Abstract
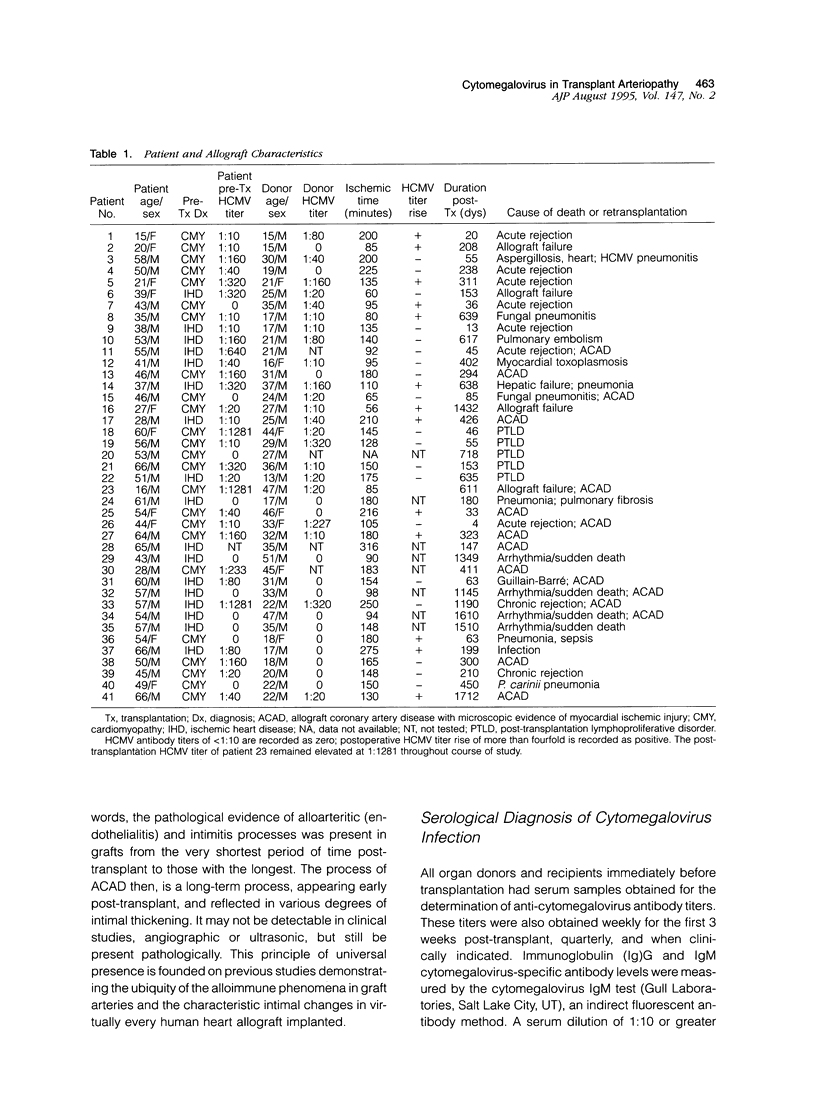
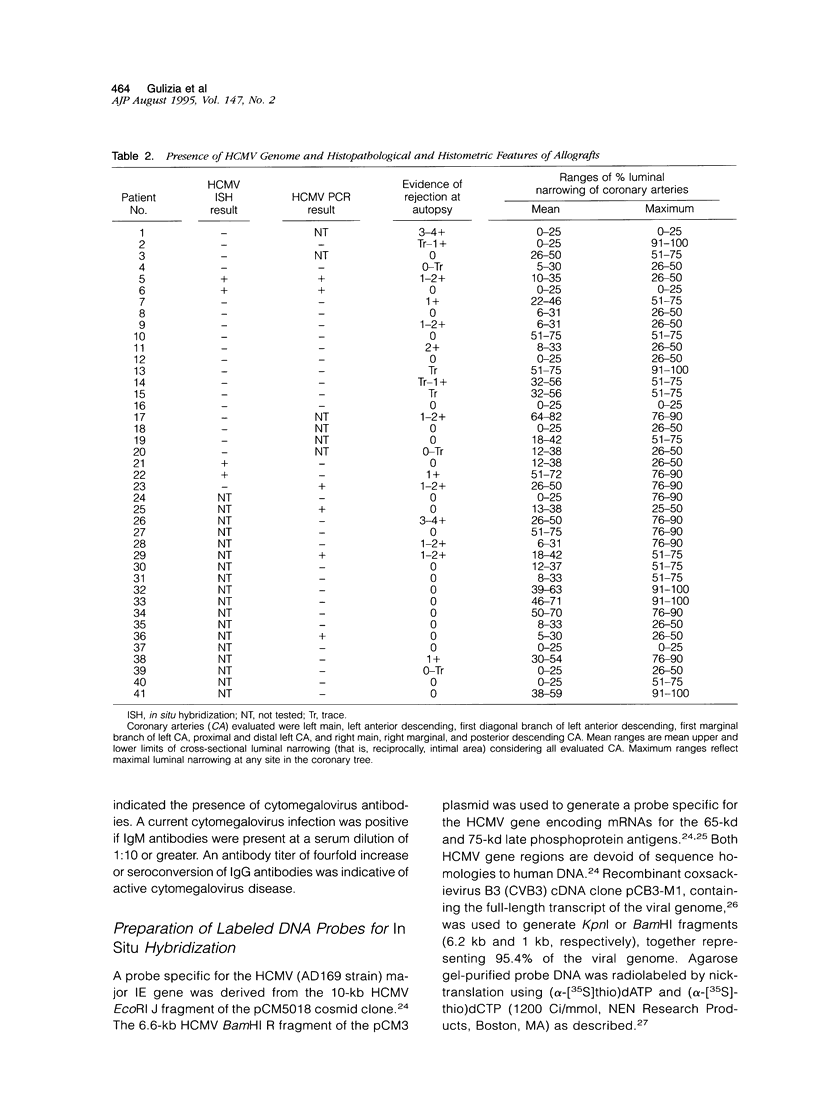
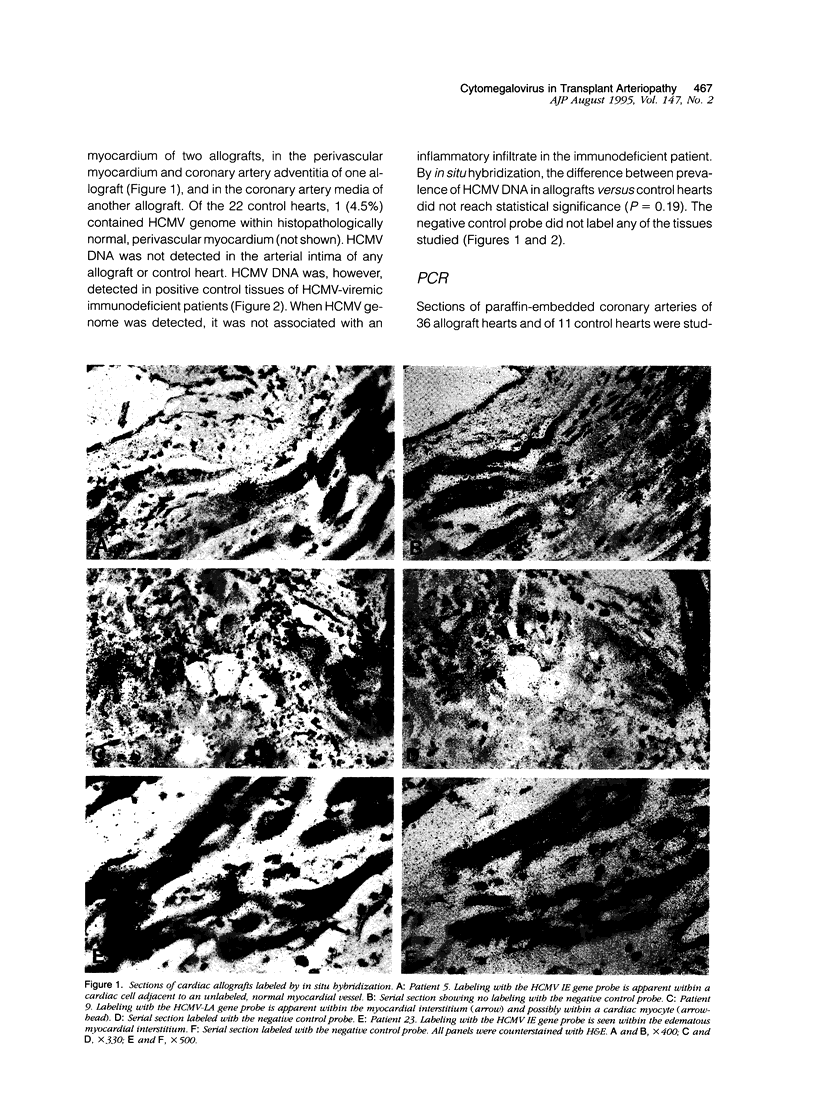
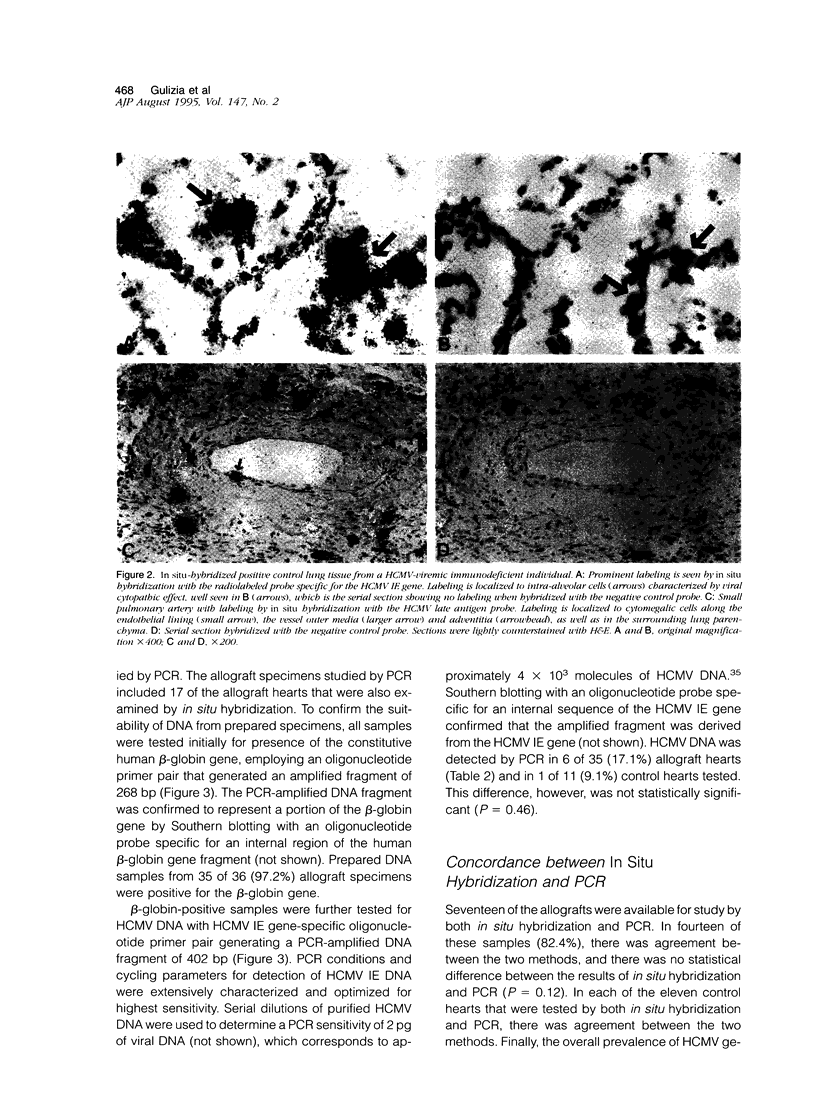
In heart transplantation, long-term engraftment success is severely limited by the rapid development of obliterative disease of the coronary arteries. Data from various groups have been suggestive of a pathogenetic role of herpesviruses, particularly human cytomegalovirus, in accelerated allograft coronary artery disease; however, results are not yet conclusive. This study examines the hypothesis that human cytomegalovirus infection of allograft tissues is related pathogenetically and directly to accelerated coronary artery disease. Using in situ DNA hybridization and polymerase chain reaction, we examined particular coronary artery segments from 41 human heart allografts (ranging from 4 days to greater than 4 years after transplantation; mean, 457 days) and 22 donor age- and gender-comparable, coronary site-matched trauma victims for presence of human cytomegalovirus DNA. Human cytomegalovirus genome was detected in 8 of 41 (19.5%) allografts and in 1 of 22 (4.5%) control hearts. This difference in positivity was not statistically significant (P = 0.10). In the human cytomegalovirus-positive hearts, viral genome was localized to perivascular myocardium or coronary artery media or adventitia. Human cytomegalovirus genome was not detected in arterial intima of any allograft or control heart, although human cytomegalovirus genome was readily identified within intima of small pulmonary arteries from lung tissue with human cytomegalovirus pneumonitis. By statistical analyses, the presence of human cytomegalovirus genome was not associated with the nature or digitized extent of transplant arteriopathy, evidence of rejection, allograft recipient or donor serological data suggestive of human cytomegalovirus infection, duration of allograft implantation, or causes of death or retransplantation. Thus, our data indicate a low frequency of detectable human cytomegalovirus genome in accelerated coronary artery disease and do not support a direct role for human cytomegalovirus in vascular wall infection or in the development of accelerated coronary artery disease.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akrigg A., Wilkinson G. W., Oram J. D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985 Mar;2(2):107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- Allen M. D., McDonald T. O., Carlos T., Himes V., Fishbein D., Aziz S., Gordon D. Endothelial adhesion molecules in heart transplantation. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S8–13. [PubMed] [Google Scholar]
