Abstract
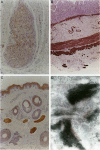
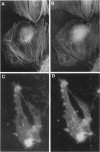
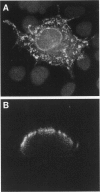
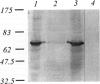
The product of the neurofibromatosis type 2 (NF2) tumor suppressor gene is a 595-amino-acid protein bearing resemblance to a family of band-4.1-related proteins. These proteins, including ezrin, radixin, and moesin, probably function as molecular linking proteins, connecting the cytoskeleton to the cell membrane. On the grounds of the homology to the ezrin, radixin, and moesin proteins and on the basis of its predicted secondary structure, the NF2 protein is also thought to act as a cytoskeleton-cell membrane linking protein. Using monoclonal antibodies to amino- and carboxyl-terminal synthetic NF2 peptides we demonstrate the co-localization of the NF2 protein with elements of the cytoskeleton in a COS cell model system and in cultured human cells. Furthermore, the presence of the NF2 protein in tissue sections is shown. The monoclonal antibodies specifically stain smooth muscle cells and the stratum granulosum of the human epidermis. In cultured smooth muscle cells the NF2 protein co-localizes with actin stress fibers. Immunoelectron microscopy demonstrates the presence of the NF2 protein associated with keratohyalin granules and to a lesser extent with intermediate filaments in the human epidermis. We conclude that the NF2 protein is indeed associated with multiple elements of the cytoskeleton.
Full text
PDF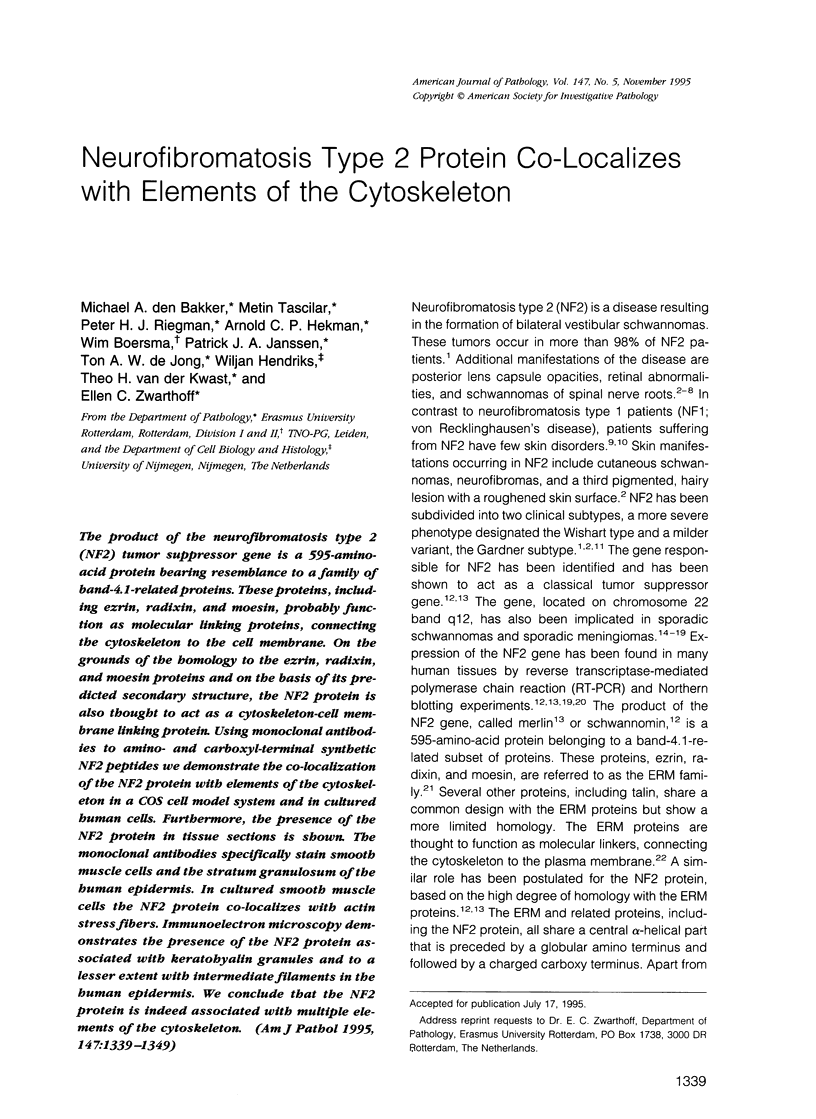



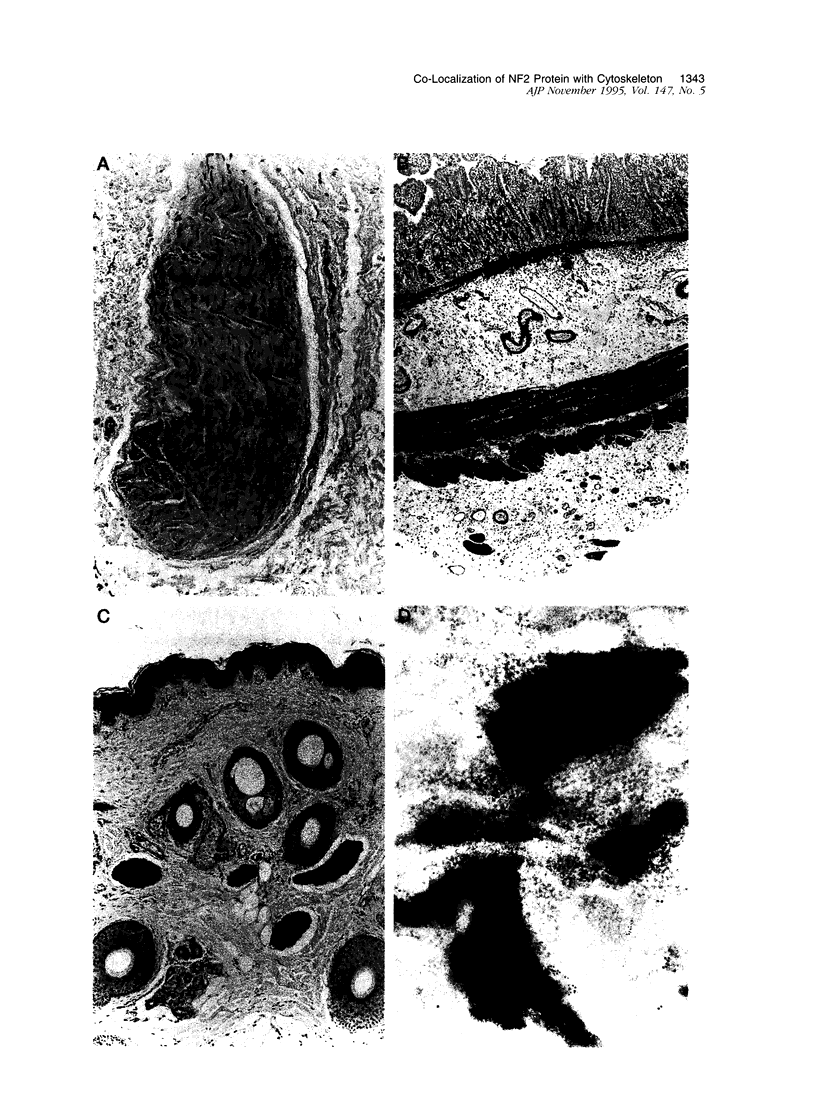
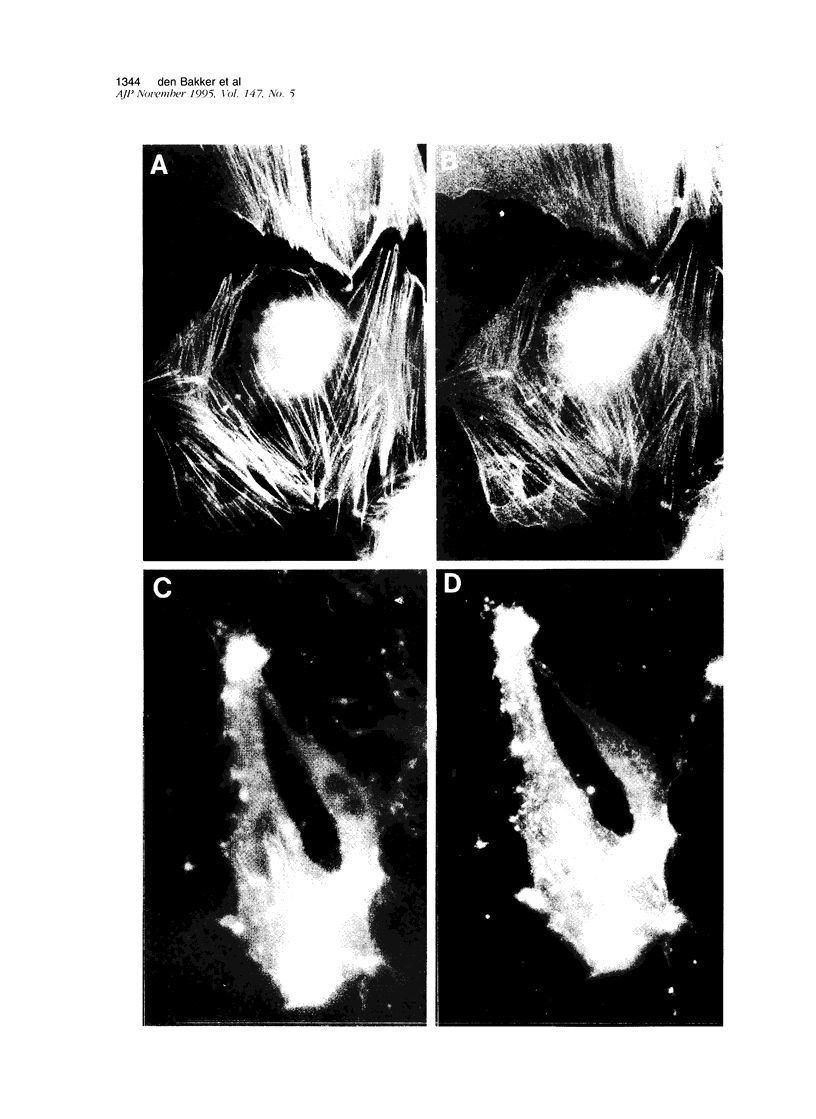
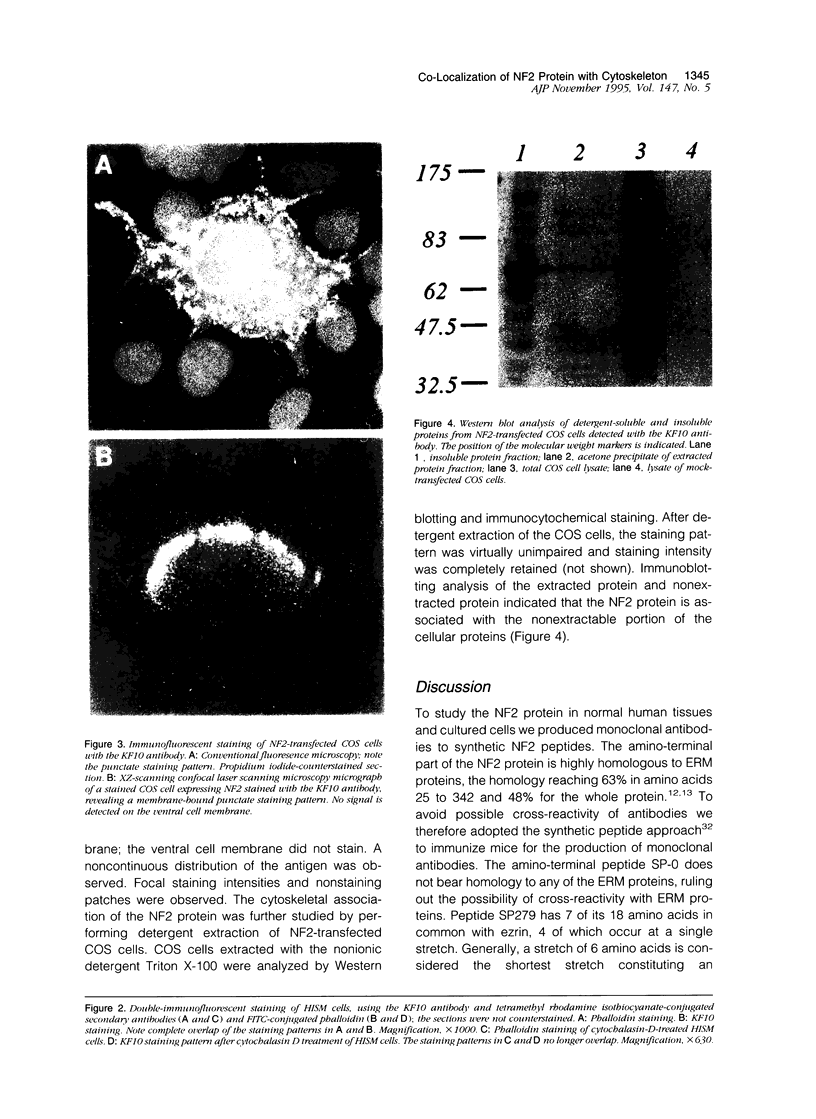




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algrain M., Turunen O., Vaheri A., Louvard D., Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993 Jan;120(1):129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H., Hayashi N., Nagase H., Ogawa M., Nakamura Y. Alternative splicing of the NF2 gene and its mutation analysis of breast and colorectal cancers. Hum Mol Genet. 1994 Apr;3(4):565–568. doi: 10.1093/hmg/3.4.565. [DOI] [PubMed] [Google Scholar]
