Abstract
This study characterized agent differential permeability, three-dimensional tumor volume, and survival in an LX-1 human small cell lung carcinoma intracerebral xenograft model in the nude rat. The percent accessible tissue space (distribution volume) and the permeability x capillary surface product for aminoisobutyric acid (M(r) 103), methotrexate (M(r) 454), dextran 10 (M(r) 10,000), and dextran 70 (M(r) 70,000) were measured between 8 and 16 days after inoculation of tumor. Magnetic resonance imaging and histology were used to quantitate intracerebral tumor volume (mm3). Accessible tissue space (ml/g) and permeability x capillary surface product in intracranial tumor, surrounding brain, and subcutaneous tumor decreased with increasing molecular weight of the agent, regardless of the number of days after inoculation. Accessible tissue space in intracranial tumor increased between 8 and 16 days for all agents except dextran 70. There was little change in the subcutaneous tumor or other tissues with time. Tumor volume calculations from imaging studies correlated with volumetric measurements from histological sections (r2 = 98.5%) and illustrated natural tumor progression (9 to 225 mm3). These results provide a basis for therapeutic design based on differential permeability of specific agents and the ability to quantitatively measure brain tumor volume for accessing tumor response.
Full text
PDF


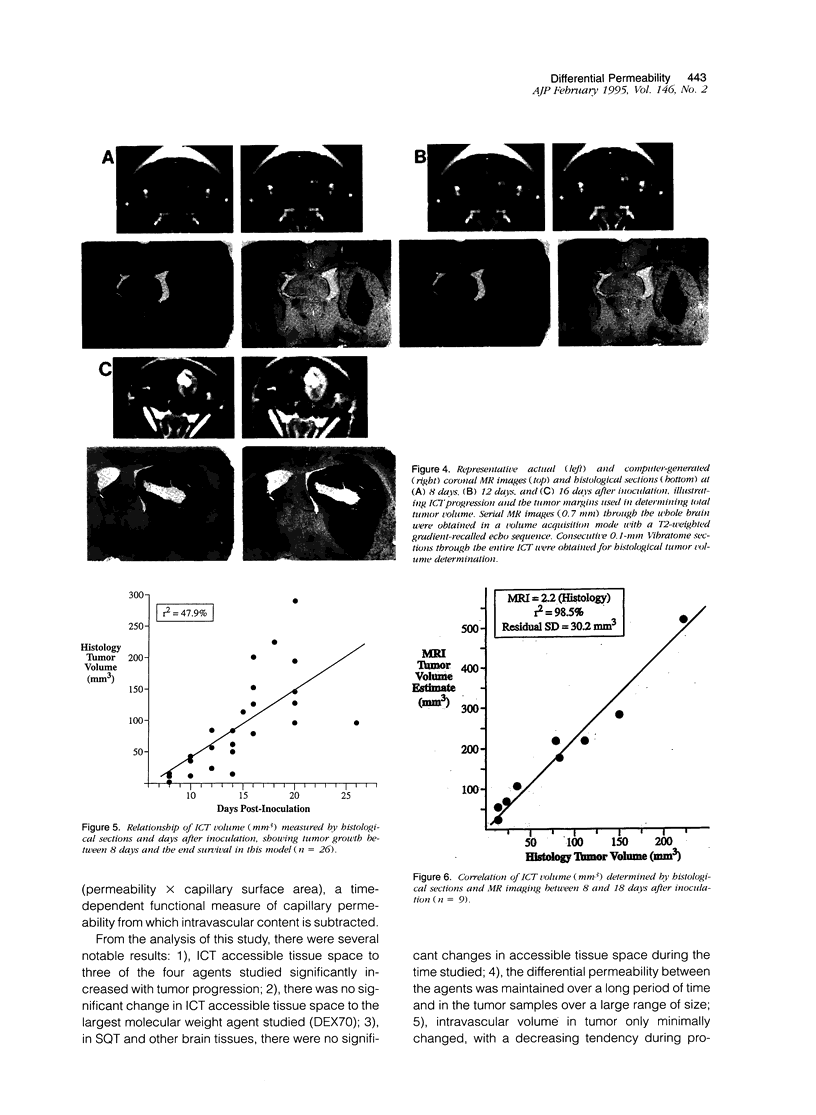






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
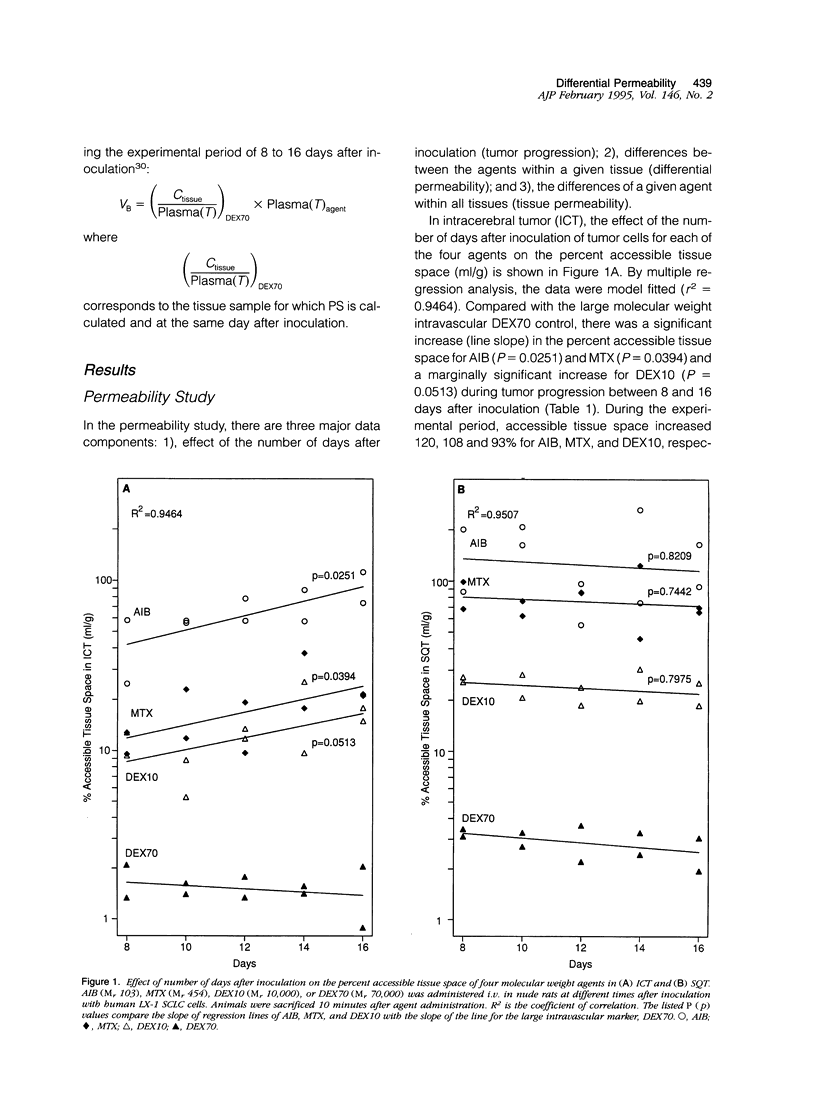
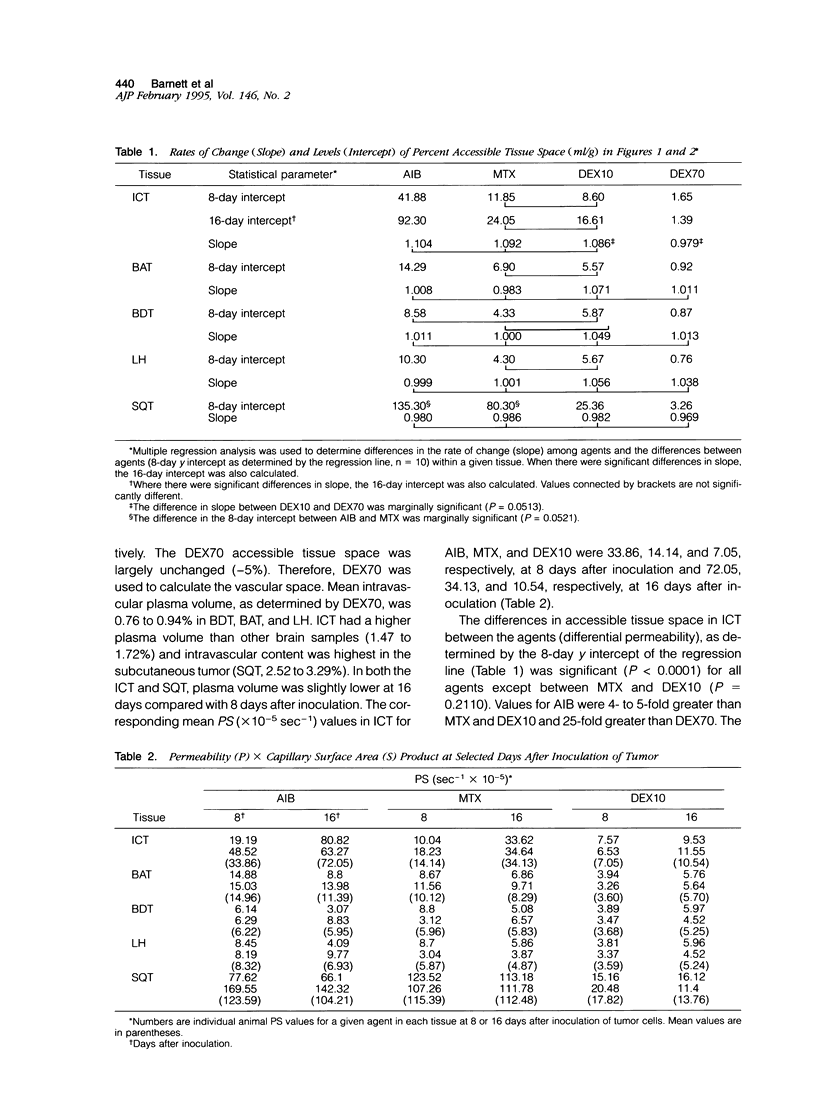
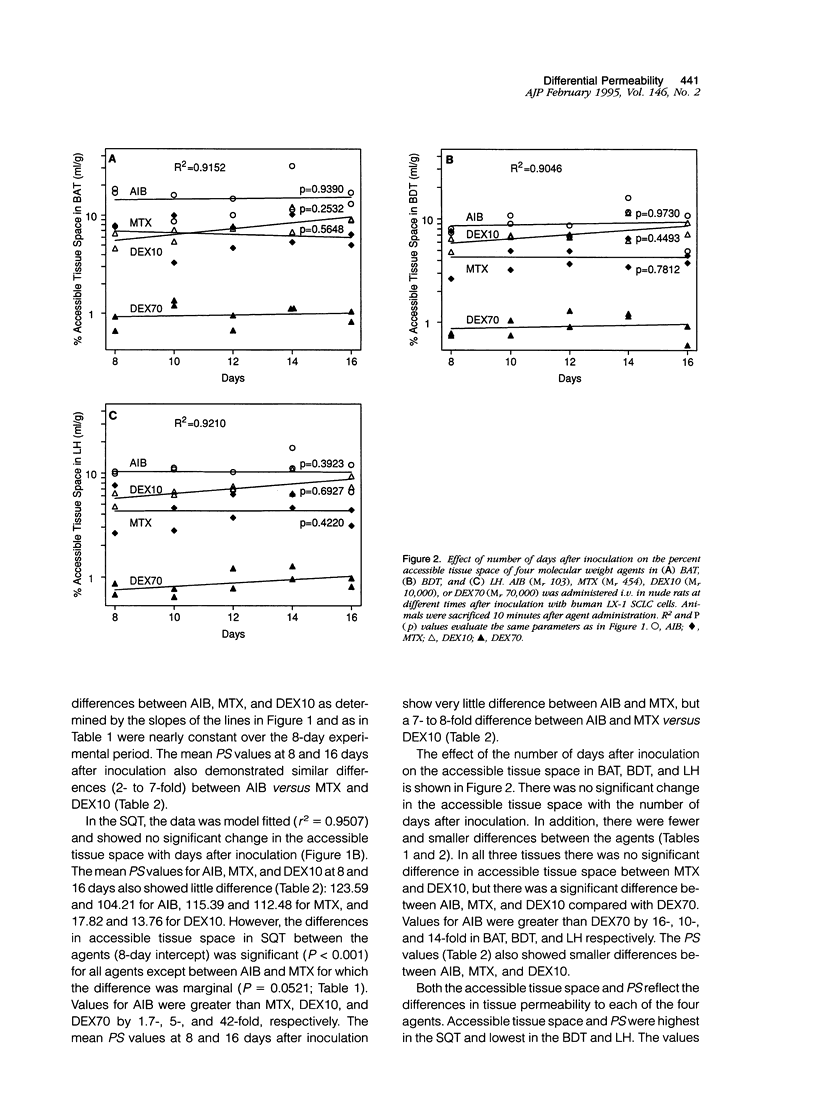
- Aicher K. P., Dupon J. W., White D. L., Aukerman S. L., Moseley M. E., Juster R., Rosenau W., Winkelhake J. L., Brasch R. C. Contrast-enhanced magnetic resonance imaging of tumor-bearing mice treated with human recombinant tumor necrosis factor alpha. Cancer Res. 1990 Nov 15;50(22):7376–7381. [PubMed] [Google Scholar]
