Abstract
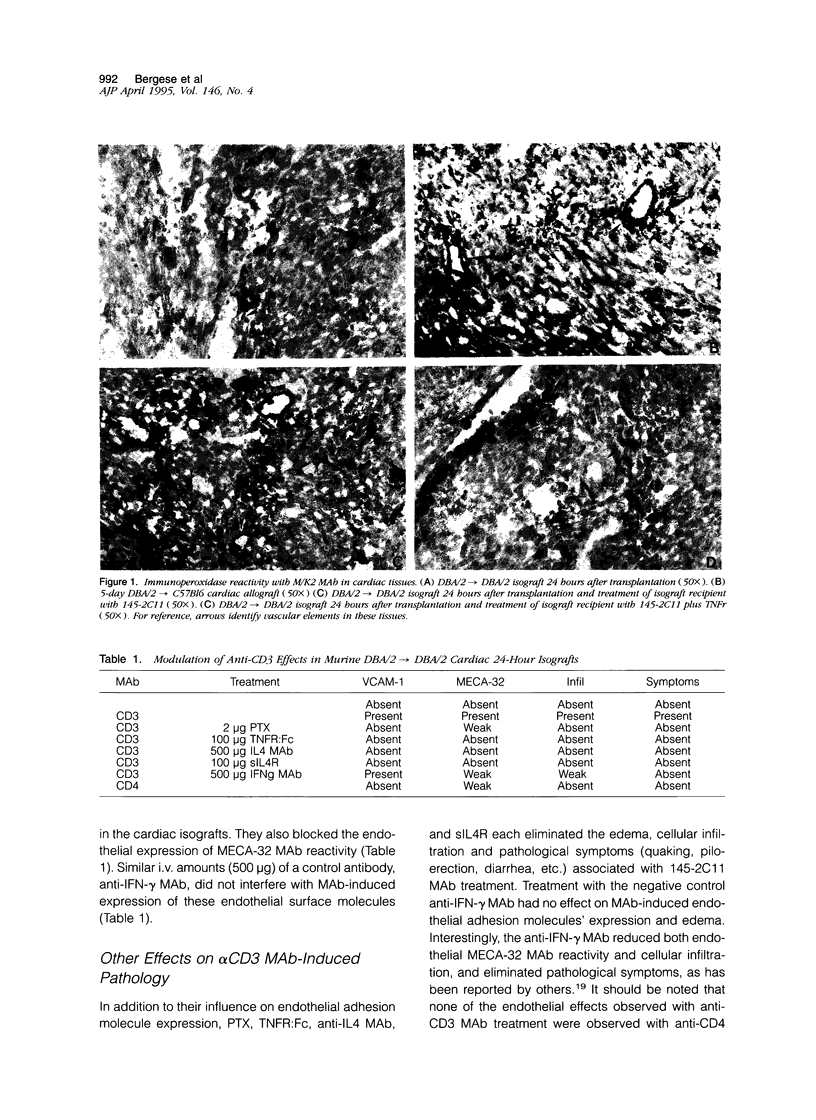
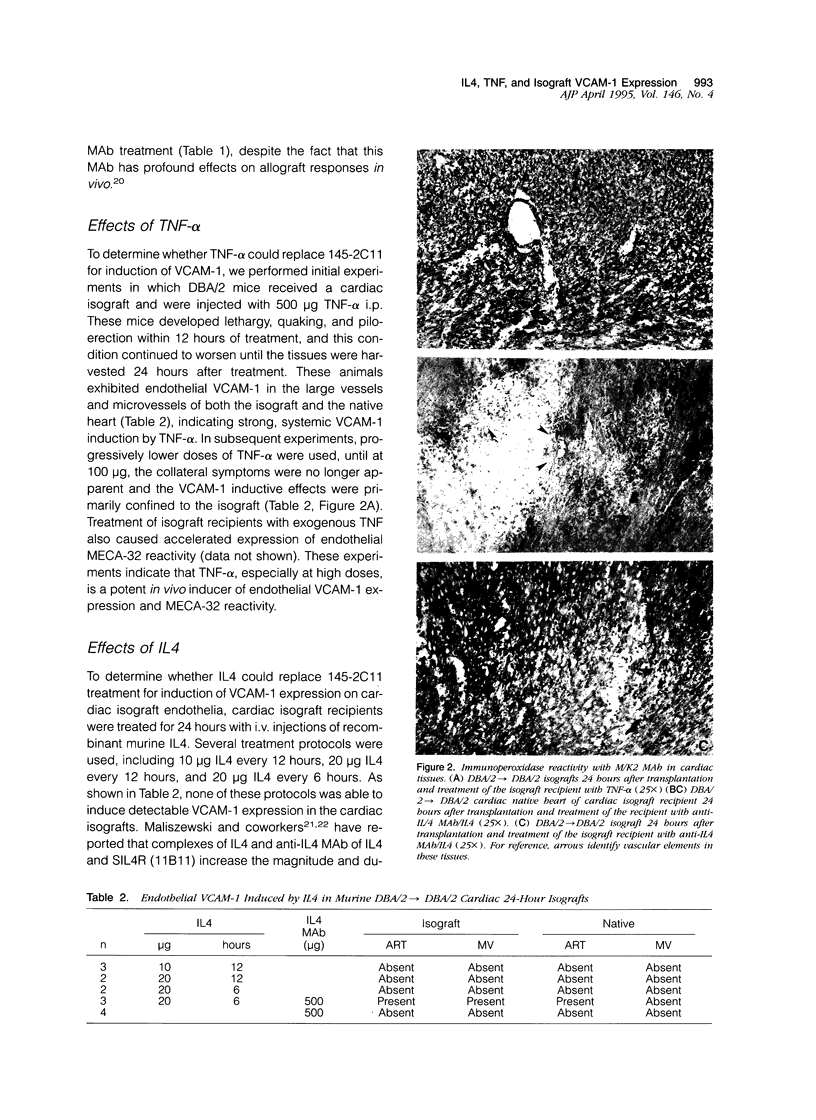
The in vivo mechanisms of vascular endothelial activation and VCAM-1 expression were studied in murine heterotopic cardiac grafts. Preliminary studies demonstrated that cardiac allograft endothelia develop reactivity with MECA-32 monoclonal antibody (MAb) and M/K-2 (anti-VCAM-1) MAb within 3 days of transplantation, whereas cardiac isografts develop MECA-32 reactivity but no M/K-2 reactivity. Additional studies demonstrated that a single treatment of cardiac isograft recipients with the anti-CD3 MAb 145-2C11 induces VCAM-1 expression on isograft microvascular endothelia within 24 hours. We have used this experimental system to identify the cytokines responsible for expression of VCAM-1 and MECA-32 MAb reactivity on graft vascular endothelia. We report that the expression of VCAM-1 on isograft endothelia that was induced with anti-CD3 MAb was blocked by simultaneous treatment with either pentoxifylline, soluble tumor necrosis factor (TNF) receptor (TNFR-Fc), anti-IL4 MAb, or soluble IL4R, but not by anti-IFN-gamma MAb. Alternatively, a similar pattern of isograft endothelial VCAM-1 expression was stimulated in the absence of anti-CD3 MAbs with a single injection of human recombinant TNF-alpha, or with recombinant murine IL4 provided as IL4/anti-IL4 MAb complexes. In addition, the IL4-induced VCAM-1 expression was completely blocked by a single intravenous treatment of the isograft recipients with TNFR:Fc. This suggests that high concentrations of TNF-alpha can stimulate endothelial VCAM-1 expression, but these concentrations are apparently not achieved in cardiac isografts. In the absence of an inducing agent such as anti-CD3 MAb, the stimulation of VCAM-1 expression with exogenous IL4 may reflect functional interaction between endogenous TNF and exogenous IL4, as suggested by the blocking experiments with TNFR:Fc. Although cardiac isograft endothelia normally develop reactivity with MECA-32 MAb within 3 days of transplantation, treatment of cardiac isograft recipients with anti-CD3 MAb accelerated MECA-32 reactivity to within 24 hours of transplantation. This accelerated expression can be experimentally manipulated in the same way as M/K-2 reactivity, suggesting that similar mechanisms may control the development of these two inflammatory endothelial phenotypical markers, despite their differential expression in cardiac isografts and allografts.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowicz D., Schandene L., Goldman M., Crusiaux A., Vereerstraeten P., De Pauw L., Wybran J., Kinnaert P., Dupont E., Toussaint C. Release of tumor necrosis factor, interleukin-2, and gamma-interferon in serum after injection of OKT3 monoclonal antibody in kidney transplant recipients. Transplantation. 1989 Apr;47(4):606–608. doi: 10.1097/00007890-198904000-00008. [DOI] [PubMed] [Google Scholar]