- Arbustini E., Grasso M., Diegoli M., Percivalle E., Grossi P., Bramerio M., Campana C., Goggi C., Gavazzi A., Vigano M. Histopathologic and molecular profile of human cytomegalovirus infections in patients with heart transplants. Am J Clin Pathol. 1992 Aug;98(2):205–213. [PubMed] [Google Scholar]
- Benditt E. P., Barrett T., McDougall J. K. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham M. E., Cary N. R., Hammond M. E., Kemnitz J., Marboe C., McCallister H. A., Snovar D. C., Winters G. L., Zerbe A. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Heart Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990 Nov-Dec;9(6):587–593. [PubMed] [Google Scholar]
- Billingham M. E. Histopathology of graft coronary disease. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S38–S44. [PubMed] [Google Scholar]
- Bours V., Franzoso G., Brown K., Park S., Azarenko V., Tomita-Yamaguchi M., Kelly K., Siebenlist U. Lymphocyte activation and the family of NF-kappa B transcription factor complexes. Curr Top Microbiol Immunol. 1992;182:411–420. doi: 10.1007/978-3-642-77633-5_52. [DOI] [PubMed] [Google Scholar]
- Bruggeman C. A., Meijer H., Bosman F., van Boven C. P. Biology of rat cytomegalovirus infection. Intervirology. 1985;24(1):1–9. doi: 10.1159/000149612. [DOI] [PubMed] [Google Scholar]
- Bruggeman C. A., Meijer H., Dormans P. H., Debie W. M., Grauls G. E., van Boven C. P. Isolation of a cytomegalovirus-like agent from wild rats. Arch Virol. 1982;73(3-4):231–241. doi: 10.1007/BF01318077. [DOI] [PubMed] [Google Scholar]
- Carlos T., Kovach N., Schwartz B., Rosa M., Newman B., Wayner E., Benjamin C., Osborn L., Lobb R., Harlan J. Human monocytes bind to two cytokine-induced adhesive ligands on cultured human endothelial cells: endothelial-leukocyte adhesion molecule-1 and vascular cell adhesion molecule-1. Blood. 1991 May 15;77(10):2266–2271. [PubMed] [Google Scholar]
- Chou S., Merigan T. C. Rapid detection and quantitation of human cytomegalovirus in urine through DNA hybridization. N Engl J Med. 1983 Apr 21;308(16):921–925. doi: 10.1056/NEJM198304213081603. [DOI] [PubMed] [Google Scholar]