- Baba T., Moriguchi M., Natori Y., Katsuki C., Inoue T., Fukui M. Magnetic resonance imaging of experimental rat brain tumors: histopathological evaluation. Surg Neurol. 1990 Dec;34(6):378–382. doi: 10.1016/0090-3019(90)90240-p. [DOI] [PubMed] [Google Scholar]
- Baer G. A., Talonen P. P., Häkkinen V. K., Frey H., Ojala J. K., Markkula H. Funktionell elstimulering av nervus phrenicus och dess kliniska betydelse. Nord Med. 1992;107(6-7):191–194. [PubMed] [Google Scholar]
- Bockhorst K., Hoehn-Berlage M., Kocher M., Hossmann K. A. MRI contrast enhancement by MnTPPS of experimental brain tumours in rats. Acta Neurochir Suppl (Wien) 1990;51:134–136. doi: 10.1007/978-3-7091-9115-6_45. [DOI] [PubMed] [Google Scholar]
- Burger P. C., Heinz E. R., Shibata T., Kleihues P. Topographic anatomy and CT correlations in the untreated glioblastoma multiforme. J Neurosurg. 1988 May;68(5):698–704. doi: 10.3171/jns.1988.68.5.0698. [DOI] [PubMed] [Google Scholar]
- Curd J., Smith T. W., Jaton J. C., Haber E. The isolation of digoxin-specific antibody and its use in reversing the effects of digoxin. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2401–2406. doi: 10.1073/pnas.68.10.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. C., Hudgins P. A., Peterman S. B., Hoffman J. C., Jr Diagnosis of cerebral metastases: double-dose delayed CT vs contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 1991 Mar-Apr;12(2):293–300. [PMC free article] [PubMed] [Google Scholar]
- Friedman H. S., Schold S. C., Jr, Bigner D. D. Chemotherapy of subcutaneous and intracranial human medulloblastoma xenografts in athymic nude mice. Cancer Res. 1986 Jan;46(1):224–228. [PubMed] [Google Scholar]
- Galloway R. L., Jr, Maciunas R. J., Failinger A. L., Whelan H. T. Volumetric measurement of canine gliomas using MRI. Magn Reson Imaging. 1990;8(2):161–165. doi: 10.1016/0730-725x(90)90249-2. [DOI] [PubMed] [Google Scholar]
- Genka S., Deutsch J., Stahle P. L., Shetty U. H., John V., Robinson C., Rapoport S. I., Greig N. H. Brain and plasma pharmacokinetics and anticancer activities of cyclophosphamide and phosphoramide mustard in the rat. Cancer Chemother Pharmacol. 1990;27(1):1–7. doi: 10.1007/BF00689268. [DOI] [PubMed] [Google Scholar]
