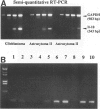
Abstract
Interleukin 10 (IL-10) was initially discovered on the basis of its ability to suppress cytokine synthesis. Additionally, it can exert immunosuppressive effects on a variety of cell types. Because patients with malignant gliomas present with a general impairment of the immune system, we investigated IL-10 expression in the glioma tissue. Because expression of IL-10 and IL-6 is associated in hematopoietic cells and IL-6 can act as an autocrine growth stimulator for glioblastoma cell lines, we looked in addition for a relationship between IL-10 and IL-6 expression. Using a quantitative reverse transcriptase polymerase chain reaction, IL-10 and IL-6 mRNA levels were determined in 37 glial tumors of different grades including 2 recurrencies, 3 specimens from normal brain tissue, and 3 glioblastoma cell lines. Expression of IL-10 mRNA was demonstrable in all tumors as well as in normal brain. High grade tumors and recurrent cases expressed significantly higher amounts of IL-10-specific mRNA compared with low grade tumors, whereas 2 of 3 cell lines showed only weak constitutive expression, mRNA for IL-6 was found in 86.5% of all gliomas with a correlation concerning the expression levels for both cytokines in 69% of gliomas. We suggest that IL-10 may contribute to the progression of astrocytomas by suppressing the patient's immune response, whereas IL-6 provides an additional growth advantage.
Full text
PDF
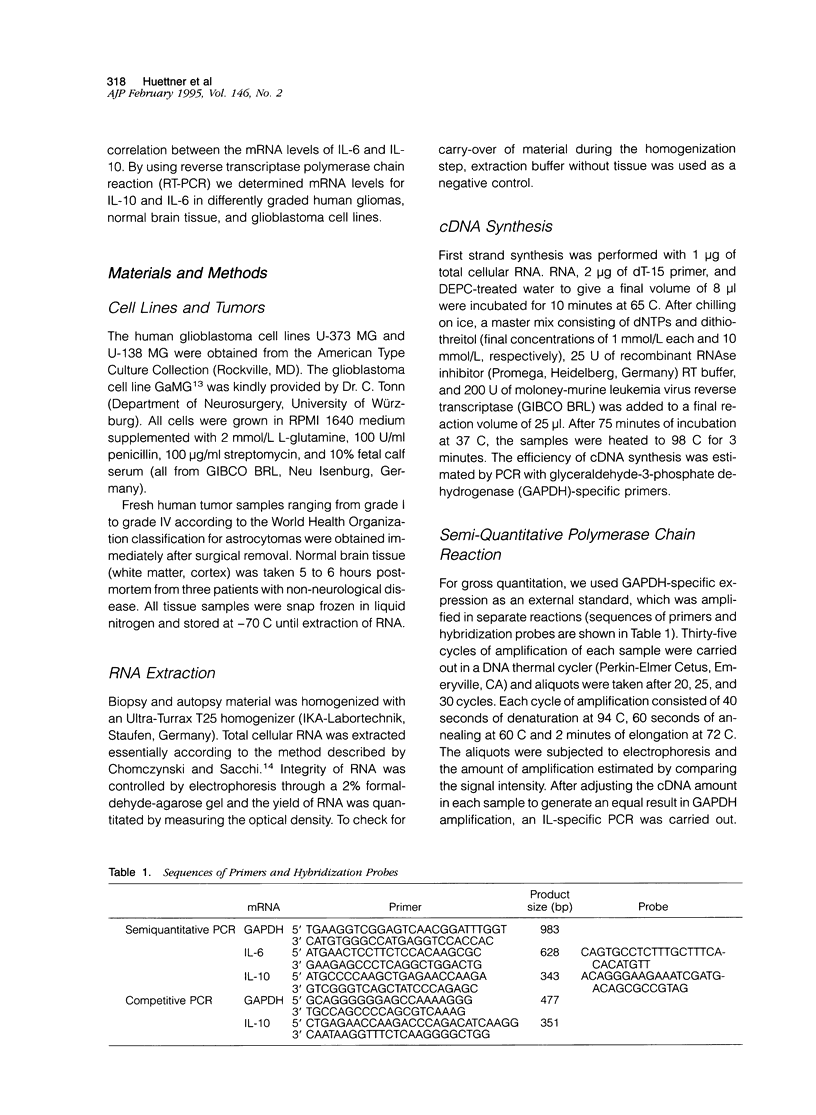
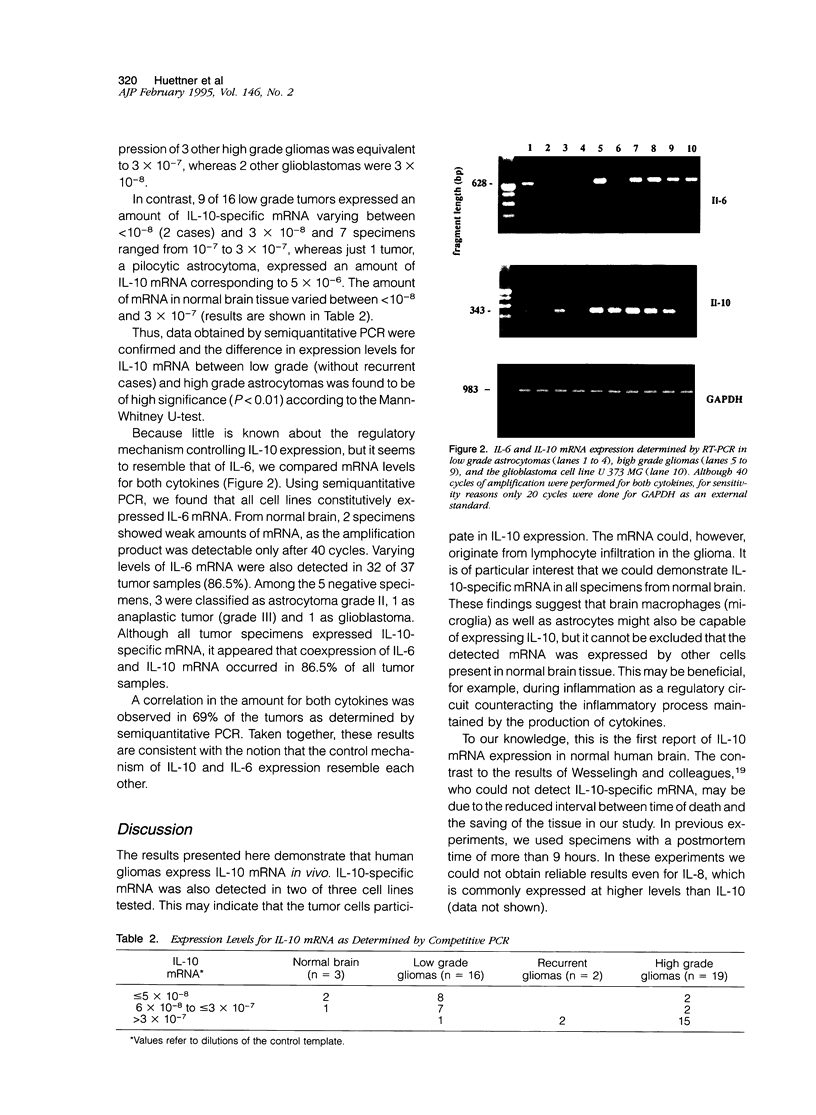


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blay J. Y., Burdin N., Rousset F., Lenoir G., Biron P., Philip T., Banchereau J., Favrot M. C. Serum interleukin-10 in non-Hodgkin's lymphoma: a prognostic factor. Blood. 1993 Oct 1;82(7):2169–2174. [PubMed] [Google Scholar]
