Abstract
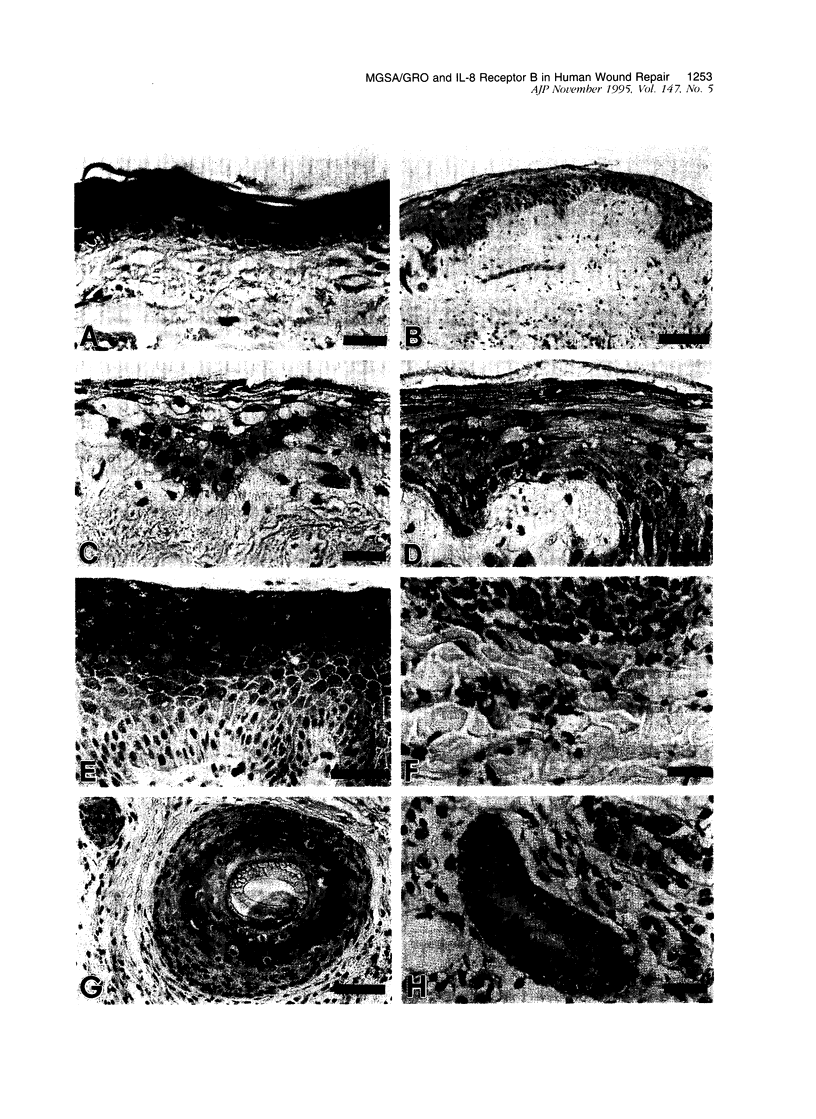
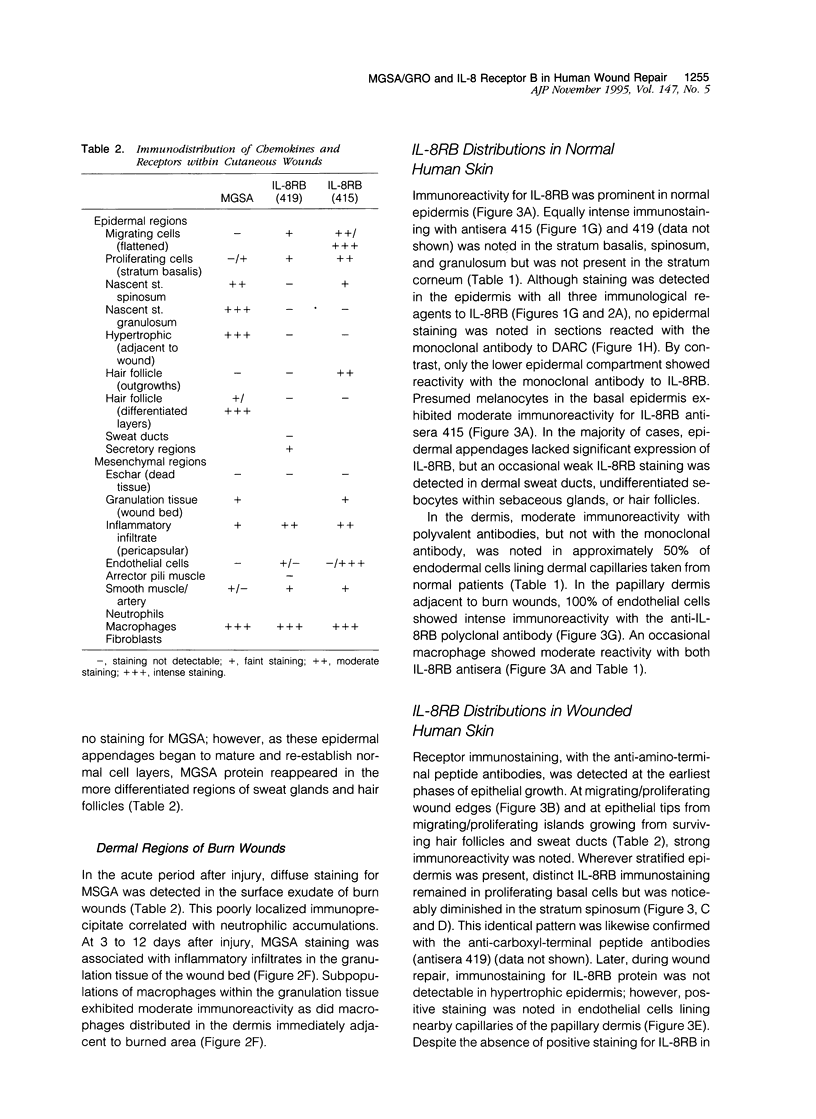
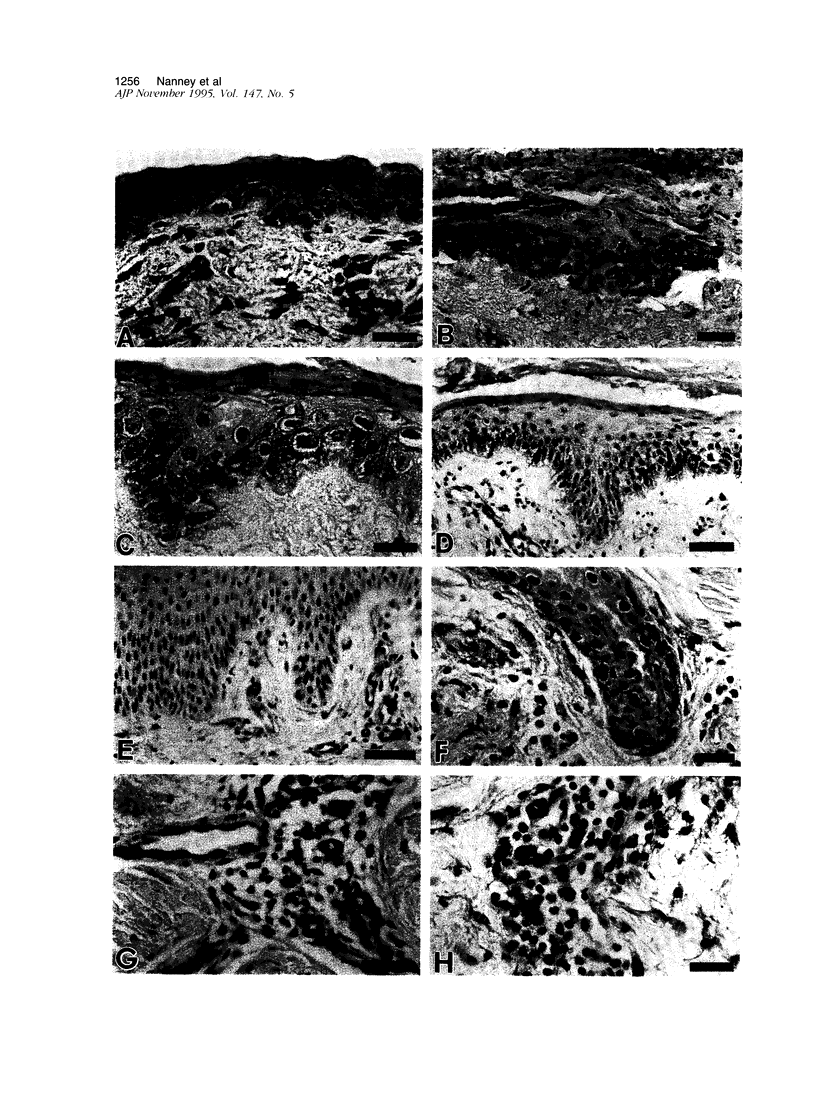
The alpha-chemokines have been implicated as regulators of proliferation and differentiation of normal keratinocytes and as mediators of keratinocyte maturation and migration in inflammatory processes that involve the skin. Using the cutaneous wound repair model, we examined the sites and temporal sequence of the appearance of melanoma growth stimulatory activity or growth-regulated gene (MGSA/GRO;ligand) and the type B interleukin (IL)-8 receptor (IL-8RB) to which MGSA/GRO binds. Human burn tissues (n = 44) representing days 2 to 12 after injury were obtained during surgical debridement, fixed in 4% paraformaldehyde, and embedded in paraffin. Immunolocalizations were performed with polyclonal antisera for both ligand and receptor, as well as a monoclonal antibody for the IL-8 RB. Western blot analysis confirmed the presence of the IL-8 RB in immunoprecipitates of epidermal keratinocyte lysates. In normal skin, MGSA/GRO protein was restricted to sites populated by differentiated keratinocytes (suprabasal compartments, inner root sheath cells, and dermal sweat ducts). MGSA/GRO protein was barely detectable within epithelial margins and islands of burn wounds where the migrating/proliferating keratinocyte populations reside, but staining intensities increased as cells matured into the outer layers. Weak diffuse staining was detected in areas of neutrophilic infiltration (granulation tissue and overlying exudates). By contrast, in normal skin the IL-8 RB was detected in specific locations within epidermal and dermal compartments of healing wounds. In the dermis, polyvalent antibodies detected receptor immunoreactivity most prominently in dermal sweat ducts and endothelium of capillaries, whereas this immunoreactivity was inconspicuous in sections stained with the monoclonal antibody. Receptor immunostaining was noted in migrating/proliferating keratinocytes in epithelial margins and islands but was in the outer layers or in hypertrophic epidermis adjacent to wounds. This same pattern was observed in epidermal appendages such as hair follicles and eccrine sweat ducts. In granulation