Abstract
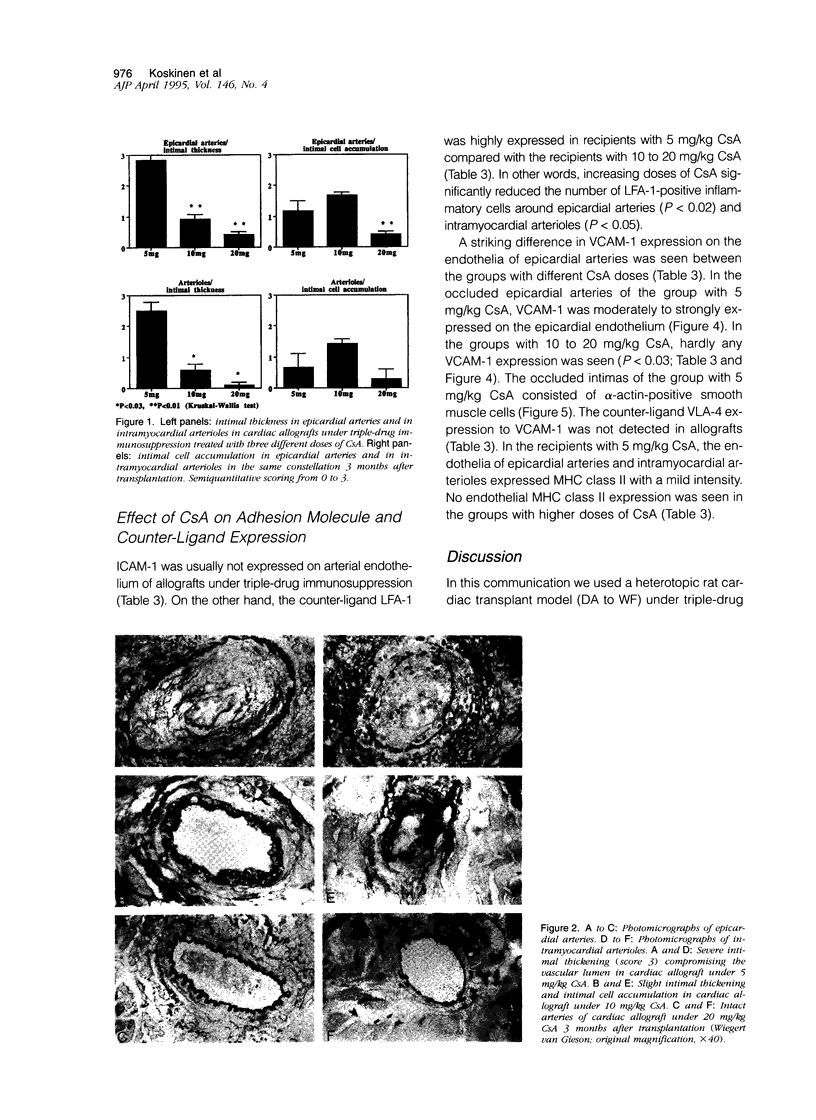
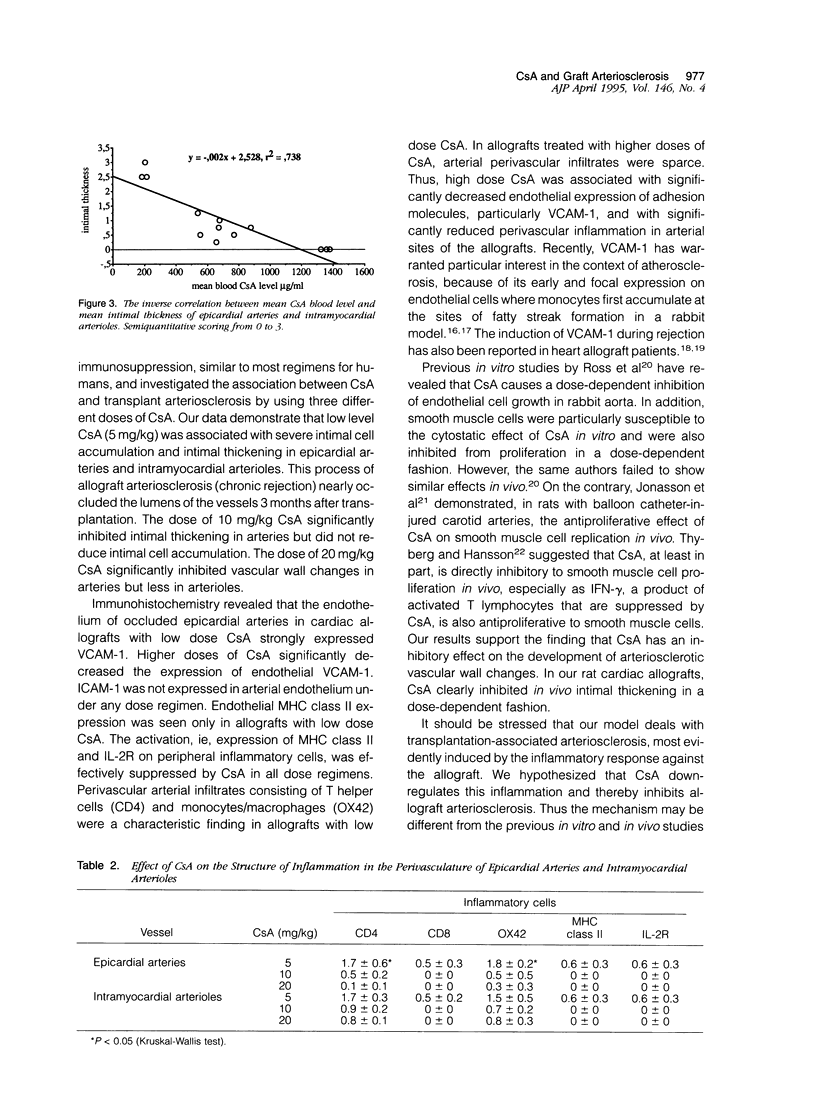
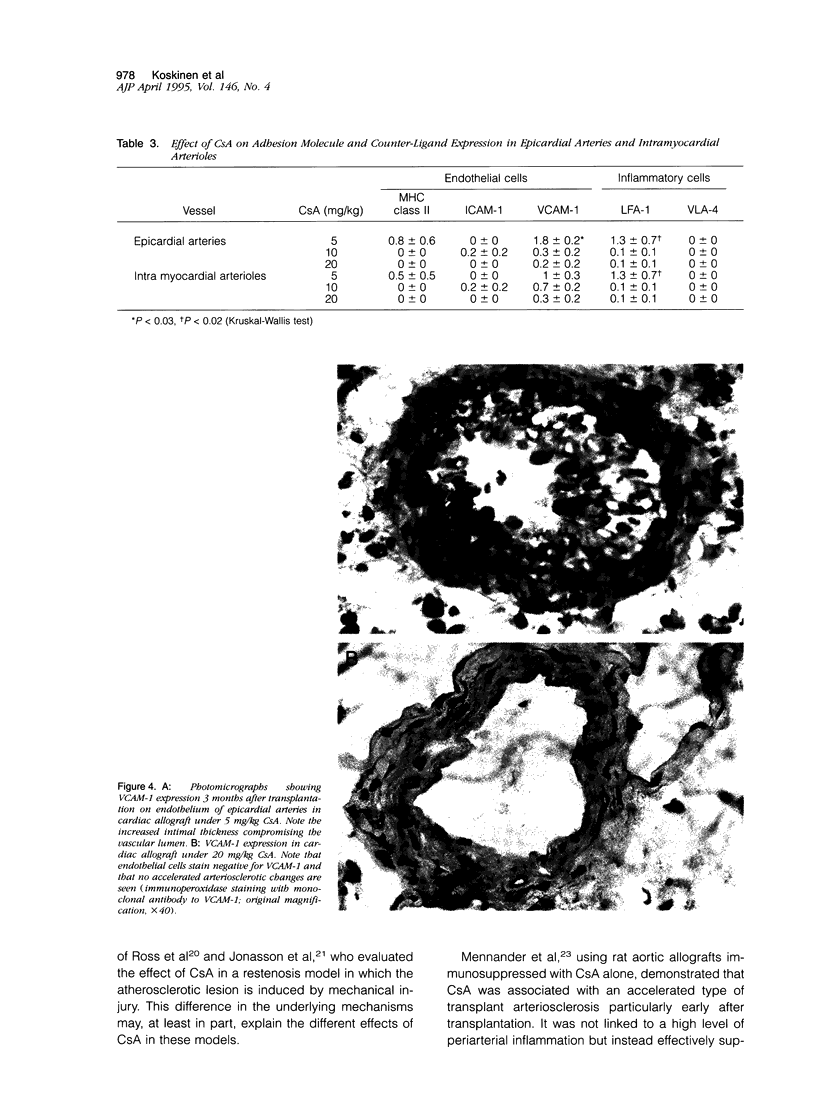
Accelerated allograft arteriosclerosis (chronic rejection) has emerged as a major factor affecting long-term survival of human cardiac allografts. The underlying mechanism of this disorder remains unclear. The purpose of this study was to investigate the effect of cyclosporine on the development of cardiac allograft arteriosclerosis at the cellular and molecular level. Heterotopic rat cardiac allografts from DA donors to WF recipients, with a strong genetic disparity in major histocompatibility complex and non-major histocompatibility complex loci, were used. The allograft recipients received triple-drug immunosuppression consisting of methylprednisolone (0.5 mg/kg/day), azathioprine (2 mg/kg/day), and three different doses of cyclosporine A (CsA; 5, 10, and 20 mg/kg/day). The grafts were removed 3 months after transplantation and processed for histology and immunohistochemistry. Low dose CsA (5 mg/kg/day) was associated with a severe form of intimal cell accumulation and intimal thickening in epicardial arteries and in smaller intramyocardial arterioles with nearly occluded vessel lumens 3 months after transplantation. The intermediate dose CsA (10 mg/kg/day) significantly inhibited arterial intimal thickening but was not efficient in reducing intimal cell accumulation. Instead, high dose CsA (20 mg/kg/day) significantly inhibited all arteriosclerotic vascular wall changes in the allografts. Immunohistochemistry revealed that the occluded epicardial arteries of cardiac allografts with low dose CsA expressed VCAM-1 on the endothelium. Higher CsA doses significantly reduced the expression of endothelial VCAM-1. Neither ICAM-1 nor major histocompatibility complex class II were expressed. Perivascular arterial infiltrates consisting of T helper cells and monocytes/macrophages were a characteristic finding in the allograft with low dose CsA. In