- Arpin M., Algrain M., Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994 Feb;6(1):136–141. doi: 10.1016/0955-0674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Bianchi A. B., Hara T., Ramesh V., Gao J., Klein-Szanto A. J., Morin F., Menon A. G., Trofatter J. A., Gusella J. F., Seizinger B. R. Mutations in transcript isoforms of the neurofibromatosis 2 gene in multiple human tumour types. Nat Genet. 1994 Feb;6(2):185–192. doi: 10.1038/ng0294-185. [DOI] [PubMed] [Google Scholar]
- Burridge K., Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 1983;3(5-6):405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Huson S. M., Donnai D., Neary W., Blair V., Newton V., Harris R. A clinical study of type 2 neurofibromatosis. Q J Med. 1992 Aug;84(304):603–618. [PubMed] [Google Scholar]
- Evans D. G., Huson S. M., Donnai D., Neary W., Blair V., Newton V., Strachan T., Harris R. A genetic study of type 2 neurofibromatosis in the United Kingdom. II. Guidelines for genetic counselling. J Med Genet. 1992 Dec;29(12):847–852. doi: 10.1136/jmg.29.12.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. G., Huson S. M., Donnai D., Neary W., Blair V., Teare D., Newton V., Strachan T., Ramsden R., Harris R. A genetic study of type 2 neurofibromatosis in the United Kingdom. I. Prevalence, mutation rate, fitness, and confirmation of maternal transmission effect on severity. J Med Genet. 1992 Dec;29(12):841–846. doi: 10.1136/jmg.29.12.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Rouse R. V., Wang M. C., Chu T. M., Herzenberg L. A. Monoclonal antibodies to a human prostate antigen. Cancer Res. 1982 Sep;42(9):3714–3718. [PubMed] [Google Scholar]
- Graham M. F., Diegelmann R. F., Elson C. O., Bitar K. N., Ehrlich H. P. Isolation and culture of human intestinal smooth muscle cells. Proc Soc Exp Biol Med. 1984 Sep;176(4):503–507. doi: 10.3181/00379727-176-4-rc1. [DOI] [PubMed] [Google Scholar]