- Collins T. Endothelial nuclear factor-kappa B and the initiation of the atherosclerotic lesion. Lab Invest. 1993 May;68(5):499–508. [PubMed] [Google Scholar]
- Cooper D. K., Novitzky D., Schlegel V., Muchmore J. S., Cucchiara A., Zuhdi N. Successful management of symptomatic cytomegalovirus disease with ganciclovir after heart transplantation. J Heart Lung Transplant. 1991 Sep-Oct;10(5 Pt 1):656–663. [PubMed] [Google Scholar]
- Costanzo-Nordin M. R. Cardiac allograft vasculopathy: relationship with acute cellular rejection and histocompatibility. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S90–103. [PubMed] [Google Scholar]
- Dolan J., Briggs J. D., Clements G. B. Antibodies to cytomegalovirus in renal allograft recipients: correlation with isolation of virus. J Clin Pathol. 1989 Oct;42(10):1070–1077. doi: 10.1136/jcp.42.10.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew W. L., Conant M. A., Miner R. C., Huang E. S., Ziegler J. L., Groundwater J. R., Gullett J. H., Volberding P., Abrams D. I., Mintz L. Cytomegalovirus and Kaposi's sarcoma in young homosexual men. Lancet. 1982 Jul 17;2(8290):125–127. doi: 10.1016/s0140-6736(82)91092-3. [DOI] [PubMed] [Google Scholar]
- Drew W. L. Diagnosis of cytomegalovirus infection. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 3):S468–S476. doi: 10.1093/clinids/10.supplement_3.s468. [DOI] [PubMed] [Google Scholar]
- Dummer J. S., Hardy A., Poorsattar A., Ho M. Early infections in kidney, heart, and liver transplant recipients on cyclosporine. Transplantation. 1983 Sep;36(3):259–267. doi: 10.1097/00007890-198309000-00007. [DOI] [PubMed] [Google Scholar]
- Dummer J. S., White L. T., Ho M., Griffith B. P., Hardesty R. L., Bahnson H. T. Morbidity of cytomegalovirus infection in recipients of heart or heart-lung transplants who received cyclosporine. J Infect Dis. 1985 Dec;152(6):1182–1191. doi: 10.1093/infdis/152.6.1182. [DOI] [PubMed] [Google Scholar]
- Dunkel E. C., de Freitas D., Scheer D. I., Siegel M. L., Zhu Q., Whitley R. J., Schaffer P. A., Pavan-Langston D. A rabbit model for human cytomegalovirus-induced chorioretinal disease. J Infect Dis. 1993 Aug;168(2):336–344. doi: 10.1093/infdis/168.2.336. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Fabricant C. G., Fabricant J., Litrenta M. M., Minick C. R. Virus-induced atherosclerosis. J Exp Med. 1978 Jul 1;148(1):335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix R. G. Cytomegalovirus antigenic heterogeneity can cause false-negative results in indirect hemagglutination and complement fixation antibody assays. J Clin Microbiol. 