the allografts treated with higher doses of CsA, arterial perivascular infiltrates were seldom seen. Our results conclusively demonstrate that sufficient immunosuppression with CsA inhibits intimal thickening and intimal cell accumulation of long-surviving rat cardiac allografts in a dose-dependent fashion. Arteriosclerotic alterations associated with increased expression of arterial endothelial VCAM-1 were totally down-regulated by high doses of CsA.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almond P. S., Matas A., Gillingham K., Dunn D. L., Payne W. D., Gores P., Gruessner R., Najarian J. S. Risk factors for chronic rejection in renal allograft recipients. Transplantation. 1993 Apr;55(4):752–757. doi: 10.1097/00007890-199304000-00013. [DOI] [PubMed] [Google Scholar]
- Barnhart G. R., Pascoe E. A., Mills A. S., Szentpetery S., Eich D. M., Mohanakumar T., Hastillo A., Thompson J. A., Hess M. L., Lower R. R. Accelerated coronary arteriosclerosis in cardiac transplant recipients. Transplant Rev (Orlando) 1987;1:31–46. doi: 10.1016/s0955-470x(87)80004-6. [DOI] [PubMed] [Google Scholar]
- Billingham M. E. Cardiac transplant atherosclerosis. Transplant Proc. 1987 Aug;19(4 Suppl 5):19–25. [PubMed] [Google Scholar]
- Billingham M. E., Cary N. R., Hammond M. E., Kemnitz J., Marboe C., McCallister H. A., Snovar D. C., Winters G. L., Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990 Nov-Dec;9(6):587–593. [PubMed] [Google Scholar]
- Billingham M. E. Graft coronary disease: the lesions and the patients. Transplant Proc. 1989 Aug;21(4):3665–3666. [PubMed] [Google Scholar]
- Carlos T., Gordon D., Fishbein D., Himes V. E., Coday A., Ross R., Allen M. D. Vascular cell adhesion molecule-1 is induced on endothelium during acute rejection in human cardiac allografts. J Heart Lung Transplant. 1992 Nov-Dec;11(6):1103–1109. [PubMed] [Google Scholar]
- Ferns G., Reidy M., Ross R. Vascular effects of cyclosporine A in vivo and in vitro. Am J Pathol. 1990 Aug;137(2):403–413. [PMC free article] [PubMed] [Google Scholar]
