Abstract
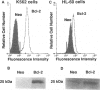
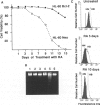
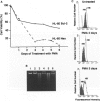
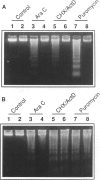
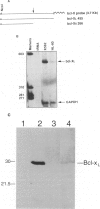
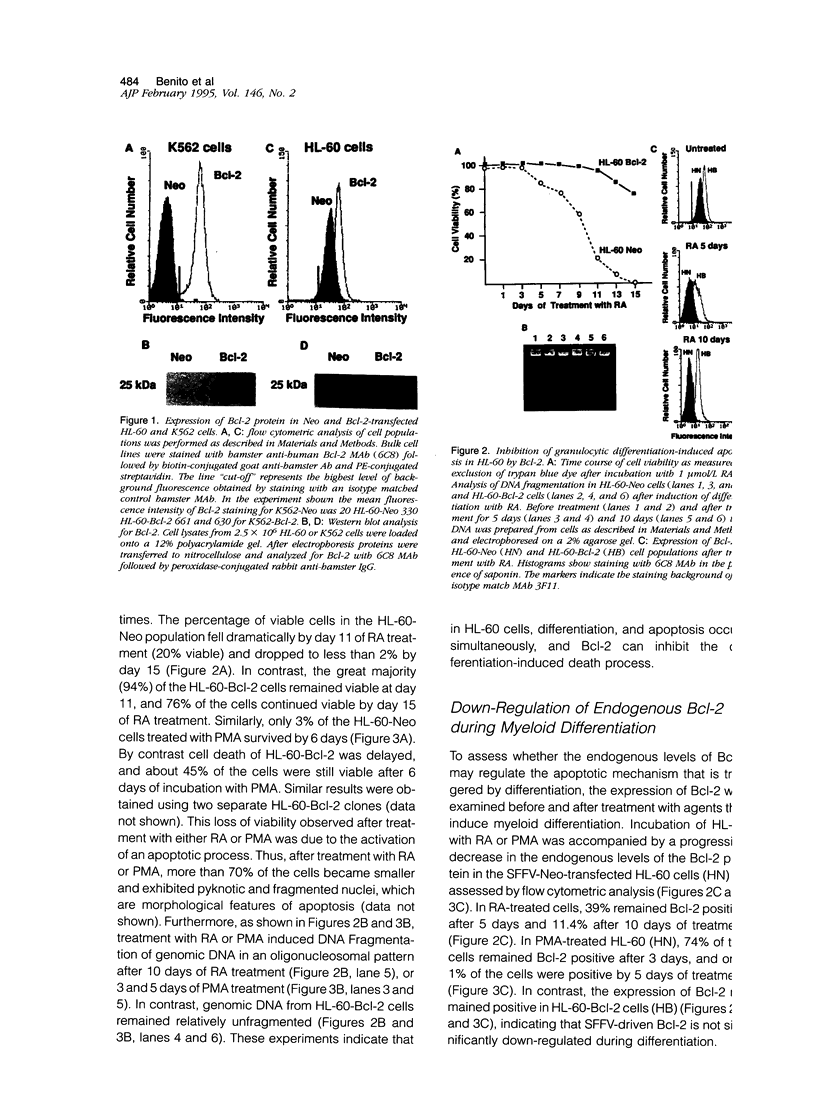
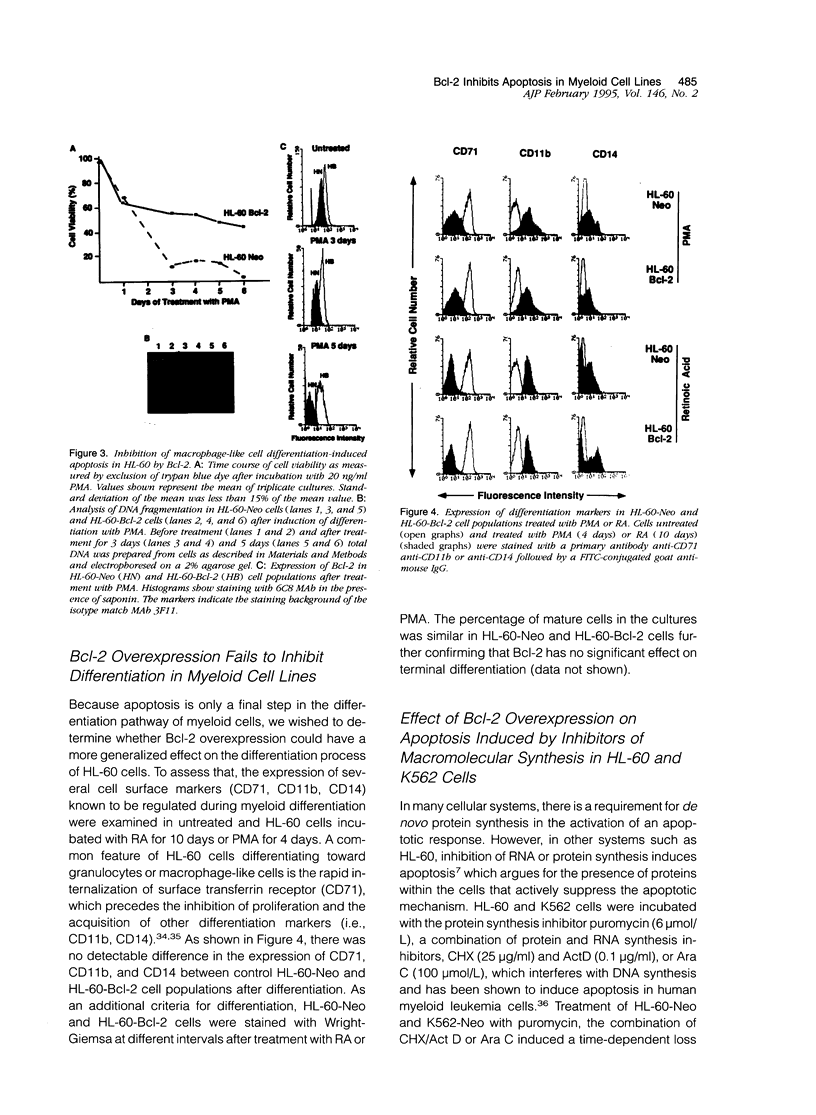
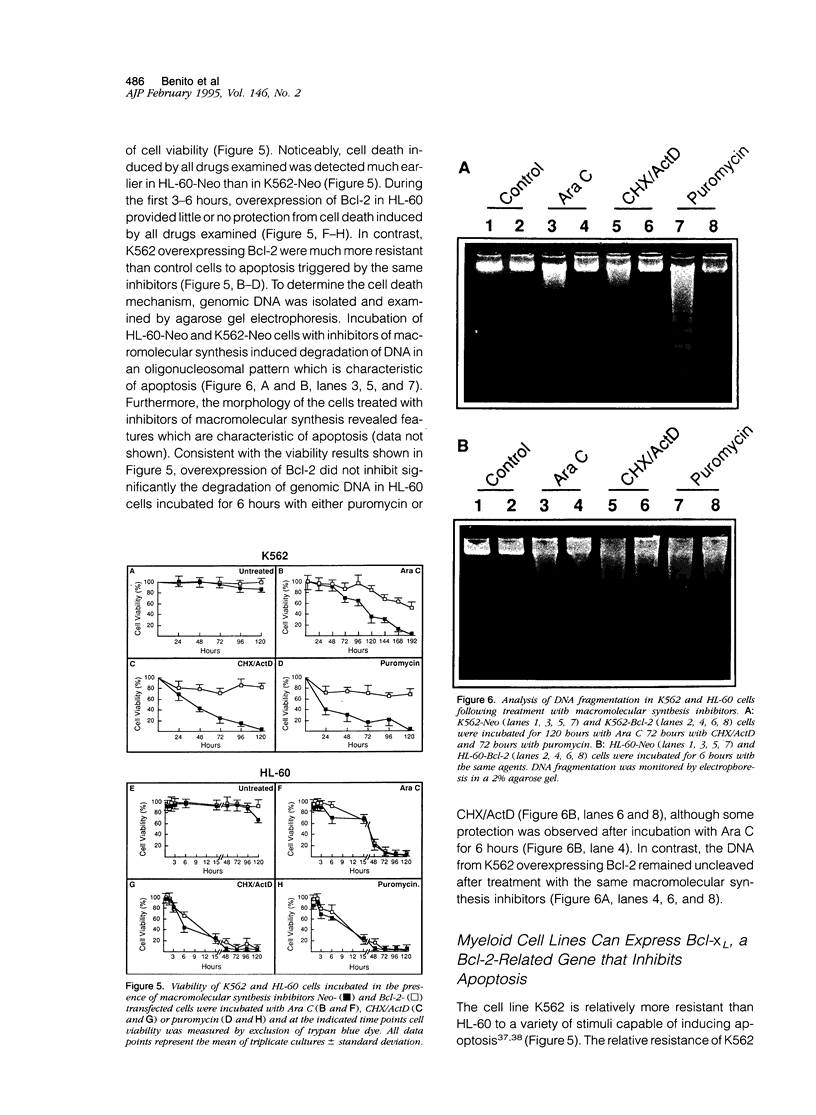
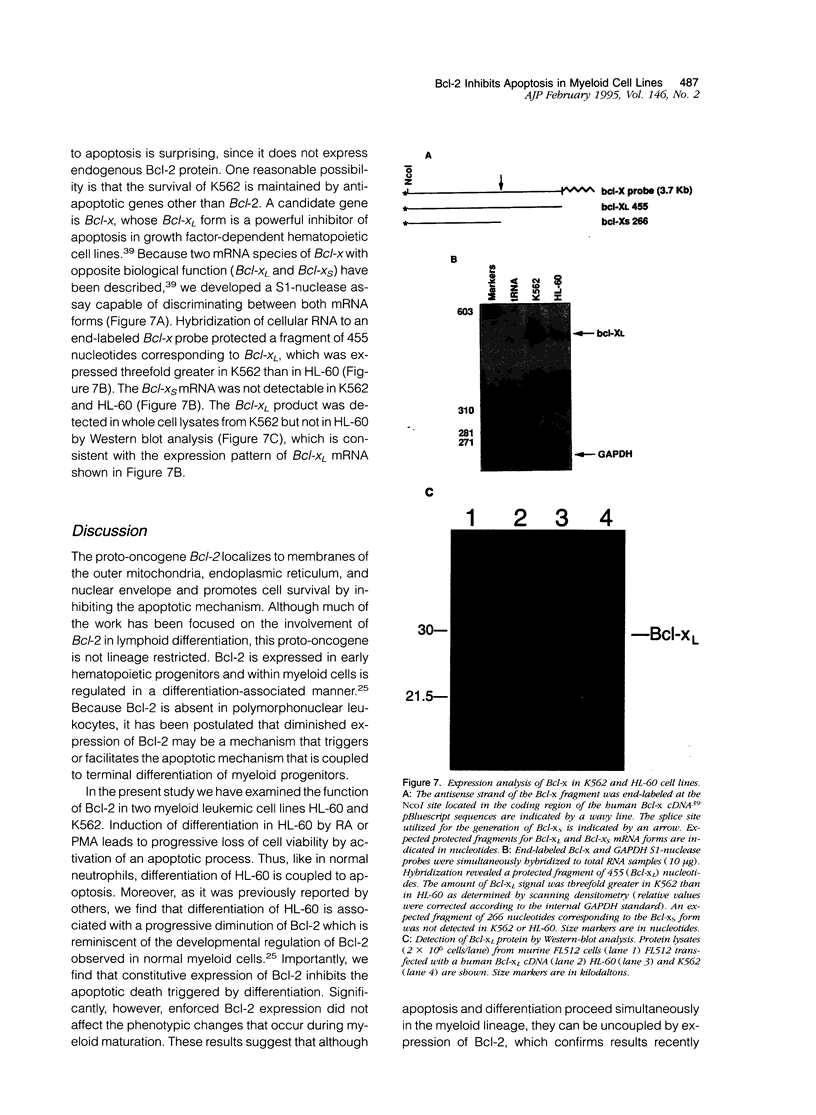
Polymorphonuclear leukocytes are generated by differentiation of early myeloid precursors. Once fully differentiated, blood neutrophils are programmed to die rapidly and are removed by tissue macrophages. In normal myeloid cells, the death mechanism seems to be coupled to the differentiation pathway and is accomplished by a process termed apoptosis. In the present study, we have examined the role of Bcl-2 in the differentiation pathways of the promyelocytic cell line HL-60. Treatment of HL-60 with retinoic acid or phorbol ester, which induced neutrophil or macrophage-like cell differentiation, respectively, resulted in progressive loss of cellular viability and internucleosomal DNA degradation. In HL-60, differentiation and apoptosis were coupled to down-regulation of the Bcl-2 protein. Overexpression of Bcl-2 by gene transfer inhibited apoptosis triggered by terminal differentiation of HL-60. Yet, Bcl-2 did not alter the expression of surface markers or other phenotypic changes that are induced upon myeloid differentiation. In contrast to HL-60, another immature myeloid cell line, K562, did not produce Bcl-2 but expressed a related protein, Bcl-xL, that functions as a repressor of apoptotic cell death. K562 has been shown to be relatively resistant to a variety of apoptotic stimuli. Incubation of HL-60 and K562 with inhibitors of macromolecular synthesis induced apoptosis, which appeared earlier in HL-60 than in K562. Interestingly, Bcl-2 overexpression protected K562 cells from apoptosis induced by inhibitor of macromolecular synthesis but it had little or no effect on HL-60 cells. We conclude that although differentiation and apoptosis proceed simultaneously, they can be uncoupled by expression of Bcl-2. Down-regulation of Bcl-2 appears to be part of the differentiation pathway and may serve to facilitate the apoptotic response.