- Hawkes R. The dot immunobinding assay. Methods Enzymol. 1986;121:484–491. doi: 10.1016/0076-6879(86)21048-4. [DOI] [PubMed] [Google Scholar]
- Hitotsumatsu T., Kitamoto T., Iwaki T., Fukui M., Tateishi J. An exon 8-spliced out transcript of neurofibromatosis 2 gene is constitutively expressed in various human tissues. J Biochem. 1994 Dec;116(6):1205–1207. doi: 10.1093/oxfordjournals.jbchem.a124664. [DOI] [PubMed] [Google Scholar]
- Irving R. M., Moffat D. A., Hardy D. G., Barton D. E., Xuereb J. H., Maher E. R. Somatic NF2 gene mutations in familial and non-familial vestibular schwannoma. Hum Mol Genet. 1994 Feb;3(2):347–350. doi: 10.1093/hmg/3.2.347. [DOI] [PubMed] [Google Scholar]
- Jacoby L. B., MacCollin M., Louis D. N., Mohney T., Rubio M. P., Pulaski K., Trofatter J. A., Kley N., Seizinger B., Ramesh V. Exon scanning for mutation of the NF2 gene in schwannomas. Hum Mol Genet. 1994 Mar;3(3):413–419. doi: 10.1093/hmg/3.3.413. [DOI] [PubMed] [Google Scholar]
- Kaiser-Kupfer M. I., Freidlin V., Datiles M. B., Edwards P. A., Sherman J. L., Parry D., McCain L. M., Eldridge R. The association of posterior capsular lens opacities with bilateral acoustic neuromas in patients with neurofibromatosis type 2. Arch Ophthalmol. 1989 Apr;107(4):541–544. doi: 10.1001/archopht.1989.01070010555030. [DOI] [PubMed] [Google Scholar]
- Kaye L. D., Rothner A. D., Beauchamp G. R., Meyers S. M., Estes M. L. Ocular findings associated with neurofibromatosis type II. Ophthalmology. 1992 Sep;99(9):1424–1429. doi: 10.1016/s0161-6420(92)31789-0. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kreis T. E. Microtubules containing detyrosinated tubulin are less dynamic. EMBO J. 1987 Sep;6(9):2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau K., Yaşargil G. M. Ocular fundus in neurofibromatosis type 2. Br J Ophthalmol. 1993 Oct;77(10):646–649. doi: 10.1136/bjo.77.10.