- Handa N., Hatanaka M., Baumgartner W. A., Reitz B. A., Sandford G., Esa A. H., Herskowitz A. Late cyclosporine treatment ameliorates established coronary graft disease in rat allografts. Transplantation. 1993 Sep;56(3):535–540. doi: 10.1097/00007890-199309000-00009. [DOI] [PubMed] [Google Scholar]
- Hruban R. H., Beschorner W. E., Baumgartner W. A., Augustine S. M., Ren H., Reitz B. A., Hutchins G. M. Accelerated arteriosclerosis in heart transplant recipients is associated with a T-lymphocyte-mediated endothelialitis. Am J Pathol. 1990 Oct;137(4):871–882. [PMC free article] [PubMed] [Google Scholar]
- Isoniemi H., Krogerus L., von Willebrand E., Taskinen E., Grönhagen-Riska C., Ahonen J., Häyry P. Renal allograft immunosuppression. VI. Triple drug therapy versus immunosuppressive double drug combinations: histopathological findings in renal allografts. Transpl Int. 1991 Sep;4(3):151–156. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Hansson G. K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2303–2306. doi: 10.1073/pnas.85.7.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen P. K., Krogerus L. A., Nieminen M. S., Mattila S. P., Häyry P. J., Lautenschlager I. T. Quantitation of cytomegalovirus infection-associated histologic findings in endomyocardial biopsies of heart allografts. J Heart Lung Transplant. 1993 May-Jun;12(3):343–354. [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993 Feb;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- Lindholm A., Kahan B. D. Influence of cyclosporine pharmacokinetics, trough concentrations, and AUC monitoring on outcome after kidney transplantation. Clin Pharmacol Ther. 1993 Aug;54(2):205–218. doi: 10.1038/clpt.1993.132. [DOI] [PubMed] [Google Scholar]
- Mennander A., Tiisala S., Paavonen T., Halttunen J., Häyry P. Chronic rejection of rat aortic allograft. II. Administration of cyclosporin induces accelerated allograft arteriosclerosis. Transpl Int. 1991 Sep;4(3):173–179. [PubMed] [Google Scholar]