- Greig N. H., Jones H. B., Cavanagh J. B. Blood-brain barrier integrity and host responses in experimental metastatic brain tumours. Clin Exp Metastasis. 1983 Jul-Sep;1(3):229–246. doi: 10.1007/BF00736407. [DOI] [PubMed] [Google Scholar]
- Groothuis D. R., Fischer J. M., Vick N. A., Bigner D. D. Comparative permeability of different glioma models to horseradish peroxidase. Cancer Treat Rep. 1981;65 (Suppl 2):13–18. [PubMed] [Google Scholar]
- Groothuis D. R., Lapin G. D., Vriesendorp F. J., Mikhael M. A., Patlak C. S. A method to quantitatively measure transcapillary transport of iodinated compounds in canine brain tumors with computed tomography. J Cereb Blood Flow Metab. 1991 Nov;11(6):939–948. doi: 10.1038/jcbfm.1991.159. [DOI] [PubMed] [Google Scholar]
- Groothuis D. R., Vriesendorp F. J., Kupfer B., Warnke P. C., Lapin G. D., Kuruvilla A., Vick N. A., Mikhael M. A., Patlak C. S. Quantitative measurements of capillary transport in human brain tumors by computed tomography. Ann Neurol. 1991 Oct;30(4):581–588. doi: 10.1002/ana.410300411. [DOI] [PubMed] [Google Scholar]
- Hagan P. L., Halpern S. E., Dillman R. O., Shawler D. L., Johnson D. E., Chen A., Krishnan L., Frincke J., Bartholomew R. M., David G. S. Tumor size: effect on monoclonal antibody uptake in tumor models. J Nucl Med. 1986 Mar;27(3):422–427. [PubMed] [Google Scholar]
- Healy M. E., Hesselink J. R., Press G. A., Middleton M. S. Increased detection of intracranial metastases with intravenous Gd-DTPA. Radiology. 1987 Dec;165(3):619–624. doi: 10.1148/radiology.165.3.3317496. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Haemodynamic and transport barriers to the treatment of solid tumours. Int J Radiat Biol. 1991 Jul-Aug;60(1-2):85–100. doi: 10.1080/09553009114551621. [DOI] [PubMed] [Google Scholar]
- Kallman R. F., Brown J. M., Denekamp J., Hill R. P., Kummermehr J. The use of rodent tumors in experimental cancer therapy. Conclusions and recommendations from an international workshop. Cancer Res. 1985 Dec;45(12 Pt 1):6541–6545. [PubMed] [Google Scholar]