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekanne Deprez R. H., Bianchi A. B., Groen N. A., Seizinger B. R., Hagemeijer A., van Drunen E., Bootsma D., Koper J. W., Avezaat C. J., Kley N. Frequent NF2 gene transcript mutations in sporadic meningiomas and vestibular schwannomas. Am J Hum Genet. 1994 Jun;54(6):1022–1029. [PMC free article] [PubMed] [Google Scholar]
- Manabe M., O'Guin W. M. Keratohyalin, trichohyalin and keratohyalin-trichohyalin hybrid granules: an overview. J Dermatol. 1992 Nov;19(11):749–755. doi: 10.1111/j.1346-8138.1992.tb03774.x. [DOI] [PubMed] [Google Scholar]
- Martuza R. L., Eldridge R. Neurofibromatosis 2 (bilateral acoustic neurofibromatosis). N Engl J Med. 1988 Mar 17;318(11):684–688. doi: 10.1056/NEJM198803173181106. [DOI] [PubMed] [Google Scholar]
- Mulvihill J. J., Parry D. M., Sherman J. L., Pikus A., Kaiser-Kupfer M. I., Eldridge R. NIH conference. Neurofibromatosis 1 (Recklinghausen disease) and neurofibromatosis 2 (bilateral acoustic neurofibromatosis). An update. Ann Intern Med. 1990 Jul 1;113(1):39–52. doi: 10.7326/0003-4819-113-1-39. [DOI] [PubMed] [Google Scholar]
- Müllauer L., Fujita H., Ishizaki A., Kuzumaki N. Tumor-suppressive function of mutated gelsolin in ras-transformed cells. Oncogene. 1993 Sep;8(9):2531–2536. [PubMed] [Google Scholar]
- Parry D. M., Eldridge R., Kaiser-Kupfer M. I., Bouzas E. A., Pikus A., Patronas N. Neurofibromatosis 2 (NF2): clinical characteristics of 63 affected individuals and clinical evidence for heterogeneity. Am J Med Genet. 1994 Oct 1;52(4):450–461. doi: 10.1002/ajmg.1320520411. [DOI] [PubMed] [Google Scholar]
- Pearson-Webb M. A., Kaiser-Kupfer M. I., Eldridge R. Eye findings in bilateral acoustic (central) neurofibromatosis: association with presenile lens opacities and cataracts but absence of Lisch nodules. N Engl J Med. 1986 Dec 11;315(24):1553–1554. doi: 10.1056/NEJM198612113152419. [DOI] [PubMed] [Google Scholar]
