Abstract
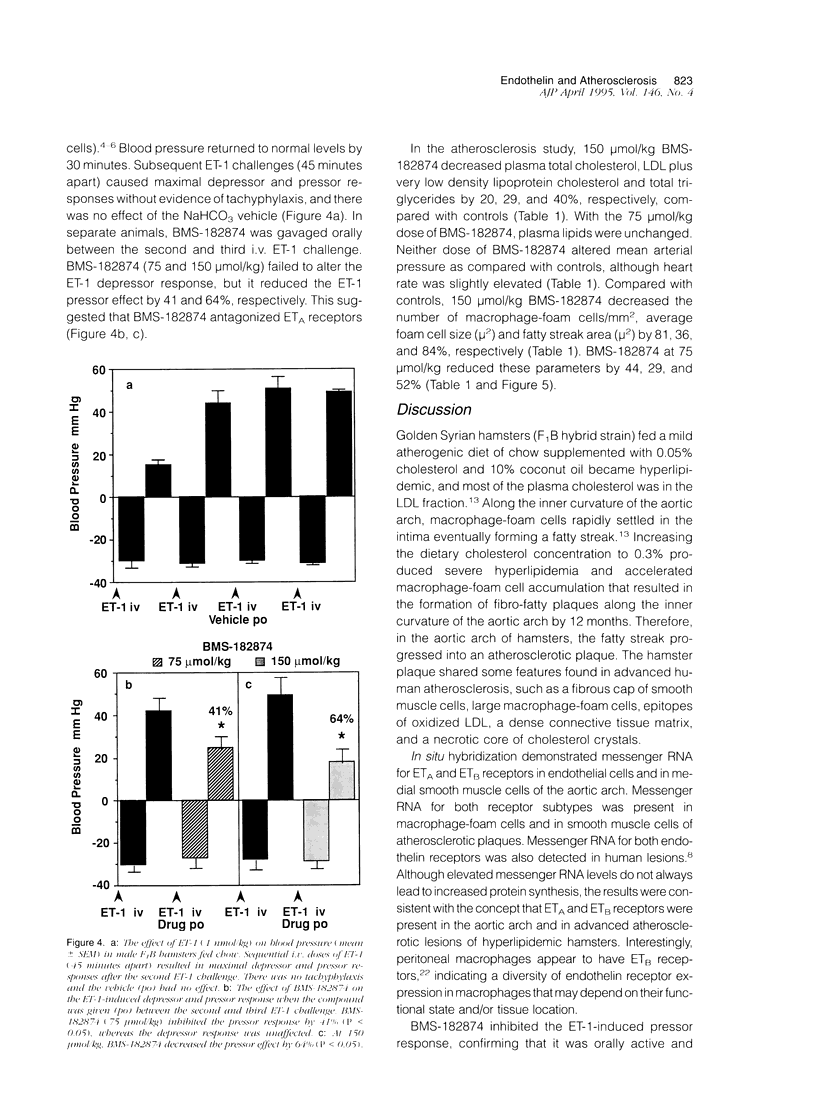
Recent studies suggest that endothelin and its receptors may be involved in atherogenesis. To test this hypothesis, cholesterol-fed hamsters were treated with a selective endothelin subtype A (ETA) receptor antagonist BMS-182874. Characterization of hamster atherosclerotic plaques indicated that they contained a fibrous cap of smooth muscle cells, large macrophage-foam cells, and epitopes of oxidized low density lipoprotein. Messenger RNA for both ETA and ETB receptors was detected in aortic endothelial cells, in medial smooth muscle cells, and in macrophage-foam cells and smooth muscle cells of the fibro-fatty plaques. BMS-182874 inhibited the endothelin-1-induced pressor response whereas the depressor effect was unaltered, suggesting that vascular ETA receptors were selectively blocked in vivo. In hyperlipidemic hamsters, BMS-182874 decreased the area of the fatty streak by reducing the number and size of macrophage-foam cells. The results indicated that ETA receptors and thus endothelin promoted the early inflammatory phase of atherosclerosis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achmad T. H., Rao G. S. Chemotaxis of human blood monocytes toward endothelin-1 and the influence of calcium channel blockers. Biochem Biophys Res Commun. 1992 Dec 15;189(2):994–1000. doi: 10.1016/0006-291x(92)92302-e. [DOI] [PubMed] [Google Scholar]
- Arai H., Hori S., Aramori I., Ohkubo H., Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990 Dec 20;348(6303):730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Boulanger C. M., Tanner F. C., Béa M. L., Hahn A. W., Werner A., Lüscher T. F. Oxidized low density lipoproteins induce mRNA expression and release of endothelin from human and porcine endothelium. Circ Res. 1992 Jun;70(6):1191–1197. doi: 10.1161/01.res.70.6.1191. [DOI] [PubMed] [Google Scholar]
- Hayzer D. J., Rose P. M., Lynch J. S., Webb M. L., Kienzle B. K., Liu E. C., Bogosian E. A., Brinson E., Runge M. S. Cloning and expression of a human endothelin receptor: subtype A. Am J Med Sci. 1992 Oct;304(4):231–238. doi: 10.1097/00000441-199210000-00002. [DOI] [PubMed] [Google Scholar]