- Kato Y., Paterson A., Langone J. J. Monoclonal antibodies to the chemotherapeutic agent methotrexate: production, properties and comparison with polyclonal antibodies. J Immunol Methods. 1984 Mar 16;67(2):321–336. doi: 10.1016/0022-1759(84)90472-1. [DOI] [PubMed] [Google Scholar]
- Kelly P. J., Daumas-Duport C., Kispert D. B., Kall B. A., Scheithauer B. W., Illig J. J. Imaging-based stereotaxic serial biopsies in untreated intracranial glial neoplasms. J Neurosurg. 1987 Jun;66(6):865–874. doi: 10.3171/jns.1987.66.6.0865. [DOI] [PubMed] [Google Scholar]
- Lee Y., Bullard D. E., Humphrey P. A., Colapinto E. V., Friedman H. S., Zalutsky M. R., Coleman R. E., Bigner D. D. Treatment of intracranial human glioma xenografts with 131I-labeled anti-tenascin monoclonal antibody 81C6. Cancer Res. 1988 May 15;48(10):2904–2910. [PubMed] [Google Scholar]
- Molnar P., Blasberg R. G., Groothuis D., Bigner D., Fenstermacher J. D. Regional blood-to-tissue transport in avian sarcoma virus (ASV)-induced brain tumors. Neurology. 1983 Jun;33(6):702–711. doi: 10.1212/wnl.33.6.702. [DOI] [PubMed] [Google Scholar]
- Nazzaro J. M., Rosenbaum L. C., Pagel M. A., Neuwelt E. A. A new model of systemic drug rescue based on permeability characteristics of the blood-brain barrier in intracerebral abscess-bearing rats. J Neurosurg. 1991 Mar;74(3):467–474. doi: 10.3171/jns.1991.74.3.0467. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Barnett P. A., Frenkel E. P. Chemotherapeutic agent permeability to normal brain and delivery to avian sarcoma virus-induced brain tumors in the rodent: observations on problems of drug delivery. Neurosurgery. 1984 Feb;14(2):154–160. doi: 10.1227/00006123-198402000-00006. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Barnett P. A., Ramsey F. L., Hellström I., Hellström K. E., McCormick C. I. Dexamethasone decreases the delivery of tumor-specific monoclonal antibody to both intracerebral and subcutaneous tumor xenografts. Neurosurgery. 1993 Sep;33(3):478–484. doi: 10.1227/00006123-199309000-00018. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Frenkel E. P., D'Agostino A. N., Carney D. N., Minna J. D., Barnett P. A., McCormick C. I. Growth of human lung tumor in the brain of the nude rat as a model to evaluate antitumor agent delivery across the blood-brain barrier. Cancer Res. 1985 Jun;45(6):2827–2833. [PubMed] [Google Scholar]
