Abstract
The tissue macrophage plays a prominent role in wound repair, yet the parameters that influence macrophage migration into the wound bed are not well understood. To better understand the process of macrophage recruitment, the production of JE, the murine homologue of monocyte chemoattractant protein 1(JE/MCP-1), was examined in a murine model of dermal wound repair. High levels of JE/MCP-1 mRNA were found in dermal punch wounds at 12 hours and 1 day (24 hours) after wounding; mRNA levels slowly decreased to undetectable by day 21. In situ hybridization analysis of wounds revealed that JE/MCP-1 was predominantly expressed by monocytic and macrophage-like cells, as well as by occasional fibroblasts and other interstitial cells. To correlate JE/MCP-1 production with macrophage migration, macrophage infiltration into the wound bed was quantitated. The number of macrophages within the wound increased to a maximum at day 3 (11.3 +/- 4.5 macrophages per high power field), began to decrease at day 5 (4.8 +/- 1.9 macrophages per high power field), and reached near base line at day 10 (3.0 +/- 1.1 macrophages per high power field). The results demonstrate that JE/MCP-1 production within wounds is closely linked to the time course and distribution of macrophage infiltration, with maximal JE/MCP-1 mRNA levels occurring 1 to 2 days before maximal macrophage infiltration. The results support a role for JE/MCP-1 in the recruitment of wound macrophages and suggest that macrophages, through the production of JE/MCP-1, may sustain the recruitment of additional monocytes and macrophages into sites of injury.
Full text
PDF

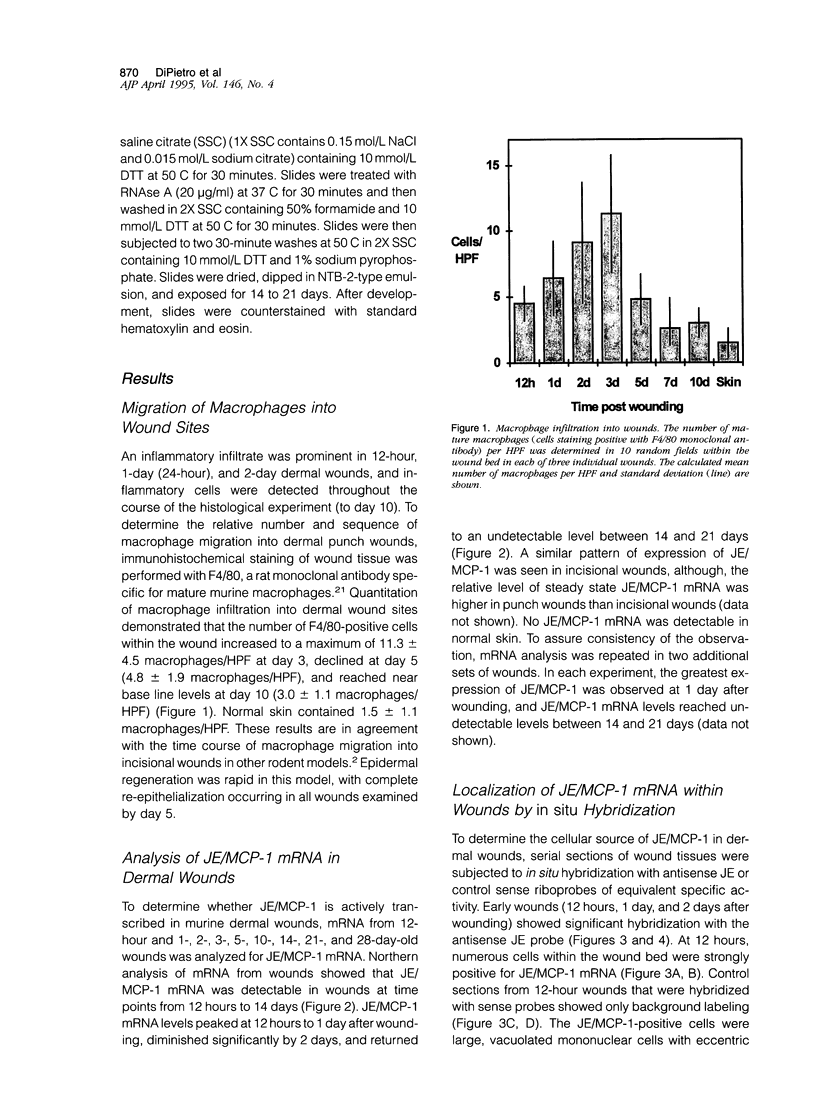
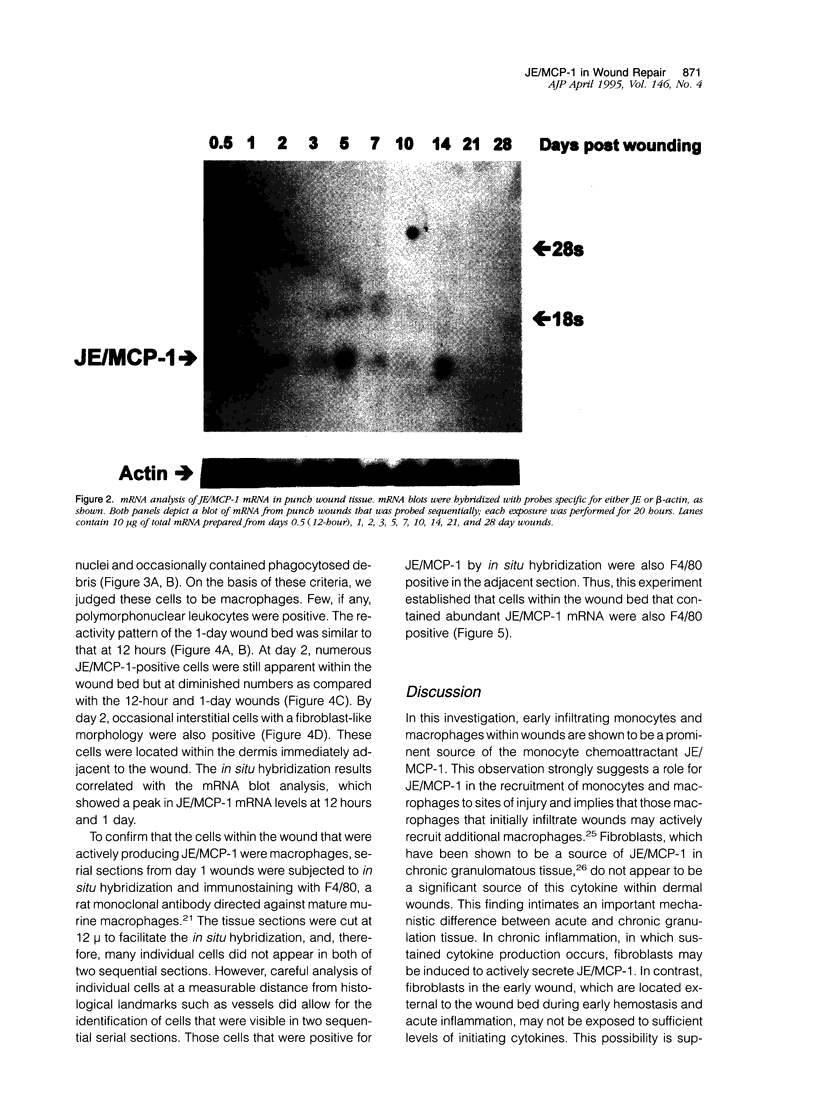




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N., Neville-Golden J., Galanopoulos T., Kradin R. L., Valente A. J., Graves D. T. Expression of monocyte chemoattractant protein 1 mRNA in human idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5371–5375. doi: 10.1073/pnas.89.12.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Brieland J. K., Jones M. L., Flory C. M., Miller G. R., Warren J. S., Phan S. H., Fantone J. C. Expression of monocyte chemoattractant protein-1 (MCP-1) by rat alveolar macrophages during chronic lung injury. Am J Respir Cell Mol Biol. 1993 Sep;9(3):300–305. doi: 10.1165/ajrcmb/9.3.300. [DOI] [PubMed] [Google Scholar]
- Carr M. W., Roth S. J., Luther E., Rose S. S., Springer T. A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Sciacca F. L., Sironi M., Luini W., Rabiet M. J., Mantovani A. Expression of monocyte chemotactic protein-1 by monocytes and endothelial cells exposed to thrombin. Am J Pathol. 1994 May;144(5):975–985. [PMC free article] [PubMed] [Google Scholar]
