Abstract
Thirty-two primary carcinomas of the lung and 17 carcinomas of the head and neck (HN) were systematically analyzed for p53 mutations in the highly conserved regions of the gene (exons 5-8). Frozen sections of the same tumors were stained immunohistochemically to assess the sensitivity and specificity of p53 expression as determined by the presence or absence of the protein. On the basis of histology, the lung tumors studied were divided into adenocarcinomas (AC; n = 15), squamous-cell carcinomas (SCC; n = 12), and large-cell carcinomas (LCC; n = 5). All the HN cancers were SCC. Mutations in the p53 gene were detected by direct sequencing of amplified polymerase chain reaction products in six AC of the lungs (40%), three SCC of the lungs (25%), and one LCC (20%), with an overall mutation frequency of 31%. Nine AC (60%) of the lungs, five SCC (42%), and four LCC (80%) were p53-positive by immunohistochemistry. Among HN cancers, p53 mutations were detected in seven tumors (41%). Nine HN tumors (53%) were positive for p53. Negative staining, despite the presence of p53 mutations, was confined to nonsense mutations with truncated p53 and to single-base mutations not causing any change in the amino acid. Although immunohistochemical staining for mutated p53 is sensitive and simple to perform as a screening method, it is not as specific for evaluation of p53 mutations in lung and HN cancers.
Full text
PDF




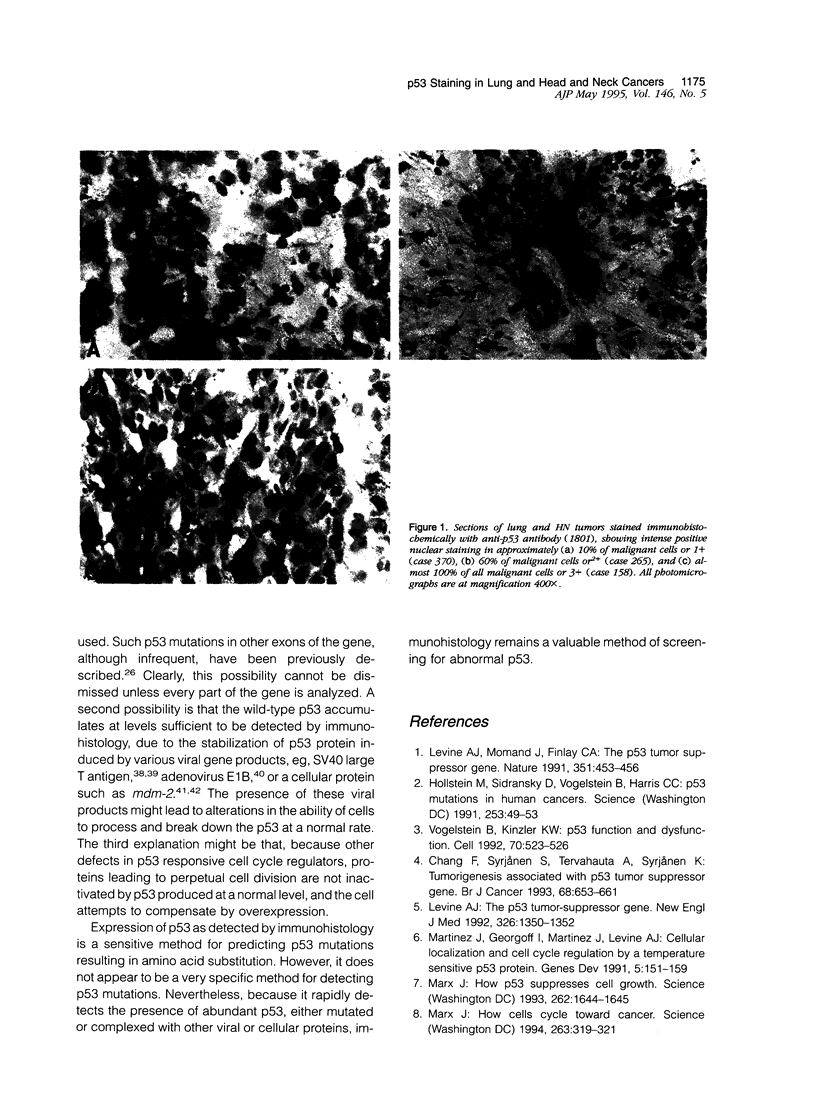


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battifora H. p53 immunohistochemistry: a word of caution. Hum Pathol. 1994 May;25(5):435–437. doi: 10.1016/0046-8177(94)90113-9. [DOI] [PubMed] [Google Scholar]
- Bennett W. P., Colby T. V., Travis W. D., Borkowski A., Jones R. T., Lane D. P., Metcalf R. A., Samet J. M., Takeshima Y., Gu J. R. p53 protein accumulates frequently in early bronchial neoplasia. Cancer Res. 1993 Oct 15;53(20):4817–4822. [PubMed] [Google Scholar]
- Boyle J. O., Hakim J., Koch W., van der Riet P., Hruban R. H., Roa R. A., Correo R., Eby Y. J., Ruppert J. M., Sidransky D. The incidence of p53 mutations increases with progression of head and neck cancer. Cancer Res. 1993 Oct 1;53(19):4477–4480. [PubMed] [Google Scholar]
- Brambilla E., Gazzeri S., Moro D., Caron de Fromentel C., Gouyer V., Jacrot M., Brambilla C. Immunohistochemical study of p53 in human lung carcinomas. Am J Pathol. 1993 Jul;143(1):199–210. [PMC free article] [PubMed] [Google Scholar]
- Burns J. E., Baird M. C., Clark L. J., Burns P. A., Edington K., Chapman C., Mitchell R., Robertson G., Soutar D., Parkinson E. K. Gene mutations and increased levels of p53 protein in human squamous cell carcinomas and their cell lines. Br J Cancer. 1993 Jun;67(6):1274–1284. doi: 10.1038/bjc.1993.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caamano J., Zhang S. Y., Rosvold E. A., Bauer B., Klein-Szanto A. J. p53 alterations in human squamous cell carcinomas and carcinoma cell lines. Am J Pathol. 1993 Apr;142(4):1131–1139. [PMC free article] [PubMed] [Google Scholar]