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baffy G., Miyashita T., Williamson J. R., Reed J. C. Apoptosis induced by withdrawal of interleukin-3 (IL-3) from an IL-3-dependent hematopoietic cell line is associated with repartitioning of intracellular calcium and is blocked by enforced Bcl-2 oncoprotein production. J Biol Chem. 1993 Mar 25;268(9):6511–6519. [PubMed] [Google Scholar]
- Bakhshi A., Jensen J. P., Goldman P., Wright J. J., McBride O. W., Epstein A. L., Korsmeyer S. J. Cloning the chromosomal breakpoint of t(14;18) human lymphomas: clustering around JH on chromosome 14 and near a transcriptional unit on 18. Cell. 1985 Jul;41(3):899–906. doi: 10.1016/s0092-8674(85)80070-2. [DOI] [PubMed] [Google Scholar]
- Boise L. H., González-García M., Postema C. E., Ding L., Lindsten T., Turka L. A., Mao X., Nuñez G., Thompson C. B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993 Aug 27;74(4):597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Brach M. A., deVos S., Gruss H. J., Herrmann F. Prolongation of survival of human polymorphonuclear neutrophils by granulocyte-macrophage colony-stimulating factor is caused by inhibition of programmed cell death. Blood. 1992 Dec 1;80(11):2920–2924. [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Smith S. D., Sklar J. Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell. 1986 Oct 10;47(1):19–28. doi: 10.1016/0092-8674(86)90362-4. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Re F., Polentarutti N., Sozzani S., Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992 Oct 15;80(8):2012–2020. [PubMed] [Google Scholar]
- Cuende E., Alés-Martínez J. E., Ding L., Gónzalez-García M., Martínez C., Nunez G. Programmed cell death by bcl-2-dependent and independent mechanisms in B lymphoma cells. EMBO J. 1993 Apr;12(4):1555–1560. doi: 10.1002/j.1460-2075.1993.tb05799.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delia D., Aiello A., Soligo D., Fontanella E., Melani C., Pezzella F., Pierotti M. A., Della Porta G. bcl-2 proto-oncogene expression in normal and neoplastic human myeloid cells. Blood. 1992 Mar 1;79(5):1291–1298. [PubMed] [Google Scholar]