- Alegre M. L., Gastaldello K., Abramowicz D., Kinnaert P., Vereerstraeten P., De Pauw L., Vandenabeele P., Moser M., Leo O., Goldman M. Evidence that pentoxifylline reduces anti-CD3 monoclonal antibody-induced cytokine release syndrome. Transplantation. 1991 Oct;52(4):674–679. doi: 10.1097/00007890-199110000-00018. [DOI] [PubMed] [Google Scholar]
- Bergese S. D., Pelletier R. P., Ohye R. G., Vallera D. A., Orosz C. G. Treatment of mice with anti-CD3 mAb induces endothelial vascular cell adhesion molecule-1 expression. Transplantation. 1994 Mar 15;57(5):711–717. doi: 10.1097/00007890-199403150-00013. [DOI] [PubMed] [Google Scholar]
- Bishop D. K., Chan S., Li W., Ensley R. D., Xu S., Eichwald E. J. CD4-positive helper T lymphocytes mediate mouse cardiac allograft rejection independent of donor alloantigen specific cytotoxic T lymphocytes. Transplantation. 1993 Oct;56(4):892–897. doi: 10.1097/00007890-199310000-00023. [DOI] [PubMed] [Google Scholar]
- Briscoe D. M., Cotran R. S., Pober J. S. Effects of tumor necrosis factor, lipopolysaccharide, and IL-4 on the expression of vascular cell adhesion molecule-1 in vivo. Correlation with CD3+ T cell infiltration. J Immunol. 1992 Nov 1;149(9):2954–2960. [PubMed] [Google Scholar]
- Briscoe D. M., Schoen F. J., Rice G. E., Bevilacqua M. P., Ganz P., Pober J. S. Induced expression of endothelial-leukocyte adhesion molecules in human cardiac allografts. Transplantation. 1991 Feb;51(2):537–539. [PubMed] [Google Scholar]
- Carlos T., Gordon D., Fishbein D., Himes V. E., Coday A., Ross R., Allen M. D. Vascular cell adhesion molecule-1 is induced on endothelium during acute rejection in human cardiac allografts. J Heart Lung Transplant. 1992 Nov-Dec;11(6):1103–1109. [PubMed] [Google Scholar]
- Chatenoud L., Ferran C., Legendre C., Thouard I., Merite S., Reuter A., Gevaert Y., Kreis H., Franchimont P., Bach J. F. In vivo cell activation following OKT3 administration. Systemic cytokine release and modulation by corticosteroids. Transplantation. 1990 Apr;49(4):697–702. doi: 10.1097/00007890-199004000-00009. [DOI] [PubMed] [Google Scholar]
- Corry R. J., Winn H. J., Russell P. S. Heart transplantation in congenic strains of mice. Transplant Proc. 1973 Mar;5(1):733–735. [PubMed] [Google Scholar]