- Chang F., Syrjänen S., Tervahauta A., Syrjänen K. Tumourigenesis associated with the p53 tumour suppressor gene. Br J Cancer. 1993 Oct;68(4):653–661. doi: 10.1038/bjc.1993.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba I., Takahashi T., Nau M. M., D'Amico D., Curiel D. T., Mitsudomi T., Buchhagen D. L., Carbone D., Piantadosi S., Koga H. Mutations in the p53 gene are frequent in primary, resected non-small cell lung cancer. Lung Cancer Study Group. Oncogene. 1990 Oct;5(10):1603–1610. [PubMed] [Google Scholar]
- Donehower L. A., Bradley A. The tumor suppressor p53. Biochim Biophys Acta. 1993 Aug 23;1155(2):181–205. doi: 10.1016/0304-419x(93)90004-v. [DOI] [PubMed] [Google Scholar]
- Field J. K., Pavelic Z. P., Spandidos D. A., Stambrook P. J., Jones A. S., Gluckman J. L. The role of the p53 tumor suppressor gene in squamous cell carcinoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1993 Oct;119(10):1118–1122. doi: 10.1001/archotol.1993.01880220064009. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Harris C. C. p53: at the crossroads of molecular carcinogenesis and risk assessment. Science. 1993 Dec 24;262(5142):1980–1981. doi: 10.1126/science.8266092. [DOI] [PubMed] [Google Scholar]
- Hirano T., Franzén B., Kato H., Ebihara Y., Auer G. Genesis of squamous cell lung carcinoma. Sequential changes of proliferation, DNA ploidy, and p53 expression. Am J Pathol. 1994 Feb;144(2):296–302. [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Hunter T. Braking the cycle. Cell. 1993 Dec 3;75(5):839–841. doi: 10.1016/0092-8674(93)90528-x. [DOI] [PubMed] [Google Scholar]
- Iggo R., Gatter K., Bartek J., Lane D., Harris A. L. Increased expression of mutant forms of p53 oncogene in primary lung cancer. Lancet. 1990 Mar 24;335(8691):675–679. doi: 10.1016/0140-6736(90)90801-b. [DOI] [PubMed] [Google Scholar]
- Kern S. E., Pietenpol J. A., Thiagalingam S., Seymour A., Kinzler K. W., Vogelstein B. Oncogenic forms of p53 inhibit p53-regulated gene expression. Science. 1992 May 8;256(5058):827–830. doi: 10.1126/science.1589764. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y., Murakami Y., Shiraishi M., Hayashi K., Sekiya T. Aberrations of the p53 tumor suppressor gene in human non-small cell carcinomas of the lung. Cancer Res. 1992 Sep 1;52(17):4799–4804. [PubMed] [Google Scholar]
- Lane D. P., Benchimol S. p53: oncogene or anti-oncogene? Genes Dev. 1990 Jan;4(1):1–8. doi: 10.1101/gad.4.1.1. [DOI] [PubMed] [Google Scholar]
- Law J. C., Strong L. C., Chidambaram A., Ferrell R. E. A germ line mutation in exon 5 of the p53 gene in an extended cancer family. Cancer Res. 1991 Dec 1;51(23 Pt 1):6385–6387. [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990 Aug;177(2):419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Levine A. J. The p53 tumor-suppressor gene. N Engl J Med. 1992 May 14;326(20):1350–1352. doi: 10.1056/NEJM199205143262008. [DOI] [PubMed] [Google Scholar]
