Abstract
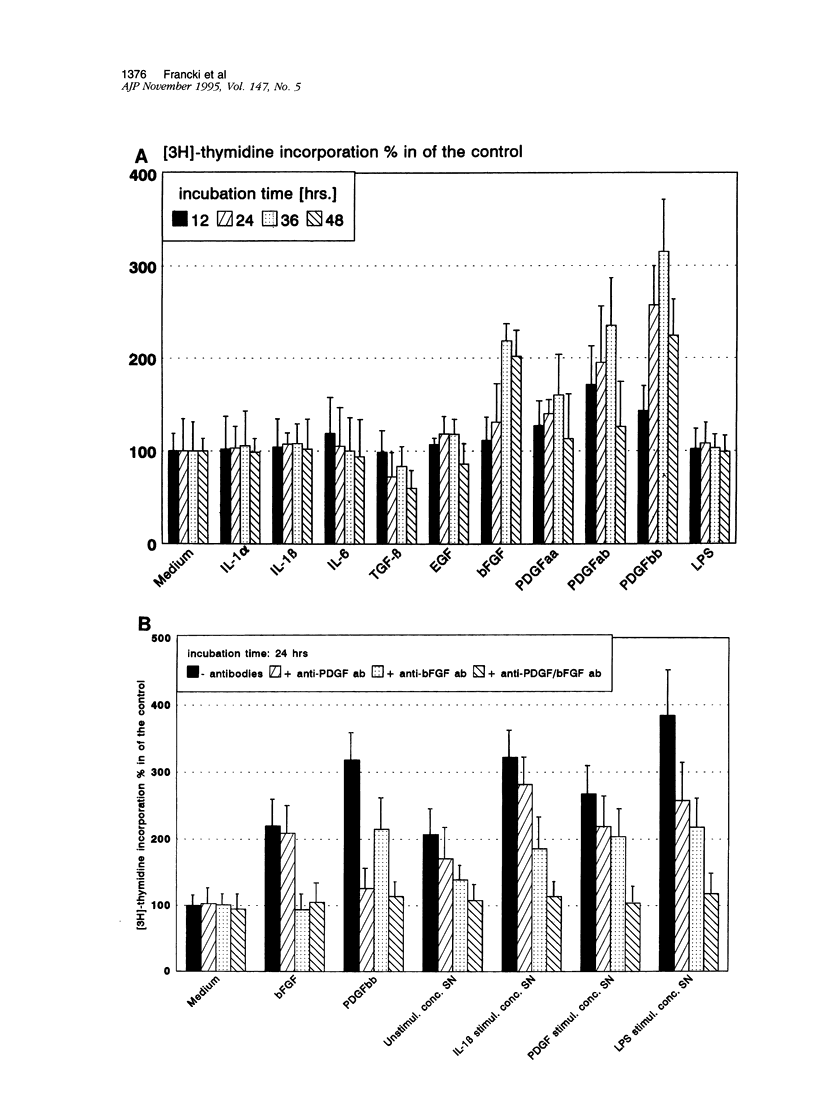
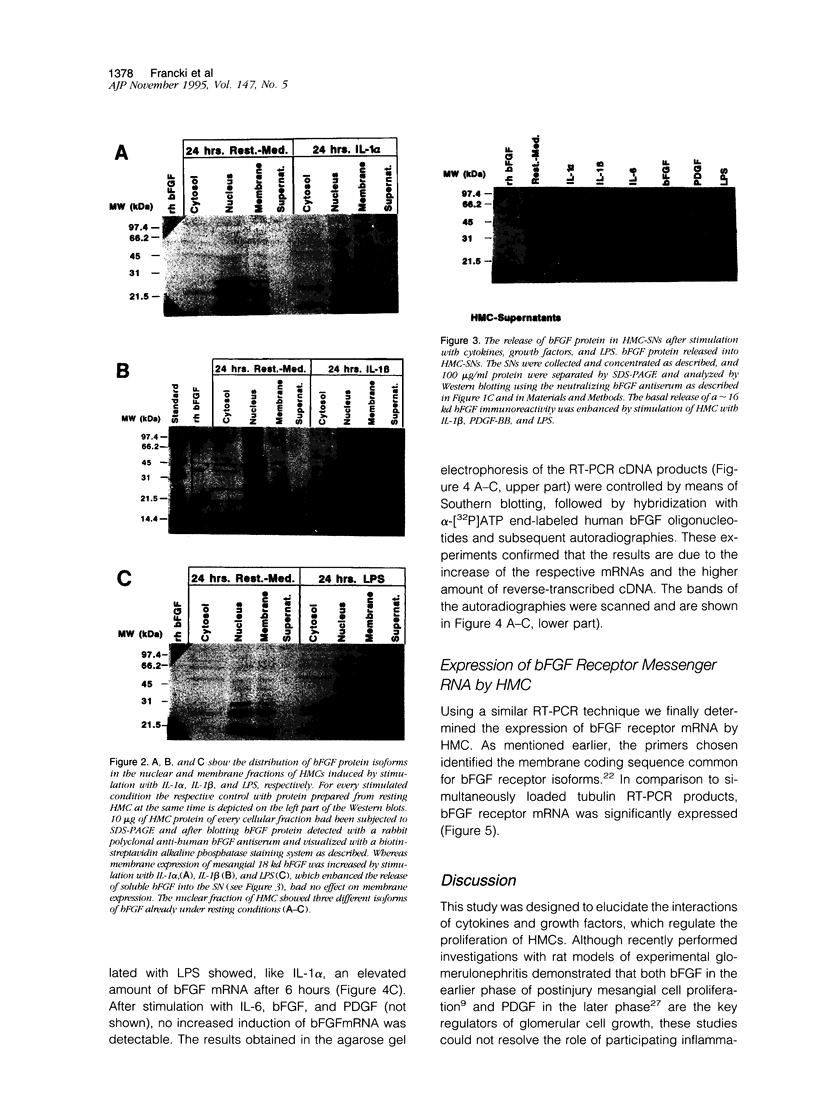
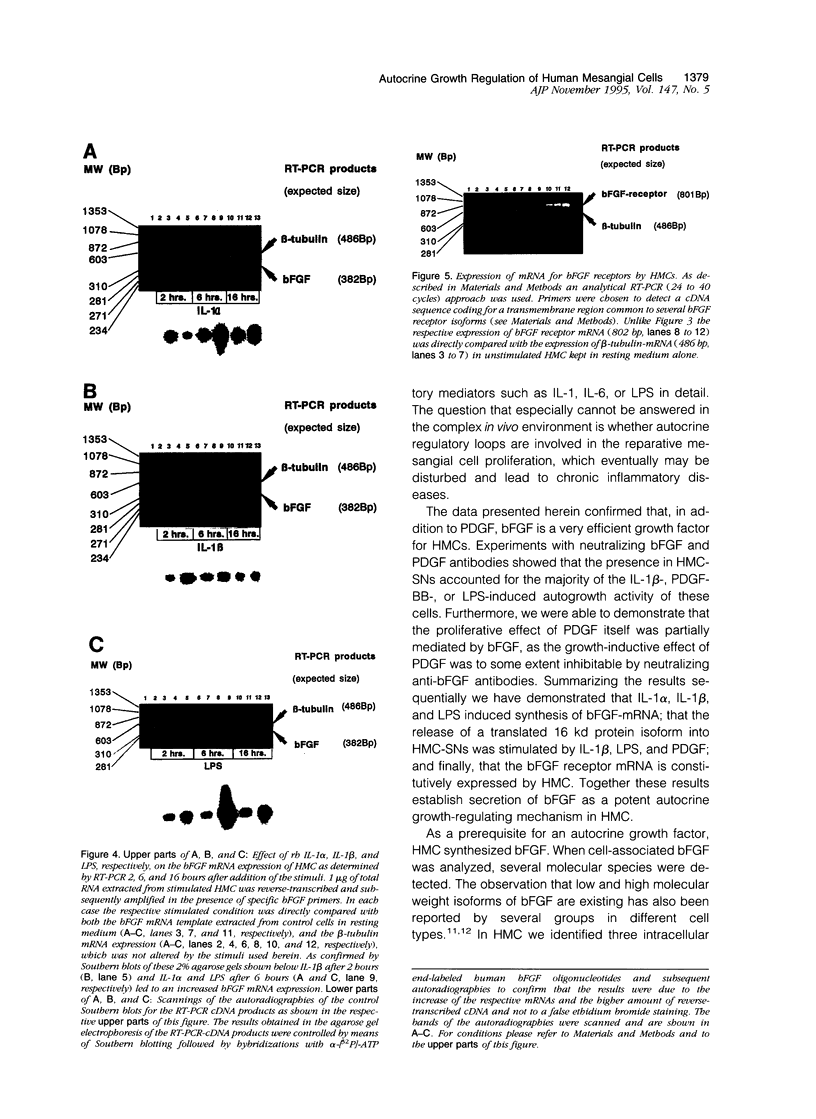
For various forms of human glomerulonephritis a close relationship between inflammatory injury and a local mesangial proliferative response has been described. Herein, we used primary cultures of human glomerular mesangial cells (HMCs) from five different donors to determine the autocrine growth-inducing capacity of their supernatants after stimulation with different cytokines and lipopolysaccharide (LPS) to determine whether this effect is due to basic fibroblast growth factor (bFGF). The basal growth-inducing capacity of supernatants collected from serum-free cultured HMC and concentrated 100-fold above a cut-off size of 10 kd was significantly increased by interleukin (IL)-1 beta, platelet-derived growth factor (PDGF), and LPS up to 15-fold, but not by IL-1 alpha, IL-6, or bFGF. An anti-human bFGF antibody blocked the majority of IL-1 or LPS-induced proliferative effect of supernatants; complete inhibition was achieved by a combination of anti-human bFGF- and anti-human platelet-derived growth factor antibodies. HMCs express different isoforms of bFGF (18, 21.5, and 24 kd) in membrane, cytosolic, and nuclear fractions. All isoforms of bFGF were found in the nuclear fraction of HMC, whether stimulated or not. Immunoblots for bFGF protein of HMC demonstrated that only a approximate to 16 kd bFGF protein was released into HMC supernatants after stimulation with IL-1 beta, platelet-derived growth factor-BB, and LPS. The 18 kd isoform of bFGF accumulated in the membranes but was not released after stimulation with IL-1 alpha, IL-6, and bFGF, suggesting that its release was a prerequisite for autocrine growth stimulation. By means of reverse transcription polymerase chain reaction controlled by Southern blots, bFGF-mRNA expression of HMC was enhanced by IL-1 alpha, IL-1 beta, and LPS. Finally, we were able to show that HMCs are expressing bFGF receptors. In summary, our data demonstrate for the first time that the autocrine proliferative response of HMC to major inflammatory factors may primarily be mediated by bFGF.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott F., Ryan J. J., Ceska M., Matsushima K., Sarraf C. E., Rees A. J. Interleukin-1 beta stimulates human mesangial cells to synthesize and release interleukins-6 and -8. Kidney Int. 1991 Oct;40(4):597–605. doi: 10.1038/ki.1991.250. [DOI] [PubMed] [Google Scholar]