- Diomede L., Colotta F., Piovani B., Re F., Modest E. J., Salmona M. Induction of apoptosis in human leukemic cells by the ether lipid 1-octadecyl-2-methyl-rac-glycero-3-phosphocholine. A possible basis for its selective action. Int J Cancer. 1993 Jan 2;53(1):124–130. doi: 10.1002/ijc.2910530123. [DOI] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Fadok V. A., Voelker D. R., Campbell P. A., Cohen J. J., Bratton D. L., Henson P. M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992 Apr 1;148(7):2207–2216. [PubMed] [Google Scholar]
- Fernandez-Sarabia M. J., Bischoff J. R. Bcl-2 associates with the ras-related protein R-ras p23. Nature. 1993 Nov 18;366(6452):274–275. doi: 10.1038/366274a0. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge R. C., Fine S. M., Unanue E. R., Chaplin D. D. Expression of membrane interleukin 1 by fibroblasts transfected with murine pro-interleukin 1 alpha cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5649–5653. doi: 10.1073/pnas.85.15.5649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratiot-Deans J., Ding L., Turka L. A., Nuñez G. bcl-2 proto-oncogene expression during human T cell development. Evidence for biphasic regulation. J Immunol. 1993 Jul 1;151(1):83–91. [PubMed] [Google Scholar]
- Gunji H., Kharbanda S., Kufe D. Induction of internucleosomal DNA fragmentation in human myeloid leukemia cells by 1-beta-D-arabinofuranosylcytosine. Cancer Res. 1991 Jan 15;51(2):741–743. [PubMed] [Google Scholar]
- HAMBURGER J., RICHET G. Enseignements tirés de la pratique du rein artificiel pour l'interprétation des désordres électrolytiques de l'urémie aiguë. Rev Fr Etud Clin Biol. 1956 Jan;1(1):39–55. [PubMed] [Google Scholar]
- Ho P. T., Ishiguro K., Sartorelli A. C. Regulation of transferrin receptor in myeloid and monocytic differentiation of HL-60 leukemia cells. Cancer Res. 1989 Apr 15;49(8):1989–1995. [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. D., Burne J. F., King M. P., Miyashita T., Reed J. C., Raff M. C. Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature. 1993 Jan 28;361(6410):365–369. doi: 10.1038/361365a0. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Wyllie A. H., Currie A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972 Aug;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozopas K. M., Yang T., Buchan H. L., Zhou P., Craig R. W. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski S., Tanaka S., Takayama S., Schibler M. J., Fenton W., Reed J. C. Investigation of the subcellular distribution of the bcl-2 oncoprotein: residence in the nuclear envelope, endoplasmic reticulum, and outer mitochondrial membranes. Cancer Res. 1993 Oct 1;53(19):4701–4714. [PubMed] [Google Scholar]
- Lagasse E., Weissman I. L. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994 Mar 1;179(3):1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Hematopoietic cytokines inhibit apoptosis induced by transforming growth factor beta 1 and cancer chemotherapy compounds in myeloid leukemic cells. Blood. 1992 Oct 1;80(7):1750–1757. [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Bradley J. G., Cotter T. G. HL-60 cells induced to differentiate towards neutrophils subsequently die via apoptosis. Clin Exp Immunol. 1990 Mar;79(3):448–453. doi: 10.1111/j.1365-2249.1990.tb08110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. J., Cotter T. G. Ultraviolet B irradiation of human leukaemia HL-60 cells in vitro induces apoptosis. Int J Radiat Biol. 1991 Apr;59(4):1001–1016. doi: 10.1080/09553009114550891. [DOI] [PubMed] [Google Scholar]
- Martin S. J., Lennon S. V., Bonham A. M., Cotter T. G. Induction of apoptosis (programmed cell death) in human leukemic HL-60 cells by inhibition of RNA or protein synthesis. J Immunol. 1990 Sep 15;145(6):1859–1867. [PubMed] [Google Scholar]
- McConkey D. J., Aguilar-Santelises M., Hartzell P., Eriksson I., Mellstedt H., Orrenius S., Jondal M. Induction of DNA fragmentation in chronic B-lymphocytic leukemia cells. J Immunol. 1991 Feb 1;146(3):1072–1076. [PubMed] [Google Scholar]
- McDonnell T. J., Nunez G., Platt F. M., Hockenberry D., London L., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2-immunoglobulin transgene expands a resting but responsive immunoglobulin M and D-expressing B-cell population. Mol Cell Biol. 1990 May;10(5):1901–1907. doi: 10.1128/mcb.10.5.