1985 Nov;22(5):768–771. doi: 10.1128/jcm.22.5.768-771.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grattan M. T., Moreno-Cabral C. E., Starnes V. A., Oyer P. E., Stinson E. B., Shumway N. E. Cytomegalovirus infection is associated with cardiac allograft rejection and atherosclerosis. JAMA. 1989 Jun 23;261(24):3561–3566. [PubMed] [Google Scholar]
- Greer C. E., Peterson S. L., Kiviat N. B., Manos M. M. PCR amplification from paraffin-embedded tissues. Effects of fixative and fixation time. Am J Clin Pathol. 1991 Feb;95(2):117–124. doi: 10.1093/ajcp/95.2.117. [DOI] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Guinn G. A., Gyorkey P., DeBakey M. E. Herpesviridae in the endothelial and smooth muscle cells of the proximal aorta in arteriosclerotic patients. Exp Mol Pathol. 1984 Jun;40(3):328–339. doi: 10.1016/0014-4800(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Hajjar D. P., Nicholson A. C., Hajjar K. A., Sando G. N., Summers B. D. Decreased messenger RNA translation in herpesvirus-infected arterial cells: effects on cholesteryl ester hydrolase. Proc Natl Acad Sci U S A. 1989 May;86(9):3366–3370. doi: 10.1073/pnas.86.9.3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix M. G., Daemen M., Bruggeman C. A. Cytomegalovirus nucleic acid distribution within the human vascular tree. Am J Pathol. 1991 Mar;138(3):563–567. [PMC free article] [PubMed] [Google Scholar]
- Hendrix M. G., Dormans P. H., Kitslaar P., Bosman F., Bruggeman C. A. The presence of cytomegalovirus nucleic acids in arterial walls of atherosclerotic and nonatherosclerotic patients. Am J Pathol. 1989 May;134(5):1151–1157. [PMC free article] [PubMed] [Google Scholar]
- Hendrix M. G., Salimans M. M., van Boven C. P., Bruggeman C. A. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am J Pathol. 1990 Jan;136(1):23–28. [PMC free article] [PubMed] [Google Scholar]
- Hruban R. H., Wu T. C., Beschorner W. E., Cameron D. E., Ambinder R. F., Baumgartner W. A., Reitz B. A., Hutchins G. M. Cytomegalovirus nucleic acids in allografted hearts. Hum Pathol. 1990 Sep;21(9):981–982. doi: 10.1016/0046-8177(90)90186-9. [DOI] [PubMed] [Google Scholar]
- Josephs S. F., Salahuddin S. Z., Ablashi D. V., Schachter F., Wong-Staal F., Gallo R. C. Genomic analysis of the human B-lymphotropic virus (HBLV). Science. 1986 Oct 31;234(4776):601–603. doi: 10.1126/science.3020691. [DOI] [PubMed] [Google Scholar]