- Neuwelt E. A., Specht H. D., Hill S. A. Permeability of human brain tumor to 99mTc-gluco-heptonate and 99mTc-albumin. Implications for monoclonal antibody therapy. J Neurosurg. 1986 Aug;65(2):194–198. doi: 10.3171/jns.1986.65.2.0194. [DOI] [PubMed] [Google Scholar]
- Nir I., Levanon D., Iosilevsky G. Permeability of blood vessels in experimental gliomas: uptake of 99mTc-glucoheptonate and alteration in blood-brain barrier as determined by cytochemistry and electron microscopy. Neurosurgery. 1989 Oct;25(4):523–532. [PubMed] [Google Scholar]
- Ohata M., Fredericks W. R., Neuwelt E. A., Sundaram U., Rapoport S. I. [3H]Methotrexate loss from the rat brain following enhanced uptake by osmotic opening of the blood-brain barrier. Cancer Res. 1985 Mar;45(3):1092–1096. [PubMed] [Google Scholar]
- REED D. J., WOODBURY D. M. KINETICS OF MOVEMENT OF IODIDE, SUCROSE, INULIN AND RADIO-IODINATED SERUM ALBUMIN IN THE CENTRAL NERVOUS SYSTEM AND CEREBROSPINAL FLUID OF THE RAT. J Physiol. 1963 Dec;169:816–850. doi: 10.1113/jphysiol.1963.sp007298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan S. S., Rosa L., Francisco J., Muraki A., Carvlin M., Tuturea E. MRI characterization of 9L-glioma in rat brain at 4.7 Tesla. Magn Reson Imaging. 1990;8(2):185–190. doi: 10.1016/0730-725x(90)90252-w. [DOI] [PubMed] [Google Scholar]
- Robinson P. J., Rapoport S. I. Model for drug uptake by brain tumors: effects of osmotic treatment and of diffusion in brain. J Cereb Blood Flow Metab. 1990 Mar;10(2):153–161. doi: 10.1038/jcbfm.1990.30. [DOI] [PubMed] [Google Scholar]
- Runge V. M., Jacobson S., Wood M. L., Kaufman D., Adelman L. S. MR imaging of rat brain glioma: Gd-DTPA versus Gd-DOTA. Radiology. 1988 Mar;166(3):835–838. doi: 10.1148/radiology.166.3.3401296. [DOI] [PubMed] [Google Scholar]