- Cushing S. D., Fogelman A. M. Monocytes may amplify their recruitment into inflammatory lesions by inducing monocyte chemotactic protein. Arterioscler Thromb. 1992 Jan;12(1):78–82. doi: 10.1161/01.atv.12.1.78. [DOI] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Chang D., Griffin G. L., Heinrikson R. L., Kaiser E. T. Platelet factor 4 is chemotactic for neutrophils and monocytes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4584–4587. doi: 10.1073/pnas.78.7.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Diamond M. S., Springer T. A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993 Jan;120(2):545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey T. J., 3rd, Sherry B., Tracey K. J., van Deventer S., Jones W. G., 2nd, Minei J. P., Morgello S., Shires G. T., Cerami A. Cytokine production in a model of wound healing: the appearance of MIP-1, MIP-2, cachectin/TNF and IL-1. Cytokine. 1990 Mar;2(2):92–99. doi: 10.1016/1043-4666(90)90002-b. [DOI] [PubMed] [Google Scholar]
- Fukasawa M., Campeau J. D., Yanagihara D. L., Rodgers K. E., Dizerega G. S. Mitogenic and protein synthetic activity of tissue repair cells: control by the postsurgical macrophage. J Invest Surg. 1989;2(2):169–180. doi: 10.3109/08941938909015348. [DOI] [PubMed] [Google Scholar]
- Hunt T. K., Knighton D. R., Thakral K. K., Goodson W. H., 3rd, Andrews W. S. Studies on inflammation and wound healing: angiogenesis and collagen synthesis stimulated in vivo by resident and activated wound macrophages. Surgery. 1984 Jul;96(1):48–54. [PubMed] [Google Scholar]
- Iida N., Grotendorst G. R. Cloning and sequencing of a new gro transcript from activated human monocytes: expression in leukocytes and wound tissue. Mol Cell Biol. 1990 Oct;10(10):5596–5599. doi: 10.1128/mcb.10.10.5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz A. C., Szpejda M. Evidence that platelet activating factor may mediate some acute inflammatory responses. Studies with the platelet-activating factor antagonist, CV3988. Lab Invest. 1986 Mar;54(3):275–281. [PubMed] [Google Scholar]
- Jiang Y., Beller D. I., Frendl G., Graves D. T. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992 Apr 15;148(8):2423–2428. [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Harlow L. A., Johnson B., Evanoff H. L., Haines G. K., Burdick M. D., Pope R. M., Strieter R. M. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 1992 Sep;90(3):772–779. doi: 10.1172/JCI115950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs E. J., DiPietro L. A. Fibrogenic cytokines and connective tissue production. FASEB J. 1994 Aug;8(11):854–861. doi: 10.1096/fasebj.8.11.7520879. [DOI] [PubMed] [Google Scholar]
- Kunkel S. L., Standiford T., Kasahara K., Strieter R. M. Stimulus specific induction of monocyte chemotactic protein-1 (MCP-1) gene expression. Adv Exp Med Biol. 1991;305:65–71. doi: 10.1007/978-1-4684-6009-4_8. [DOI] [PubMed] [Google Scholar]
- Landis R. C., Bennett R. I., Hogg N. A novel LFA-1 activation epitope maps to the I domain. J Cell Biol. 1993 Mar;120(6):1519–1527. doi: 10.1083/jcb.120.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Leonard E. J., Skeel A., Yoshimura T. Biological aspects of monocyte chemoattractant protein-1 (MCP-1). Adv Exp Med Biol. 1991;305:57–64. doi: 10.1007/978-1-4684-6009-4_7. [DOI] [PubMed] [Google Scholar]