- Jäkel K. T., Löning T., Arndt R., Rödiger W. Rejection, herpesvirus infection, and Ki-67 expression in endomyocardial biopsy specimens from heart transplant recipients. Pathol Res Pract. 1992 Feb;188(1-2):27–36. doi: 10.1016/s0344-0338(11)81152-0. [DOI] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Hofschneider P. H. Molecular cloning of the genome of a cardiotropic Coxsackie B3 virus: full-length reverse-transcribed recombinant cDNA generates infectious virus in mammalian cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4818–4822. doi: 10.1073/pnas.82.14.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keh W. C., Gerber M. A. In situ hybridization for cytomegalovirus DNA in AIDS patients. Am J Pathol. 1988 Jun;131(3):490–496. [PMC free article] [PubMed] [Google Scholar]
- Kendall T. J., Wilson J. E., Radio S. J., Kandolf R., Gulizia J. M., Winters G. L., Costanzo-Nordin M. R., Malcom G. T., Thieszen S. L., Miller L. W. Cytomegalovirus and other herpesviruses: do they have a role in the development of accelerated coronary arterial disease in human heart allografts? J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S14–S20. [PubMed] [Google Scholar]
- Keogh A., Simons L., Spratt P., Esmore D., Chang V., Hickie J., Baron D. Hyperlipidemia after heart transplantation. J Heart Transplant. 1988 May-Jun;7(3):171–175. [PubMed] [Google Scholar]
- Klingel K., Hohenadl C., Canu A., Albrecht M., Seemann M., Mall G., Kandolf R. Ongoing enterovirus-induced myocarditis is associated with persistent heart muscle infection: quantitative analysis of virus replication, tissue damage, and inflammation. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):314–318. doi: 10.1073/pnas.89.1.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen P. K., Nieminen M. S., Mattila S. P., Häyry P. J., Lautenschlager I. T. The correlation between symptomatic CMV infection and CMV antigenemia in heart allograft recipients. Transplantation. 1993 Mar;55(3):547–551. doi: 10.1097/00007890-199303000-00017. [DOI] [PubMed] [Google Scholar]
- Kriett J. M., Kaye M. P. The Registry of the International Society for Heart and Lung Transplantation: eighth official report--1991. J Heart Lung Transplant. 1991 Jul-Aug;10(4):491–498. [PubMed] [Google Scholar]