- Russell E. J., Geremia G. K., Johnson C. E., Huckman M. S., Ramsey R. G., Washburn-Bleck J., Turner D. A., Norusis M. Multiple cerebral metastases: detectability with Gd-DTPA-enhanced MR imaging. Radiology. 1987 Dec;165(3):609–617. doi: 10.1148/radiology.165.3.3317495. [DOI] [PubMed] [Google Scholar]
- Schmiedl U. P., Kenney J., Maravilla K. R. Dyke Award Paper. Kinetics of pathologic blood-brain-barrier permeability in an astrocytic glioma using contrast-enhanced MR. AJNR Am J Neuroradiol. 1992 Jan-Feb;13(1):5–14. [PMC free article] [PubMed] [Google Scholar]
- Schold S. C., Jr, Rawlings C. E., 3rd, Bigner S. H., Bigner D. D. Intracerebral growth of a human glioma tumor line in athymic mice and treatment with procarbazine, 1,3-bis(2-chloroethyl)-1-nitrosourea, aziridinylbenzoquinone, and cis-platinum. Neurosurgery. 1983 Jun;12(6):672–677. doi: 10.1227/00006123-198306000-00014. [DOI] [PubMed] [Google Scholar]
- Senter P. D., Saulnier M. G., Schreiber G. J., Hirschberg D. L., Brown J. P., Hellström I., Hellström K. E. Anti-tumor effects of antibody-alkaline phosphatase conjugates in combination with etoposide phosphate. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4842–4846. doi: 10.1073/pnas.85.13.4842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senter P. D., Schreiber G. J., Hirschberg D. L., Ashe S. A., Hellström K. E., Hellström I. Enhancement of the in vitro and in vivo antitumor activities of phosphorylated mitomycin C and etoposide derivatives by monoclonal antibody-alkaline phosphatase conjugates. Cancer Res. 1989 Nov 1;49(21):5789–5792. [PubMed] [Google Scholar]
- Symon L. Management of giant intracranial aneurysms. Acta Neurochir (Wien) 1992;116(2-4):107–118. doi: 10.1007/BF01540863. [DOI] [PubMed] [Google Scholar]
- Sze G., Milano E., Johnson C., Heier L. Detection of brain metastases: comparison of contrast-enhanced MR with unenhanced MR and enhanced CT. AJNR Am J Neuroradiol. 1990 Jul-Aug;11(4):785–791. [PMC free article] [PubMed] [Google Scholar]