tissues, IL-8 RB was noted in numerous fibroblasts and in subpopulations of macrophages and smooth muscle. The presence of both MGSA/GRO and its receptor in human burn wounds implicate this cytokine as an autocrine or paracrine mediator of epidermal regeneration in both the inflammatory and proliferative phases of cutaneous wound repair.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. B., Manthey C. L., Hand A. R., Ohura K., Ellingsworth L., Wahl S. M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor beta. J Exp Med. 1990 Jan 1;171(1):231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila H. S., Reitamo S., Erkko P., Ceska M., Moser B., Baggiolini M. Interleukin-8 immunoreactivity in the skin of healthy subjects and patients with palmoplantar pustulosis and psoriasis. J Invest Dermatol. 1992 Jan;98(1):96–101. doi: 10.1111/1523-1747.ep12495817. [DOI] [PubMed] [Google Scholar]
- Beckmann M. P., Munger W. E., Kozlosky C., VandenBos T., Price V., Lyman S., Gerard N. P., Gerard C., Cerretti D. P. Molecular characterization of the interleukin-8 receptor. Biochem Biophys Res Commun. 1991 Sep 16;179(2):784–789. doi: 10.1016/0006-291x(91)91885-g. [DOI] [PubMed] [Google Scholar]
- Boorsma D. M., de Haan P., Willemze R., Stoof T. J. Human growth factor (huGRO), interleukin-8 (IL-8) and interferon-gamma-inducible protein (gamma-IP-10) gene expression in cultured normal human keratinocytes. Arch Dermatol Res. 1994;286(8):471–475. doi: 10.1007/BF00371574. [DOI] [PubMed] [Google Scholar]
- Bucala R., Ritchlin C., Winchester R., Cerami A. Constitutive production of inflammatory and mitogenic cytokines by rheumatoid synovial fibroblasts. J Exp Med. 1991 Mar 1;173(3):569–574. doi: 10.1084/jem.173.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerretti D. P., Kozlosky C. J., Vanden Bos T., Nelson N., Gearing D. P., Beckmann M. P. Molecular characterization of receptors for human interleukin-8, GRO/melanoma growth-stimulatory activity and neutrophil activating peptide-2. Mol Immunol. 1993 Mar;30(4):359–367. doi: 10.1016/0161-5890(93)90065-j. [DOI] [PubMed] [Google Scholar]