- Lemström K. B., Bruning J. H., Bruggeman C. A., Lautenschlager I. T., Häyry P. J. Cytomegalovirus infection enhances smooth muscle cell proliferation and intimal thickening of rat aortic allografts. J Clin Invest. 1993 Aug;92(2):549–558. doi: 10.1172/JCI116622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Wilson J. E., Kendall T. J., Radio S. J., Cornhill F. J., Herderick E., Winters G. L., Costanzo M. R., Porter T., Thieszen S. L. Comparable proximal and distal severity of intimal thickening and size of epicardial coronary arteries in transplant arteriopathy of human cardiac allografts. J Heart Lung Transplant. 1994 Sep-Oct;13(5):824–833. [PubMed] [Google Scholar]
- Loebe M., Schüler S., Zais O., Warnecke H., Fleck E., Hetzer R. Role of cytomegalovirus infection in the development of coronary artery disease in the transplanted heart. J Heart Transplant. 1990 Nov-Dec;9(6):707–711. [PubMed] [Google Scholar]
- Löning T., Milde K., Foss H. D. In situ hybridization for the detection of cytomegalovirus (CMV) infection. Application of biotinylated CMV-DNA probes on paraffin-embedded specimens. Virchows Arch A Pathol Anat Histopathol. 1986;409(6):777–790. doi: 10.1007/BF00710763. [DOI] [PubMed] [Google Scholar]
- McDonald K., Rector T. S., Braulin E. A., Kubo S. H., Olivari M. T. Association of coronary artery disease in cardiac transplant recipients with cytomegalovirus infection. Am J Cardiol. 1989 Aug 1;64(5):359–362. doi: 10.1016/0002-9149(89)90535-3. [DOI] [PubMed] [Google Scholar]
- Melnick J. L., Petrie B. L., Dreesman G. R., Burek J., McCollum C. H., DeBakey M. E. Cytomegalovirus antigen within human arterial smooth muscle cells. Lancet. 1983 Sep 17;2(8351):644–647. doi: 10.1016/s0140-6736(83)92529-1. [DOI] [PubMed] [Google Scholar]
- Minick C. R., Fabricant C. G., Fabricant J., Litrenta M. M. Atheroarteriosclerosis induced by infection with a herpesvirus. Am J Pathol. 1979 Sep;96(3):673–706. [PMC free article] [PubMed] [Google Scholar]
- Myerson D., Hackman R. C., Nelson J. A., Ward D. C., McDougall J. K. Widespread presence of histologically occult cytomegalovirus. Hum Pathol. 1984 May;15(5):430–439. doi: 10.1016/s0046-8177(84)80076-3. [DOI] [PubMed] [Google Scholar]
- Nadasdy T., Smith J., Laszik Z., Waner J. L., Johnson L. D., Silva F. G. Absence of association between cytomegalovirus infection and obliterative transplant arteriopathy in renal allograft rejection. Mod Pathol. 1994 Apr;7(3):289–294. [PubMed] [Google Scholar]
- Niedt G. W., Schinella R. A. Acquired immunodeficiency syndrome. Clinicopathologic study of 56 autopsies. Arch Pathol Lab Med. 1985 Aug;109(8):727–734. [PubMed] [Google Scholar]