- Vriesendorp F. J., Peagram C., Bigner D. D., Groothuis D. R. Concurrent measurements of blood flow and transcapillary transport in xenotransplanted human gliomas in immunosuppressed rats. J Natl Cancer Inst. 1987 Jul;79(1):123–130. [PubMed] [Google Scholar]
- Warnke P. C., Friedman H. S., Bigner D. D., Groothuis D. R. Simultaneous measurements of blood flow and blood-to-tissue transport in xenotransplanted medulloblastomas. Cancer Res. 1987 Mar 15;47(6):1687–1690. [PubMed] [Google Scholar]
- Wedeking P., Kumar K., Tweedle M. F. Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging. 1992;10(4):641–648. doi: 10.1016/0730-725x(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Wheeler K. T. Review of factors influencing the response of experimental brain tumors to therapy. Cancer Treat Rep. 1981;65 (Suppl 2):75–81. [PubMed] [Google Scholar]
- Whelan H. T., Clanton J. A., Moore P. M., Tolner D. J., Kessler R. M., Whetsell W. O., Jr Magnetic resonance brain tumor imaging in canine glioma. Neurology. 1987 Jul;37(7):1235–1239. doi: 10.1212/wnl.37.7.1235. [DOI] [PubMed] [Google Scholar]
- Yamada K., Hayakawa T., Ushio Y., Arita N., Kato A., Mogami H. Regional blood flow and capillary permeability in the ethylnitrosourea-induced rat glioma. J Neurosurg. 1981 Dec;55(6):922–928. doi: 10.3171/jns.1981.55.6.0922. [DOI] [PubMed] [Google Scholar]
- Yamada K., Ushio Y., Hayakawa T., Kato A., Yamada N., Mogami H. Quantitative autoradiographic measurements of blood-brain barrier permeability in the rat glioma model. J Neurosurg. 1982 Sep;57(3):394–398. doi: 10.3171/jns.1982.57.3.0394. [DOI] [PubMed] [Google Scholar]
- Yoshida K., Furuse M., Kaneoke Y., Saso K., Inao S., Motegi Y., Ichihara K., Izawa A. Assessment of T1 time course changes and tissue-blood ratios after Gd-DTPA administration in brain tumors. Magn Reson Imaging. 1989 Jan-Feb;7(1):9–15. doi: 10.1016/0730-725x(89)90319-6. [DOI] [PubMed] [Google Scholar]
- Zhang R. D., Price J. E., Fujimaki T., Bucana C. D., Fidler I. J. Differential permeability of the blood-brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992 Nov;141(5):1115–1124. [PMC free article] [PubMed] [Google Scholar]