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meikrantz W., Gisselbrecht S., Tam S. W., Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino R., Ding L., Veis D. J., Korsmeyer S. J., Nuñez G. Developmental regulation of the Bcl-2 protein and susceptibility to cell death in B lymphocytes. EMBO J. 1994 Feb 1;13(3):683–691. doi: 10.1002/j.1460-2075.1994.tb06307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993 Jan 1;81(1):151–157. [PubMed] [Google Scholar]
- Monaghan P., Robertson D., Amos T. A., Dyer M. J., Mason D. Y., Greaves M. F. Ultrastructural localization of bcl-2 protein. J Histochem Cytochem. 1992 Dec;40(12):1819–1825. doi: 10.1177/40.12.1453000. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Cleary M. L. Bcl2 inhibits apoptosis associated with terminal differentiation of HL-60 myeloid leukemia cells. Blood. 1994 Apr 15;83(8):2261–2267. [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Oltvai Z. N., Milliman C. L., Korsmeyer S. J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993 Aug 27;74(4):609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- Park J. R., Robertson K., Hickstein D. D., Tsai S., Hockenbery D. M., Collins S. J. Dysregulated bcl-2 expression inhibits apoptosis but not differentiation of retinoic acid-induced HL-60 granulocytes. Blood. 1994 Jul 15;84(2):440–445. [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. J., Prochownik E., Gottlieb C. A., Apel I. J., Merino R., Nuñez G., Clarke M. F. c-myc and bcl-2 modulate p53 function by altering p53 subcellular trafficking during the cell cycle. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5878–5882. doi: 10.1073/pnas.91.13.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J. S., Wyllie A. H., Henson J. E., Walport M. J., Henson P. M., Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest. 1989 Mar;83(3):865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Trayner I. D., Clemens M. J. Phorbol ester-induced macrophage-like differentiation of human promyelocytic leukemia (HL-60) cells occurs independently of transferrin availability. Cancer Res. 1990 Nov 15;50(22):7221–7225. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Walker N. I., Harmon B. V., Gobé G. C., Kerr J. F. Patterns of cell death. Methods Achiev Exp Pathol. 1988;13:18–54. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]