- Olivari M. T., Kubo S. H., Braunlin E. A., Bolman R. M., Ring W. S. Five-year experience with triple-drug immunosuppressive therapy in cardiac transplantation. Circulation. 1990 Nov;82(5 Suppl):IV276–IV280. [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pahl E., Fricker F. J., Armitage J., Griffith B. P., Taylor S., Uretsky B. F., Beerman L. B., Zuberbuhler J. R. Coronary arteriosclerosis in pediatric heart transplant survivors: limitation of long-term survival. J Pediatr. 1990 Feb;116(2):177–183. doi: 10.1016/s0022-3476(05)82871-9. [DOI] [PubMed] [Google Scholar]
- Petrie B. L., Melnick J. L., Adam E., Burek J., McCollum C. H., DeBakey M. E. Nucleic acid sequences of cytomegalovirus in cells cultured from human arterial tissue. J Infect Dis. 1987 Jan;155(1):158–159. doi: 10.1093/infdis/155.1.158. [DOI] [PubMed] [Google Scholar]
- Pollard R. B. Cytomegalovirus infections in renal, heart, heart-lung and liver transplantation. Pediatr Infect Dis J. 1988 May;7(5 Suppl):S97–102. [PubMed] [Google Scholar]
- Reichert C. M., O'Leary T. J., Levens D. L., Simrell C. R., Macher A. M. Autopsy pathology in the acquired immune deficiency syndrome. Am J Pathol. 1983 Sep;112(3):357–382. [PMC free article] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger R., Bornkamm G. W., Fleckenstein B. Human cytomegalovirus DNA sequences with homologies to the cellular genome. J Gen Virol. 1984 Aug;65(Pt 8):1351–1364. doi: 10.1099/0022-1317-65-8-1351. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sambucetti L. C., Cherrington J. M., Wilkinson G. W., Mocarski E. S. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989 Dec 20;8(13):4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharples L. D., Caine N., Mullins P., Scott J. P., Solis E., English T. A., Large S. R., Schofield P. M., Wallwork J. Risk factor analysis for the major hazards following heart transplantation--rejection, infection, and coronary occlusive disease. Transplantation. 1991 Aug;52(2):244–252. doi: 10.1097/00007890-199108000-00012. [DOI] [PubMed] [Google Scholar]
- Skowronski E. W., Mendoza A., Smith S. C., Jr, Jaski B. E. Detection of cytomegalovirus in paraffin-embedded postmortem coronary artery specimens of heart transplant recipients by the polymerase chain reaction: implications of cytomegalovirus association with graft atherosclerosis. J Heart Lung Transplant. 1993 Sep-Oct;12(5):717–723. [PubMed] [Google Scholar]