- Dallman M. J., Larsen C. P., Morris P. J. Cytokine gene transcription in vascularised organ grafts: analysis using semiquantitative polymerase chain reaction. J Exp Med. 1991 Aug 1;174(2):493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestijn A. M., Kerkhove M., Bargatze R. F., Butcher E. C. Lymphoid tissue- and inflammation-specific endothelial cell differentiation defined by monoclonal antibodies. J Immunol. 1987 Feb 1;138(3):713–719. [PubMed] [Google Scholar]
- Edwards M. J., Abney D. L., Miller F. N. Pentoxifylline inhibits interleukin-2-induced leukocyte-endothelial adherence and reduces systemic toxicity. Surgery. 1991 Aug;110(2):199–204. [PubMed] [Google Scholar]
- Ferran C., Dy M., Sheehan K., Merite S., Schreiber R., Landais P., Grau G., Bluestone J., Bach J. F., Chatenoud L. Inter-mouse strain differences in the in vivo anti-CD3 induced cytokine release. Clin Exp Immunol. 1991 Dec;86(3):537–543. doi: 10.1111/j.1365-2249.1991.tb02966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran C., Sheehan K., Dy M., Schreiber R., Merite S., Landais P., Noel L. H., Grau G., Bluestone J., Bach J. F. Cytokine-related syndrome following injection of anti-CD3 monoclonal antibody: further evidence for transient in vivo T cell activation. Eur J Immunol. 1990 Mar;20(3):509–515. doi: 10.1002/eji.1830200308. [DOI] [PubMed] [Google Scholar]
- Finkelman F. D., Madden K. B., Morris S. C., Holmes J. M., Boiani N., Katona I. M., Maliszewski C. R. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993 Aug 1;151(3):1235–1244. [PubMed] [Google Scholar]
- Hession C., Moy P., Tizard R., Chisholm P., Williams C., Wysk M., Burkly L., Miyake K., Kincade P., Lobb R. Cloning of murine and rat vascular cell adhesion molecule-1. Biochem Biophys Res Commun. 1992 Feb 28;183(1):163–169. doi: 10.1016/0006-291x(92)91623-x. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppink D. M., Bishop D. K., Sedmak D. D., Henry M. L., Ferguson R. M., Streeter P. R., Butcher E. C., Orosz C. G. Inducible expression of an endothelial cell antigen on murine myocardial vasculature in association with interstitial cellular infiltration. Transplantation. 1989 Nov;48(5):874–877. doi: 10.1097/00007890-198911000-00032. [DOI] [PubMed] [Google Scholar]
- Miyake K., Weissman I. L., Greenberger J. S., Kincade P. W. Evidence for a role of the integrin VLA-4 in lympho-hemopoiesis. J Exp Med. 1991 Mar 1;173(3):599–607. doi: 10.1084/jem.173.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler K. M., Torrance D. S., Smith C. A., Goodwin R. G., Stremler K. E., Fung V. P., Madani H., Widmer M. B. Soluble tumor necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both TNF carriers and TNF antagonists. J Immunol. 1993 Aug 1;151(3):1548–1561. [PubMed] [Google Scholar]