- Alpers C. E., Hudkins K. L., Gown A. M., Johnson R. J. Enhanced expression of "muscle-specific" actin in glomerulonephritis. Kidney Int. 1992 May;41(5):1134–1142. doi: 10.1038/ki.1992.173. [DOI] [PubMed] [Google Scholar]
- Baldin V., Roman A. M., Bosc-Bierne I., Amalric F., Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990 May;9(5):1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore P. A. Modes of FGF release in vivo and in vitro. Cancer Metastasis Rev. 1990 Nov;9(3):227–238. doi: 10.1007/BF00046362. [DOI] [PubMed] [Google Scholar]
- Dono R., Zeller R. Cell-type-specific nuclear translocation of fibroblast growth factor-2 isoforms during chicken kidney and limb morphogenesis. Dev Biol. 1994 Jun;163(2):316–330. doi: 10.1006/dbio.1994.1151. [DOI] [PubMed] [Google Scholar]
- Floege J., Burns M. W., Alpers C. E., Yoshimura A., Pritzl P., Gordon K., Seifert R. A., Bowen-Pope D. F., Couser W. G., Johnson R. J. Glomerular cell proliferation and PDGF expression precede glomerulosclerosis in the remnant kidney model. Kidney Int. 1992 Feb;41(2):297–309. doi: 10.1038/ki.1992.42. [DOI] [PubMed] [Google Scholar]
- Floege J., Eng E., Lindner V., Alpers C. E., Young B. A., Reidy M. A., Johnson R. J. Rat glomerular mesangial cells synthesize basic fibroblast growth factor. Release, upregulated synthesis, and mitogenicity in mesangial proliferative glomerulonephritis. J Clin Invest. 1992 Dec;90(6):2362–2369. doi: 10.1172/JCI116126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Eng E., Young B. A., Johnson R. J. Factors involved in the regulation of mesangial cell proliferation in vitro and in vivo. Kidney Int Suppl. 1993 Jan;39:S47–S54. [PubMed] [Google Scholar]