- Chabot-Fletcher M., Breton J., Lee J., Young P., Griswold D. E. Interleukin-8 production is regulated by protein kinase C in human keratinocytes. J Invest Dermatol. 1994 Oct;103(4):509–515. doi: 10.1111/1523-1747.ep12395658. [DOI] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Bascom C. C., Sipes N. J., Graves-Deal R., Weissman B. E., Moses H. L. Selective inhibition of growth-related gene expression in murine keratinocytes by transforming growth factor beta. Mol Cell Biol. 1988 Aug;8(8):3088–3093. doi: 10.1128/mcb.8.8.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubitt C. L., Tang Q., Monteiro C. A., Lausch R. N., Oakes J. E. IL-8 gene expression in cultures of human corneal epithelial cells and keratocytes. Invest Ophthalmol Vis Sci. 1993 Oct;34(11):3199–3206. [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Zhang Q. Y., Eisen D., Krust A., Kastner P., Chambon P., Voorhees J. J. Retinoic acid receptor gene expression in human skin. J Invest Dermatol. 1991 Apr;96(4):425–433. doi: 10.1111/1523-1747.ep12469889. [DOI] [PubMed] [Google Scholar]
- Fava R. A., Olsen N. J., Postlethwaite A. E., Broadley K. N., Davidson J. M., Nanney L. B., Lucas C., Townes A. S. Transforming growth factor beta 1 (TGF-beta 1) induced neutrophil recruitment to synovial tissues: implications for TGF-beta-driven synovial inflammation and hyperplasia. J Exp Med. 1991 May 1;173(5):1121–1132. doi: 10.1084/jem.173.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner W. L., Rodriguez J. L., Miller C. G., Till G. O., Rees R. S., Smith D. J., Remick D. G. Acute skin injury releases neutrophil chemoattractants. Surgery. 1994 Jul;116(1):42–48. [PubMed] [Google Scholar]
- Glick A. B., Lee M. M., Darwiche N., Kulkarni A. B., Karlsson S., Yuspa S. H. Targeted deletion of the TGF-beta 1 gene causes rapid progression to squamous cell carcinoma. Genes Dev. 1994 Oct 15;8(20):2429–2440. doi: 10.1101/gad.8.20.2429. [DOI] [PubMed] [Google Scholar]
- Holmes W. E., Lee J., Kuang W. J., Rice G. C., Wood W. I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1278–1280. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- Horuk R., Chitnis C. E., Darbonne W. C., Colby T. J., Rybicki A., Hadley T. J., Miller L. H. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993 Aug 27;261(5125):1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- Horuk R., Colby T. J., Darbonne W. C., Schall T. J., Neote K. The human erythrocyte inflammatory peptide (chemokine) receptor. Biochemical characterization, solubilization, and development of a binding assay for the soluble receptor. Biochemistry. 1993 Jun 8;32(22):5733–5738. doi: 10.1021/bi00073a002. [DOI] [PubMed] [Google Scholar]