- Prasad G. L., Fuldner R. A., Cooper H. L. Expression of transduced tropomyosin 1 cDNA suppresses neoplastic growth of cells transformed by the ras oncogene. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7039–7043. doi: 10.1073/pnas.90.15.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pykett M. J., Murphy M., Harnish P. R., George D. L. The neurofibromatosis 2 (NF2) tumor suppressor gene encodes multiple alternatively spliced transcripts. Hum Mol Genet. 1994 Apr;3(4):559–564. doi: 10.1093/hmg/3.4.559. [DOI] [PubMed] [Google Scholar]
- Ragge N. K. Clinical and genetic patterns of neurofibromatosis 1 and 2. Br J Ophthalmol. 1993 Oct;77(10):662–672. doi: 10.1136/bjo.77.10.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez Fernández J. L., Geiger B., Salomon D., Sabanay I., Zöller M., Ben-Ze'ev A. Suppression of tumorigenicity in transformed cells after transfection with vinculin cDNA. J Cell Biol. 1992 Oct;119(2):427–438. doi: 10.1083/jcb.119.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau G. A., Merel P., Lutchman M., Sanson M., Zucman J., Marineau C., Hoang-Xuan K., Demczuk S., Desmaze C., Plougastel B. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993 Jun 10;363(6429):515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Ruttledge M. H., Sarrazin J., Rangaratnam S., Phelan C. M., Twist E., Merel P., Delattre O., Thomas G., Nordenskjöld M., Collins V. P. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat Genet. 1994 Feb;6(2):180–184. doi: 10.1038/ng0294-180. [DOI] [PubMed] [Google Scholar]
- Sato N., Funayama N., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992 Sep;103(Pt 1):131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Takakuwa Y., Koizumi H., Ishibashi T., Ohkawara A. Presence and localization of proteins immunologically related to erythrocyte protein 4.1 in human skin. Histochemistry. 1991;95(6):549–554. doi: 10.1007/BF00266740. [DOI] [PubMed] [Google Scholar]