- Bouaboula M., Legoux P., Pességué B., Delpech B., Dumont X., Piechaczyk M., Casellas P., Shire D. Standardization of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J Biol Chem. 1992 Oct 25;267(30):21830–21838. [PubMed] [Google Scholar]
- Castelli M. G., Chiabrando C., Fanelli R., Martelli L., Butti G., Gaetani P., Paoletti P. Prostaglandin and thromboxane synthesis by human intracranial tumors. Cancer Res. 1989 Mar 15;49(6):1505–1508. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Enk A. H., Katz S. I. Identification and induction of keratinocyte-derived IL-10. J Immunol. 1992 Jul 1;149(1):92–95. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastl G. A., Abrams J. S., Nanus D. M., Oosterkamp R., Silver J., Liu F., Chen M., Albino A. P., Bander N. H. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int J Cancer. 1993 Aug 19;55(1):96–101. doi: 10.1002/ijc.2910550118. [DOI] [PubMed] [Google Scholar]
- Gilliland G., Perrin S., Blanchard K., Bunn H. F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb W. H., Abrams J. S., Watson J. M., Velu T. J., Berek J. S., Martínez-Maza O. Presence of interleukin 10 (IL-10) in the ascites of patients with ovarian and other intra-abdominal cancers. Cytokine. 1992 Sep;4(5):385–390. doi: 10.1016/1043-4666(92)90082-3. [DOI] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Kim J. M., Brannan C. I., Copeland N. G., Jenkins N. A., Khan T. A., Moore K. W. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992 Jun 1;148(11):3618–3623. [PubMed] [Google Scholar]
- Lichtor T., Dohrmann G. J., Gurney M. E. Cytokine gene expression by human gliomas. Neurosurgery. 1990 May;26(5):788–793. doi: 10.1097/00006123-199005000-00009. [DOI] [PubMed] [Google Scholar]