- Horuk R., Yansura D. G., Reilly D., Spencer S., Bourell J., Henzel W., Rice G., Unemori E. Purification, receptor binding analysis, and biological characterization of human melanoma growth stimulating activity (MGSA). Evidence for a novel MGSA receptor. J Biol Chem. 1993 Jan 5;268(1):541–546. [PubMed] [Google Scholar]
- Kemény L., Ruzicka T., Dobozy A., Michel G. Role of interleukin-8 receptor in skin. Int Arch Allergy Immunol. 1994 Aug;104(4):317–322. doi: 10.1159/000236686. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kojima T., Cromie M. A., Fisher G. J., Voorhees J. J., Elder J. T. GRO-alpha mRNA is selectively overexpressed in psoriatic epidermis and is reduced by cyclosporin A in vivo, but not in cultured keratinocytes. J Invest Dermatol. 1993 Dec;101(6):767–772. doi: 10.1111/1523-1747.ep12371692. [DOI] [PubMed] [Google Scholar]
- Lee J., Horuk R., Rice G. C., Bennett G. L., Camerato T., Wood W. I. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992 Aug 15;267(23):16283–16287. [PubMed] [Google Scholar]
- Michel G., Kemény L., Peter R. U., Beetz A., Ried C., Arenberger P., Ruzicka T. Interleukin-8 receptor-mediated chemotaxis of normal human epidermal cells. FEBS Lett. 1992 Jul 6;305(3):241–243. doi: 10.1016/0014-5793(92)80677-9. [DOI] [PubMed] [Google Scholar]
- Moser B., Barella L., Mattei S., Schumacher C., Boulay F., Colombo M. P., Baggiolini M. Expression of transcripts for two interleukin 8 receptors in human phagocytes, lymphocytes and melanoma cells. Biochem J. 1993 Aug 15;294(Pt 1):285–292. doi: 10.1042/bj2940285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller S. G., Schraw W. P., Richmond A. Melanoma growth stimulatory activity enhances the phosphorylation of the class II interleukin-8 receptor in non-hematopoietic cells. J Biol Chem. 1994 Jan 21;269(3):1973–1980. [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991 Sep 13;253(5025):1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- Neote K., Mak J. Y., Kolakowski L. F., Jr, Schall T. J. Functional and biochemical analysis of the cloned Duffy antigen: identity with the red blood cell chemokine receptor. Blood. 1994 Jul 1;84(1):44–52. [PubMed] [Google Scholar]