- Floege J., Topley N., Hoppe J., Barrett T. B., Resch K. Mitogenic effect of platelet-derived growth factor in human glomerular mesangial cells: modulation and/or suppression by inflammatory cytokines. Clin Exp Immunol. 1991 Nov;86(2):334–341. doi: 10.1111/j.1365-2249.1991.tb05819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floege J., Topley N., Resch K. Regulation of mesangial cell proliferation. Am J Kidney Dis. 1991 Jun;17(6):673–676. doi: 10.1016/s0272-6386(12)80349-0. [DOI] [PubMed] [Google Scholar]
- Floege J., Topley N., Wessel K., Kaever V., Radeke H., Hoppe J., Kishimoto T., Resch K. Monokines and platelet-derived growth factor modulate prostanoid production in growth arrested, human mesangial cells. Kidney Int. 1990 Mar;37(3):859–869. doi: 10.1038/ki.1990.59. [DOI] [PubMed] [Google Scholar]
- Gibran N. S., Isik F. F., Heimbach D. M., Gordon D. Basic fibroblast growth factor in the early human burn wound. J Surg Res. 1994 Mar;56(3):226–234. doi: 10.1006/jsre.1994.1036. [DOI] [PubMed] [Google Scholar]
- Isacchi A., Bergonzoni L., Sarmientos P. Complete sequence of a human receptor for acidic and basic fibroblast growth factors. Nucleic Acids Res. 1990 Apr 11;18(7):1906–1906. doi: 10.1093/nar/18.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. J., Raines E. W., Floege J., Yoshimura A., Pritzl P., Alpers C., Ross R. Inhibition of mesangial cell proliferation and matrix expansion in glomerulonephritis in the rat by antibody to platelet-derived growth factor. J Exp Med. 1992 May 1;175(5):1413–1416. doi: 10.1084/jem.175.5.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashgarian M., Sterzel R. B. The pathobiology of the mesangium. Kidney Int. 1992 Mar;41(3):524–529. doi: 10.1038/ki.1992.74. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Smith S., Sullivan R., Shing Y., Davidson S., Smith J. A., Sasse J. Multiple forms of basic fibroblast growth factor: amino-terminal cleavages by tumor cell- and brain cell-derived acid proteinases. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1839–1843. doi: 10.1073/pnas.84.7.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahr S., Schreiner G., Ichikawa I. The progression of renal disease. N Engl J Med. 1988 Jun 23;318(25):1657–1666. doi: 10.1056/NEJM198806233182505. [DOI] [PubMed] [Google Scholar]
