992 Mar;66(3):378–389. [PubMed] [Google Scholar]
- Yoshioka K., Takemura T., Tohda M., Akano N., Miyamoto H., Ooshima A., Maki S. Glomerular localization of type III collagen in human kidney disease. Kidney Int. 1989 May;35(5):1203–1211. doi: 10.1038/ki.1989.111. [DOI] [PubMed] [Google Scholar]
- Yumura W., Sugino N., Nagasawa R., Kubo S., Hirokawa K., Maruyama N. Age-associated changes in renal glomeruli of mice. Exp Gerontol. 1989;24(3):237–249. doi: 10.1016/0531-5565(89)90015-6. [DOI] [PubMed] [Google Scholar]