- Nickoloff B. J., Karabin G. D., Barker J. N., Griffiths C. E., Sarma V., Mitra R. S., Elder J. T., Kunkel S. L., Dixit V. M. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. Am J Pathol. 1991 Jan;138(1):129–140. [PMC free article] [PubMed] [Google Scholar]
- Odland G., Ross R. Human wound repair. I. Epidermal regeneration. J Cell Biol. 1968 Oct;39(1):135–151. doi: 10.1083/jcb.39.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper S. C., Wang Z. X., Neote K., Martin A. W., Showell H. J., Conklyn M. J., Ogborne K., Hadley T. J., Lu Z. H., Hesselgesser J. The Duffy antigen/receptor for chemokines (DARC) is expressed in endothelial cells of Duffy negative individuals who lack the erythrocyte receptor. J Exp Med. 1995 Apr 1;181(4):1311–1317. doi: 10.1084/jem.181.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond A., Thomas H. G. Melanoma growth stimulatory activity: isolation from human melanoma tumors and characterization of tissue distribution. J Cell Biochem. 1988 Feb;36(2):185–198. doi: 10.1002/jcb.240360209. [DOI] [PubMed] [Google Scholar]
- Salo T., Lyons J. G., Rahemtulla F., Birkedal-Hansen H., Larjava H. Transforming growth factor-beta 1 up-regulates type IV collagenase expression in cultured human keratinocytes. J Biol Chem. 1991 Jun 25;266(18):11436–11441. [PubMed] [Google Scholar]
- Schröder J. M., Gregory H., Young J., Christophers E. Neutrophil-activating proteins in psoriasis. J Invest Dermatol. 1992 Feb;98(2):241–247. doi: 10.1111/1523-1747.ep12556058. [DOI] [PubMed] [Google Scholar]
- Schulz B. S., Michel G., Wagner S., Süss R., Beetz A., Peter R. U., Kemény L., Ruzicka T. Increased expression of epidermal IL-8 receptor in psoriasis. Down-regulation by FK-506 in vitro. J Immunol. 1993 Oct 15;151(8):4399–4406. [PubMed] [Google Scholar]
- Sticherling M., Bornscheuer E., Schröder J. M., Christophers E. Localization of neutrophil-activating peptide-1/interleukin-8-immunoreactivity in normal and psoriatic skin. J Invest Dermatol. 1991 Jan;96(1):26–30. doi: 10.1111/1523-1747.ep12514689. [DOI] [PubMed] [Google Scholar]