- Li Y., Koga M., Kasayama S., Matsumoto K., Arita N., Hayakawa T., Sato B. Identification and characterization of high molecular weight forms of basic fibroblast growth factor in human pituitary adenomas. J Clin Endocrinol Metab. 1992 Dec;75(6):1436–1441. doi: 10.1210/jcem.75.6.1464644. [DOI] [PubMed] [Google Scholar]
- Mené P., Simonson M. S., Dunn M. J. Physiology of the mesangial cell. Physiol Rev. 1989 Oct;69(4):1347–1424. doi: 10.1152/physrev.1989.69.4.1347. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Morimoto T., Rifkin D. B. Basic fibroblast growth factor, a protein devoid of secretory signal sequence, is released by cells via a pathway independent of the endoplasmic reticulum-Golgi complex. J Cell Physiol. 1992 Apr;151(1):81–93. doi: 10.1002/jcp.1041510113. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Sato Y., Knee R. S. Phosphorothioate antisense oligonucleotides against basic fibroblast growth factor inhibit anchorage-dependent and anchorage-independent growth of a malignant glioblastoma cell line. Mol Endocrinol. 1992 Jun;6(6):877–884. doi: 10.1210/mend.6.6.1323055. [DOI] [PubMed] [Google Scholar]
- Quarto N., Finger F. P., Rifkin D. B. The NH2-terminal extension of high molecular weight bFGF is a nuclear targeting signal. J Cell Physiol. 1991 May;147(2):311–318. doi: 10.1002/jcp.1041470217. [DOI] [PubMed] [Google Scholar]
- Radeke H. H., Cross A. R., Hancock J. T., Jones O. T., Nakamura M., Kaever V., Resch K. Functional expression of NADPH oxidase components (alpha- and beta-subunits of cytochrome b558 and 45-kDa flavoprotein) by intrinsic human glomerular mesangial cells. J Biol Chem. 1991 Nov 5;266(31):21025–21029. [PubMed] [Google Scholar]
- Radeke H. H., Gessner J. E., Uciechowski P., Mägert H. J., Schmidt R. E., Resch K. Intrinsic human glomerular mesangial cells can express receptors for IgG complexes (hFc gamma RIII-A) and the associated Fc epsilon RI gamma-chain. J Immunol. 1994 Aug 1;153(3):1281–1292. [PubMed] [Google Scholar]
- Radeke H. H., Meier B., Topley N., Flöge J., Habermehl G. G., Resch K. Interleukin 1-alpha and tumor necrosis factor-alpha induce oxygen radical production in mesangial cells. Kidney Int. 1990 Feb;37(2):767–775. doi: 10.1038/ki.1990.44. [DOI] [PubMed] [Google Scholar]