- Span A. H., Grauls G., Bosman F., van Boven C. P., Bruggeman C. A. Cytomegalovirus infection induces vascular injury in the rat. Atherosclerosis. 1992 Mar;93(1-2):41–52. doi: 10.1016/0021-9150(92)90198-p. [DOI] [PubMed] [Google Scholar]
- Span A. H., Mullers W., Miltenburg A. M., Bruggeman C. A. Cytomegalovirus induced PMN adherence in relation to an ELAM-1 antigen present on infected endothelial cell monolayers. Immunology. 1991 Mar;72(3):355–360. [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Rua J. A., Spector D. H., McMillan R. Detection of human cytomegalovirus in clinical specimens by DNA-DNA hybridization. J Infect Dis. 1984 Jul;150(1):121–126. doi: 10.1093/infdis/150.1.121. [DOI] [PubMed] [Google Scholar]
- Stovin P. G., Wreghitt T. G., English T. A., Wallwork J. Lack of association between cytomegalovirus infection of heart and rejection-like inflammation. J Clin Pathol. 1989 Jan;42(1):81–83. doi: 10.1136/jcp.42.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uretsky B. F., Murali S., Reddy P. S., Rabin B., Lee A., Griffith B. P., Hardesty R. L., Trento A., Bahnson H. T. Development of coronary artery disease in cardiac transplant patients receiving immunosuppressive therapy with cyclosporine and prednisone. Circulation. 1987 Oct;76(4):827–834. doi: 10.1161/01.cir.76.4.827. [DOI] [PubMed] [Google Scholar]
- Volpi A., Britt W. J. Serological heterogeneity of CMV isolates with a monoclonal antibody. J Infect Dis. 1985 Sep;152(3):648–649. doi: 10.1093/infdis/152.3.648. [DOI] [PubMed] [Google Scholar]
- Winters G. L., Kendall T. J., Radio S. J., Wilson J. E., Costanzo-Nordin M. R., Switzer B. L., Remmenga J. A., McManus B. M. Posttransplant obesity and hyperlipidemia: major predictors of severity of coronary arteriopathy in failed human heart allografts. J Heart Transplant. 1990 Jul-Aug;9(4):364–371. [PubMed] [Google Scholar]
- Wu T. C., Hruban R. H., Ambinder R. F., Pizzorno M., Cameron D. E., Baumgartner W. A., Reitz B. A., Hayward G. S., Hutchins G. M. Demonstration of cytomegalovirus nucleic acids in the coronary arteries of transplanted hearts. Am J Pathol. 1992 Mar;140(3):739–747. [PMC free article] [PubMed] [Google Scholar]
- Yamashiroya H. M., Ghosh L., Yang R., Robertson A. L., Jr Herpesviridae in the coronary arteries and aorta of young trauma victims. Am J Pathol. 1988 Jan;130(1):71–79. [PMC free article] [PubMed] [Google Scholar]