- Strange P., Cooper K. D., Hansen E. R., Fisher G., Larsen J. K., Fox D., Krag C., Voorhees J. J., Baadsgaard O. T-lymphocyte clones initiated from lesional psoriatic skin release growth factors that induce keratinocyte proliferation. J Invest Dermatol. 1993 Nov;101(5):695–700. doi: 10.1111/1523-1747.ep12371678. [DOI] [PubMed] [Google Scholar]
- Szekanecz Z., Shah M. R., Harlow L. A., Pearce W. H., Koch A. E. Interleukin-8 and tumor necrosis factor-alpha are involved in human aortic endothelial cell migration. The possible role of these cytokines in human aortic aneurysmal blood vessel growth. Pathobiology. 1994;62(3):134–139. doi: 10.1159/000163891. [DOI] [PubMed] [Google Scholar]
- Tettelbach W., Nanney L., Ellis D., King L., Richmond A. Localization of MGSA/GRO protein in cutaneous lesions. J Cutan Pathol. 1993 Jun;20(3):259–266. doi: 10.1111/j.1600-0560.1993.tb00653.x. [DOI] [PubMed] [Google Scholar]
- Tuschil A., Lam C., Haslberger A., Lindley I. Interleukin-8 stimulates calcium transients and promotes epidermal cell proliferation. J Invest Dermatol. 1992 Sep;99(3):294–298. doi: 10.1111/1523-1747.ep12616634. [DOI] [PubMed] [Google Scholar]
- Wilmer J. L., Burleson F. G., Kayama F., Kanno J., Luster M. I. Cytokine induction in human epidermal keratinocytes exposed to contact irritants and its relation to chemical-induced inflammation in mouse skin. J Invest Dermatol. 1994 Jun;102(6):915–922. doi: 10.1111/1523-1747.ep12383512. [DOI] [PubMed] [Google Scholar]
- Yang Y. C., Yin T. Interleukin-11 and its receptor. Biofactors. 1992 Dec;4(1):15–21. [PubMed] [Google Scholar]
- Yue T. L., Wang X., Sung C. P., Olson B., McKenna P. J., Gu J. L., Feuerstein G. Z. Interleukin-8. A mitogen and chemoattractant for vascular smooth muscle cells. Circ Res. 1994 Jul;75(1):1–7. doi: 10.1161/01.res.75.1.1. [DOI] [PubMed] [Google Scholar]
- de Vos S., Brach M., Budnik A., Grewe M., Herrmann F., Krutmann J. Post-transcriptional regulation of interleukin-6 gene expression in human keratinocytes by ultraviolet B radiation. J Invest Dermatol. 1994 Jul;103(1):92–96. doi: 10.1111/1523-1747.ep12391818. [DOI] [PubMed] [Google Scholar]