- Sternberger L. A., Joseph S. A. The unlabeled antibody method. Contrasting color staining of paired pituitary hormones without antibody removal. J Histochem Cytochem. 1979 Nov;27(11):1424–1429. doi: 10.1177/27.11.92498. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Izawa I., Lee P. S., Safdar N., Levin V. A., Saya H. Detection of cellular proteins that interact with the NF2 tumor suppressor gene product. Oncogene. 1994 Aug;9(8):2135–2144. [PubMed] [Google Scholar]
- Tikoo A., Varga M., Ramesh V., Gusella J., Maruta H. An anti-Ras function of neurofibromatosis type 2 gene product (NF2/Merlin). J Biol Chem. 1994 Sep 23;269(38):23387–23390. [PubMed] [Google Scholar]
- Trofatter J. A., MacCollin M. M., Rutter J. L., Murrell J. R., Duyao M. P., Parry D. M., Eldridge R., Kley N., Menon A. G., Pulaski K. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993 Mar 12;72(5):791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Turunen O., Wahlström T., Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994 Sep;126(6):1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers N. D., Claassen E., Neelen C., Mulder E., van Laar J. H., Voorhorst M. M., Berrevoets C. A., Brinkmann A. O., van der Kwast T. H., Ruizeveld de Winter J. A. Epitope prediction and confirmation for the human androgen receptor: generation of monoclonal antibodies for multi-assay performance following the synthetic peptide strategy. Biochim Biophys Acta. 1991 Jan 23;1073(1):23–32. doi: 10.1016/0304-4165(91)90178-j. [DOI] [PubMed] [Google Scholar]
- Zondervan P. E., van der Kwast T. H., de Jong A., Visser W. J., de Bruijn W. C. Lysosomal localization of secretory prostatic acid phosphatase in human hyperplastic prostate epithelium. Urol Res. 1986;14(6):331–335. doi: 10.1007/BF00262386. [DOI] [PubMed] [Google Scholar]
- den Bakker M. A., Riegman P. H., Hekman R. A., Boersma W., Janssen P. J., van der Kwast T. H., Zwarthoff E. C. The product of the NF2 tumour suppressor gene localizes near the plasma membrane and is highly expressed in muscle cells. Oncogene. 1995 Feb 16;10(4):757–763. [PubMed] [Google Scholar]