- Morgan C. J., Pelletier R. P., Hernandez C. J., Teske D. L., Huang E., Ohye R., Orosz C. G., Ferguson R. M. Alloantigen-dependent endothelial phenotype and lymphokine mRNA expression in rejecting murine cardiac allografts. Transplantation. 1993 Apr;55(4):919–924. doi: 10.1097/00007890-199304000-00042. [DOI] [PubMed] [Google Scholar]
- Mosley B., Beckmann M. P., March C. J., Idzerda R. L., Gimpel S. D., VandenBos T., Friend D., Alpert A., Anderson D., Jackson J. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989 Oct 20;59(2):335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- Pelletier R. P., Morgan C. J., Sedmak D. D., Miyake K., Kincade P. W., Ferguson R. M., Orosz C. G. Analysis of inflammatory endothelial changes, including VCAM-1 expression, in murine cardiac grafts. Transplantation. 1993 Feb;55(2):315–320. doi: 10.1097/00007890-199302000-00017. [DOI] [PubMed] [Google Scholar]
- Pober J. S., Cotran R. S. Cytokines and endothelial cell biology. Physiol Rev. 1990 Apr;70(2):427–451. doi: 10.1152/physrev.1990.70.2.427. [DOI] [PubMed] [Google Scholar]
- Sato T. A., Widmer M. B., Finkelman F. D., Madani H., Jacobs C. A., Grabstein K. H., Maliszewski C. R. Recombinant soluble murine IL-4 receptor can inhibit or enhance IgE responses in vivo. J Immunol. 1993 Apr 1;150(7):2717–2723. [PubMed] [Google Scholar]
- Singer J. W., Bianco J. A., Takahashi G., Simrell C., Petersen J., Andrews D. F., 3rd Effect of methylxanthine derivatives on T cell activation. Bone Marrow Transplant. 1992 Jul;10(1):19–25. [PubMed] [Google Scholar]
- Swerlick R. A., Lee K. H., Li L. J., Sepp N. T., Caughman S. W., Lawley T. J. Regulation of vascular cell adhesion molecule 1 on human dermal microvascular endothelial cells. J Immunol. 1992 Jul 15;149(2):698–705. [PubMed] [Google Scholar]
- Taylor P. M., Rose M. L., Yacoub M. H., Pigott R. Induction of vascular adhesion molecules during rejection of human cardiac allografts. Transplantation. 1992 Sep;54(3):451–457. doi: 10.1097/00007890-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Thornhill M. H., Wellicome S. M., Mahiouz D. L., Lanchbury J. S., Kyan-Aung U., Haskard D. O. Tumor necrosis factor combines with IL-4 or IFN-gamma to selectively enhance endothelial cell adhesiveness for T cells. The contribution of vascular cell adhesion molecule-1-dependent and -independent binding mechanisms. J Immunol. 1991 Jan 15;146(2):592–598. [PubMed] [Google Scholar]