- Ono K., Lindsey E. S. Improved technique of heart transplantation in rats. J Thorac Cardiovasc Surg. 1969 Feb;57(2):225–229. [PubMed] [Google Scholar]
- Richardson M., Hadcock S. J., DeReske M., Cybulsky M. I. Increased expression in vivo of VCAM-1 and E-selectin by the aortic endothelium of normolipemic and hyperlipemic diabetic rabbits. Arterioscler Thromb. 1994 May;14(5):760–769. doi: 10.1161/01.atv.14.5.760. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Vaughan D. E., Rudd M. A., Mudge G. H., Kirshenbaum J., Young P., Alexander R. W., Loscalzo J. Frequency of hypercholesterolemia after cardiac transplantation. Am J Cardiol. 1988 Dec 1;62(17):1268–1272. doi: 10.1016/0002-9149(88)90272-x. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Rose M. L., Yacoub M. H., Pigott R. Induction of vascular adhesion molecules during rejection of human cardiac allografts. Transplantation. 1992 Sep;54(3):451–457. doi: 10.1097/00007890-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hansson G. K. Cyclosporine A inhibits induction of DNA synthesis by PDGF and other peptide mitogens in cultured rat aortic smooth muscle cells and dermal fibroblasts. Growth Factors. 1991;4(3):209–219. doi: 10.3109/08977199109104817. [DOI] [PubMed] [Google Scholar]
- Turunen J. P., Mattila P., Halttunen J., Häyry P., Renkonen R. Evidence that lymphocyte traffic into rejecting cardiac allografts is CD11a- and CD49d-dependent. Transplantation. 1992 Dec;54(6):1053–1058. doi: 10.1097/00007890-199212000-00020. [DOI] [PubMed] [Google Scholar]
- Uretsky B. F., Murali S., Reddy P. S., Rabin B., Lee A., Griffith B. P., Hardesty R. L., Trento A., Bahnson H. T. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- Yilmaz S., Taskinen E., Paavonen T., Mennander A., Häyry P. Chronic rejection of rat renal allograft. I. Histological differentiation between chronic rejection and cyclosporin nephrotoxicity. Transpl Int. 1992 May;5(2):85–95. [PubMed] [Google Scholar]