- Miki S., Iwano M., Miki Y., Yamamoto M., Tang B., Yokokawa K., Sonoda T., Hirano T., Kishimoto T. Interleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomas. FEBS Lett. 1989 Jul 3;250(2):607–610. doi: 10.1016/0014-5793(89)80805-1. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Moore K. W. The role of IL-10 in crossregulation of TH1 and TH2 responses. Immunol Today. 1991 Mar;12(3):A49–A53. doi: 10.1016/S0167-5699(05)80015-5. [DOI] [PubMed] [Google Scholar]
- Muñoz-Fernández M. A., Fresno M. Involvement of nitric oxide on the cytokine induced growth of glial cell. Biochem Biophys Res Commun. 1993 Jul 15;194(1):319–325. doi: 10.1006/bbrc.1993.1822. [DOI] [PubMed] [Google Scholar]
- Phipps R. P., Stein S. H., Roper R. L. A new view of prostaglandin E regulation of the immune response. Immunol Today. 1991 Oct;12(10):349–352. doi: 10.1016/0167-5699(91)90064-Z. [DOI] [PubMed] [Google Scholar]
- Roszman T., Elliott L., Brooks W. Modulation of T-cell function by gliomas. Immunol Today. 1991 Oct;12(10):370–374. doi: 10.1016/0167-5699(91)90068-5. [DOI] [PubMed] [Google Scholar]
- Van Meir E., Sawamura Y., Diserens A. C., Hamou M. F., de Tribolet N. Human glioblastoma cells release interleukin 6 in vivo and in vitro. Cancer Res. 1990 Oct 15;50(20):6683–6688. [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira P., de Waal-Malefyt R., Dang M. N., Johnson K. E., Kastelein R., Fiorentino D. F., deVries J. E., Roncarolo M. G., Mosmann T. R., Moore K. W. Isolation and expression of human cytokine synthesis inhibitory factor cDNA clones: homology to Epstein-Barr virus open reading frame BCRFI. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1172–1176. doi: 10.1073/pnas.88.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselingh S. L., Power C., Glass J. D., Tyor W. R., McArthur J. C., Farber J. M., Griffin J. W., Griffin D. E. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993 Jun;33(6):576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]