Abstract
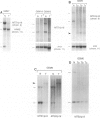
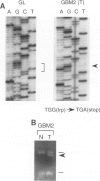
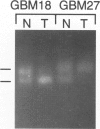
The multiple tumor suppressor 1 (MTS1) gene encoding the p16 inhibitor of cyclin-dependent kinase 4 is deleted or mutated in a wide variety of human tumor cell lines, but the importance of this gene as a tumor suppressor in vivo appears to be highly dependent on tumor type. Because MTS1/p16/CDKN2 and the homologous MTS2/p15 gene map to a region of chromosome 9p21, which is frequently deleted in malignant gliomas, we searched for lesions of these genes in primary biopsies of glioblastoma multiforme (GBM). Our analysis confirms a sizable frequency of homozygous deletion of MTS1/p16/CDKN2 (9/27 cases) and also reveals a low but detectable frequency of intragenic DNA lesions (one point mutation in exon 2 leading to premature termination) among GBMs that retain one or both copies of the gene. No mutations were found in exon 2 of MTS2/p15 (12 cases examined), and one GBM showed a DNA deletion breakpoint in the 30 kb between MTS1/p16/CDKN2 and MTS2/p15 resulting in deletion of MTS1/p16/CDKN2 with retention of MTS2/p15. In contrast to the high-grade tumors, none of 12 low-grade gliomas showed MTS1/p16/CDKN2 deletions. These data support a role for MTS1/p16/CDKN2 as a tumor suppressor gene in the in vivo evolution of GBMs. Given that two tumors with hemizygous MTS1/p16/CDKN2 deletions and loss of heterozygosity for chromosome 9p21 did not contain detectable intragenic mutations, there may be one or more additional relevant 9p21 tumor suppressor genes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigner S. H., Mark J., Bigner D. D. Cytogenetics of human brain tumors. Cancer Genet Cytogenet. 1990 Jul 15;47(2):141–154. doi: 10.1016/0165-4608(90)90024-5. [DOI] [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Cairns P., Shaw M. E., Knowles M. A. Preliminary mapping of the deleted region of chromosome 9 in bladder cancer. Cancer Res. 1993 Mar 15;53(6):1230–1232. [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Collins V. P., James C. D. Gene and chromosomal alterations associated with the development of human gliomas. FASEB J. 1993 Jul;7(10):926–930. doi: 10.1096/fasebj.7.10.8344489. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. Hypomethylation of ras oncogenes in primary human cancers. Biochem Biophys Res Commun. 1983 Feb 28;111(1):47–54. doi: 10.1016/s0006-291x(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- He J., Allen J. R., Collins V. P., Allalunis-Turner M. J., Godbout R., Day R. S., 3rd, James C. D. CDK4 amplification is an alternative mechanism to p16 gene homozygous deletion in glioma cell lines. Cancer Res. 1994 Nov 15;54(22):5804–5807. [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Ichimura K., Schmidt E. E., Yamaguchi N., James C. D., Collins V. P. A common region of homozygous deletion in malignant human gliomas lies between the IFN alpha/omega gene cluster and the D9S171 locus. Cancer Res. 1994 Jun 15;54(12):3127–3130. [PubMed] [Google Scholar]
- James C. D., He J., Carlbom E., Nordenskjold M., Cavenee W. K., Collins V. P. Chromosome 9 deletion mapping reveals interferon alpha and interferon beta-1 gene deletions in human glial tumors. Cancer Res. 1991 Mar 15;51(6):1684–1688. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Miyakoshi J., Dobler K. D., Allalunis-Turner J., McKean J. D., Petruk K., Allen P. B., Aronyk K. N., Weir B., Huyser-Wierenga D., Fulton D. Absence of IFNA and IFNB genes from human malignant glioma cell lines and lack of correlation with cellular sensitivity to interferons. Cancer Res. 1990 Jan 15;50(2):278–283. [PubMed] [Google Scholar]
- Mori T., Miura K., Aoki T., Nishihira T., Mori S., Nakamura Y. Frequent somatic mutation of the MTS1/CDK4I (multiple tumor suppressor/cyclin-dependent kinase 4 inhibitor) gene in esophageal squamous cell carcinoma. Cancer Res. 1994 Jul 1;54(13):3396–3397. [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Bohlander S. K., Pomykala H., Maltepe E., Van Melle E., Le Beau M. M., Diaz M. O. Mapping of the shortest region of overlap of deletions of the short arm of chromosome 9 associated with human neoplasia. Genomics. 1992 Oct;14(2):437–443. doi: 10.1016/s0888-7543(05)80238-1. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Buchhagen D. L., Malik K., Sherman J., Nobori T., Bader S., Nau M. M., Gazdar A. F., Minna J. D., Diaz M. O. Homozygous loss of the interferon genes defines the critical region on 9p that is deleted in lung cancers. Cancer Res. 1993 May 15;53(10 Suppl):2410–2415. [PubMed] [Google Scholar]
- Olopade O. I., Jenkins R. B., Ransom D. T., Malik K., Pomykala H., Nobori T., Cowan J. M., Rowley J. D., Diaz M. O. Molecular analysis of deletions of the short arm of chromosome 9 in human gliomas. Cancer Res. 1992 May 1;52(9):2523–2529. [PubMed] [Google Scholar]
- Pata I., Metspalu A. A dinucleotide repeat polymorphism at the ribosomal protein S6 (RPS6) gene. Hum Mol Genet. 1993 Oct;2(10):1749–1749. doi: 10.1093/hmg/2.10.1749-a. [DOI] [PubMed] [Google Scholar]
- Reifenberger G., Reifenberger J., Ichimura K., Meltzer P. S., Collins V. P. Amplification of multiple genes from chromosomal region 12q13-14 in human malignant gliomas: preliminary mapping of the amplicons shows preferential involvement of CDK4, SAS, and MDM2. Cancer Res. 1994 Aug 15;54(16):4299–4303. [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Ueki K., Rubio M. P., Ramesh V., Correa K. M., Rutter J. L., von Deimling A., Buckler A. J., Gusella J. F., Louis D. N. MTS1/CDKN2 gene mutations are rare in primary human astrocytomas with allelic loss of chromosome 9p. Hum Mol Genet. 1994 Oct;3(10):1841–1845. doi: 10.1093/hmg/3.10.1841. [DOI] [PubMed] [Google Scholar]
- Wilkie P. J., Krizman D. B., Weber J. L. Linkage map of human chromosome 9 microsatellite polymorphisms. Genomics. 1992 Mar;12(3):607–609. doi: 10.1016/0888-7543(92)90456-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shields T., Crenshaw T., Hao Y., Moulton T., Tycko B. Imprinting of human H19: allele-specific CpG methylation, loss of the active allele in Wilms tumor, and potential for somatic allele switching. Am J Hum Genet. 1993 Jul;53(1):113–124. [PMC free article] [PubMed] [Google Scholar]