- Ihara M., Noguchi K., Saeki T., Fukuroda T., Tsuchida S., Kimura S., Fukami T., Ishikawa K., Nishikibe M., Yano M. Biological profiles of highly potent novel endothelin antagonists selective for the ETA receptor. Life Sci. 1992;50(4):247–255. doi: 10.1016/0024-3205(92)90331-i. [DOI] [PubMed] [Google Scholar]
- Kowala M. C., Nunnari J. J., Durham S. K., Nicolosi R. J. Doxazosin and cholestyramine similarly decrease fatty streak formation in the aortic arch of hyperlipidemic hamsters. Atherosclerosis. 1991 Nov;91(1-2):35–49. doi: 10.1016/0021-9150(91)90185-6. [DOI] [PubMed] [Google Scholar]
- Lerman A., Edwards B. S., Hallett J. W., Heublein D. M., Sandberg S. M., Burnett J. C., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991 Oct 3;325(14):997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Martin-Nizard F., Houssaini H. S., Lestavel-Delattre S., Duriez P., Fruchart J. C. Modified low density lipoproteins activate human macrophages to secrete immunoreactive endothelin. FEBS Lett. 1991 Nov 18;293(1-2):127–130. doi: 10.1016/0014-5793(91)81167-7. [DOI] [PubMed] [Google Scholar]
- Masaki T., Kimura S., Yanagisawa M., Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991 Oct;84(4):1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- Ohlstein E. H., Arleth A., Bryan H., Elliott J. D., Sung C. P. The selective endothelin ETA receptor antagonist BQ123 antagonizes endothelin-1-mediated mitogenesis. Eur J Pharmacol. 1992 Apr 10;225(4):347–350. doi: 10.1016/0922-4106(92)90109-9. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Sakurai T., Yanagisawa M., Takuwa Y., Miyazaki H., Kimura S., Goto K., Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990 Dec 20;348(6303):732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Stein P. D., Hunt J. T., Floyd D. M., Moreland S., Dickinson K. E., Mitchell C., Liu E. C., Webb M. L., Murugesan N., Dickey J. The discovery of sulfonamide endothelin antagonists and the development of the orally active ETA antagonist 5-(dimethylamino)-N-(3,4-dimethyl-5-isoxazolyl)-1-naphthalenesulf onamide. J Med Chem. 1994 Feb 4;37(3):329–331. doi: 10.1021/jm00029a001. [DOI] [PubMed] [Google Scholar]
- Winkles J. A., Alberts G. F., Brogi E., Libby P. Endothelin-1 and endothelin receptor mRNA expression in normal and atherosclerotic human arteries. Biochem Biophys Res Commun. 1993 Mar 31;191(3):1081–1088. doi: 10.1006/bbrc.1993.1327. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]