- Li Z. H., Zheng J., Weiss L. M., Shibata D. c-k-ras and p53 mutations occur very early in adenocarcinoma of the lung. Am J Pathol. 1994 Feb;144(2):303–309. [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Georgoff I., Martinez J., Levine A. J. Cellular localization and cell cycle regulation by a temperature-sensitive p53 protein. Genes Dev. 1991 Feb;5(2):151–159. doi: 10.1101/gad.5.2.151. [DOI] [PubMed] [Google Scholar]
- Marx J. How cells cycle toward cancer. Science. 1994 Jan 21;263(5145):319–321. doi: 10.1126/science.8278804. [DOI] [PubMed] [Google Scholar]
- Marx J. How p53 suppresses cell growth. Science. 1993 Dec 10;262(5140):1644–1645. doi: 10.1126/science.8259506. [DOI] [PubMed] [Google Scholar]
- Miller C. W., Simon K., Aslo A., Kok K., Yokota J., Buys C. H., Terada M., Koeffler H. P. p53 mutations in human lung tumors. Cancer Res. 1992 Apr 1;52(7):1695–1698. [PubMed] [Google Scholar]
- Momand J., Zambetti G. P., Olson D. C., George D., Levine A. J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992 Jun 26;69(7):1237–1245. doi: 10.1016/0092-8674(92)90644-r. [DOI] [PubMed] [Google Scholar]
- Nees M., Homann N., Discher H., Andl T., Enders C., Herold-Mende C., Schuhmann A., Bosch F. X. Expression of mutated p53 occurs in tumor-distant epithelia of head and neck cancer patients: a possible molecular basis for the development of multiple tumors. Cancer Res. 1993 Sep 15;53(18):4189–4196. [PubMed] [Google Scholar]
- Ogden G. R., Kiddie R. A., Lunny D. P., Lane D. P. Assessment of p53 protein expression in normal, benign, and malignant oral mucosa. J Pathol. 1992 Apr;166(4):389–394. doi: 10.1002/path.1711660411. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Porter P. L., Gown A. M., Kramp S. G., Coltrera M. D. Widespread p53 overexpression in human malignant tumors. An immunohistochemical study using methacarn-fixed, embedded tissue. Am J Pathol. 1992 Jan;140(1):145–153. [PMC free article] [PubMed] [Google Scholar]
- Quinlan D. C., Davidson A. G., Summers C. L., Warden H. E., Doshi H. M. Accumulation of p53 protein correlates with a poor prognosis in human lung cancer. Cancer Res. 1992 Sep 1;52(17):4828–4831. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Schmieg F. I., Simmons D. T. Characterization of the in vitro interaction between SV40 T antigen and p53: mapping the p53 binding site. Virology. 1988 May;164(1):132–140. doi: 10.1016/0042-6822(88)90628-9. [DOI] [PubMed] [Google Scholar]
- Shin D. M., Kim J., Ro J. Y., Hittelman J., Roth J. A., Hong W. K., Hittelman W. N. Activation of p53 gene expression in premalignant lesions during head and neck tumorigenesis. Cancer Res. 1994 Jan 15;54(2):321–326. [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- Wynford-Thomas D. P53 in tumour pathology: can we trust immunocytochemistry? J Pathol. 1992 Apr;166(4):329–330. doi: 10.1002/path.1711660402. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]