- Radeke H. H., Resch K. The inflammatory function of renal glomerular mesangial cells and their interaction with the cellular immune system. Clin Investig. 1992 Sep;70(9):825–842. doi: 10.1007/BF00180754. [DOI] [PubMed] [Google Scholar]
- Speir E., Sasse J., Shrivastav S., Casscells W. Culture-induced increase in acidic and basic fibroblast growth factor activities and their association with the nuclei of vascular endothelial and smooth muscle cells. J Cell Physiol. 1991 May;147(2):362–373. doi: 10.1002/jcp.1041470223. [DOI] [PubMed] [Google Scholar]
- Sterzel R. B., Schulze-Lohoff E., Marx M. Cytokines and mesangial cells. Kidney Int Suppl. 1993 Jan;39:S26–S31. [PubMed] [Google Scholar]
- Stevenson F. T., Torrano F., Locksley R. M., Lovett D. H. Interleukin 1: the patterns of translation and intracellular distribution support alternative secretory mechanisms. J Cell Physiol. 1992 Aug;152(2):223–231. doi: 10.1002/jcp.1041520202. [DOI] [PubMed] [Google Scholar]
- Striker L. J., Peten E. P., Elliot S. J., Doi T., Striker G. E. Mesangial cell turnover: effect of heparin and peptide growth factors. Lab Invest. 1991 Apr;64(4):446–456. [PubMed] [Google Scholar]
- Vlodavsky I., Bar-Shavit R., Ishai-Michaeli R., Bashkin P., Fuks Z. Extracellular sequestration and release of fibroblast growth factor: a regulatory mechanism? Trends Biochem Sci. 1991 Jul;16(7):268–271. doi: 10.1016/0968-0004(91)90102-2. [DOI] [PubMed] [Google Scholar]
- Werber H. I., Emancipator S. N., Tykocinski M. L., Sedor J. R. The interleukin 1 gene is expressed by rat glomerular mesangial cells and is augmented in immune complex glomerulonephritis. J Immunol. 1987 May 15;138(10):3207–3212. [PubMed] [Google Scholar]
- Zoja C., Wang J. M., Bettoni S., Sironi M., Renzi D., Chiaffarino F., Abboud H. E., Van Damme J., Mantovani A., Remuzzi G. Interleukin-1 beta and tumor necrosis factor-alpha induce gene expression and production of leukocyte chemotactic factors, colony-stimulating factors, and interleukin-6 in human mesangial cells. Am J Pathol. 1991 Apr;138(4):991–1003. [PMC free article] [PubMed] [Google Scholar]