- Lukacs N. W., Chensue S. W., Smith R. E., Strieter R. M., Warmington K., Wilke C., Kunkel S. L. Production of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 alpha by inflammatory granuloma fibroblasts. Am J Pathol. 1994 Apr;144(4):711–718. [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Reuterdahl C., Sundberg C., Rubin K., Funa K., Gerdin B. Tissue localization of beta receptors for platelet-derived growth factor and platelet-derived growth factor B chain during wound repair in humans. J Clin Invest. 1993 May;91(5):2065–2075. doi: 10.1172/JCI116429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., Morrison E. D., Stiles C. D. Cloning and expression of JE, a gene inducible by platelet-derived growth factor and whose product has cytokine-like properties. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3738–3742. doi: 10.1073/pnas.85.11.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Odland G. Human wound repair. II. Inflammatory cells, epithelial-mesenchymal interrelations, and fibrogenesis. J Cell Biol. 1968 Oct;39(1):152–168. doi: 10.1083/jcb.39.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M. E., Adams D. H., Wyner L. R., Yamashita Y., Halnon N. J., Karnovsky M. J. Early and persistent induction of monocyte chemoattractant protein 1 in rat cardiac allografts. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6086–6090. doi: 10.1073/pnas.90.13.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Ford-Hutchinson A. W., Bray M. A. Leukotriene B: a potential mediator of inflammation. J Pharm Pharmacol. 1980 Jul;32(7):517–518. doi: 10.1111/j.2042-7158.1980.tb12985.x. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Gewurz H., Mergenhagen S. E. Interactions of the complement system with endotoxic lipopolysaccharide. Generation of a factor chemotactic for polymorphonuclear leukocytes. J Exp Med. 1968 Aug 1;128(2):259–275. doi: 10.1084/jem.128.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., Assoian R. K. Transforming growth factor-beta: biological function and chemical structure. Science. 1986 Aug 1;233(4763):532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stiernberg J., Redin W. R., Warner W. S., Carney D. H. The role of thrombin and thrombin receptor activating peptide (TRAP-508) in initiation of tissue repair. Thromb Haemost. 1993 Jul 1;70(1):158–162. [PubMed] [Google Scholar]
- Valente A. J., Graves D. T., Vialle-Valentin C. E., Delgado R., Schwartz C. J. Purification of a monocyte chemotactic factor secreted by nonhuman primate vascular cells in culture. Biochemistry. 1988 May 31;27(11):4162–4168. doi: 10.1021/bi00411a039. [DOI] [PubMed] [Google Scholar]
- Weksler B. B., Coupal C. E. Platelet-dependent generation of chemotactic activity in serum. J Exp Med. 1973 Jun 1;137(6):1419–1430. doi: 10.1084/jem.137.6.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitby D. J., Ferguson M. W. Immunohistochemical localization of growth factors in fetal wound healing. Dev Biol. 1991 Sep;147(1):207–215. doi: 10.1016/s0012-1606(05)80018-1. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Leonard E. J. Secretion by human fibroblasts of monocyte chemoattractant protein-1, the product of gene JE. J Immunol. 1990 Mar 15;144(6):2377–2383. [PubMed] [Google Scholar]