Abstract
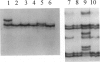
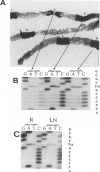
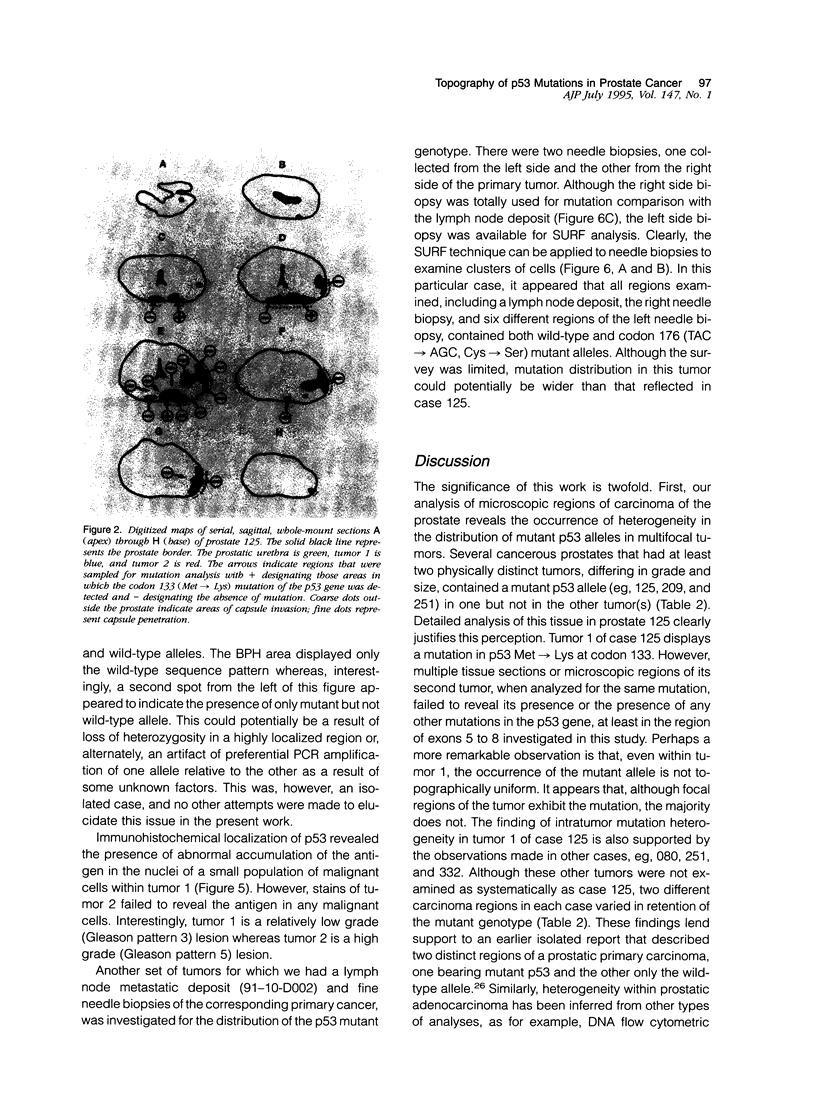
Prostatic carcinoma from 65 patients have been examined for the occurrence of point mutations in the p53 tumor suppressor gene locus within the region of exons 5 to 8. Overall, only a small fraction of tumors (12.3%) was found to contain p53 mutations. No significant correlation was detected between the presence of the mutant gene and either tumor volume or histopathological grade. However, metastatic prostatic tumors are found to display a higher percentage (21.4%) of p53 mutations compared with primary adenocarcinomas (9.8%). Analysis of the topographical distribution of the p53 mutant genotype revealed two remarkable findings. First, multifocal tumors within a prostate appear to differ in harboring the mutant gene, and second, evidence is obtained for intratumor heterogeneity in the distribution of the mutant p53 allele. Together these findings appear to explain, at least in part, why there has been a wide discrepancy in the reported detection frequency of p53 mutations in prostate cancer specimens. It appears that the outcome of mutation analysis would depend not only on which tumors but also which regions of the tumors are included in the study. Furthermore, the observed heterogeneous topographical distribution of the mutation, if confirmed to be unique to prostate cancer, may have important implications in the understanding of the biology of prostate carcinogenesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bookstein R., MacGrogan D., Hilsenbeck S. G., Sharkey F., Allred D. C. p53 is mutated in a subset of advanced-stage prostate cancers. Cancer Res. 1993 Jul 15;53(14):3369–3373. [PubMed] [Google Scholar]
- Caron de Fromentel C., Soussi T. TP53 tumor suppressor gene: a model for investigating human mutagenesis. Genes Chromosomes Cancer. 1992 Jan;4(1):1–15. doi: 10.1002/gcc.2870040102. [DOI] [PubMed] [Google Scholar]
- Carter B. S., Ewing C. M., Ward W. S., Treiger B. F., Aalders T. W., Schalken J. A., Epstein J. I., Isaacs W. B. Allelic loss of chromosomes 16q and 10q in human prostate cancer. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8751–8755. doi: 10.1073/pnas.87.22.8751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S. G., deVere White R. W., Meyers F. J., Siders D. B., Lee F., Gumerlock P. H. p53 in prostate cancer: frequent expressed transition mutations. J Natl Cancer Inst. 1994 Jun 15;86(12):926–933. doi: 10.1093/jnci/86.12.926. [DOI] [PubMed] [Google Scholar]
- Deitch A. D., Miller G. J., deVere White R. W. Significance of abnormal diploid DNA histograms in localized prostate cancer and adjacent benign prostatic tissue. Cancer. 1993 Sep 1;72(5):1692–1700. doi: 10.1002/1097-0142(19930901)72:5<1692::aid-cncr2820720533>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Effert P. J., Neubauer A., Walther P. J., Liu E. T. Alterations of the P53 gene are associated with the progression of a human prostate carcinoma. J Urol. 1992 Mar;147(3 Pt 2):789–793. doi: 10.1016/s0022-5347(17)37387-1. [DOI] [PubMed] [Google Scholar]
- Epstein J. I., Carmichael M. J., Partin A. W., Walsh P. C. Small high grade adenocarcinoma of the prostate in radical prostatectomy specimens performed for nonpalpable disease: pathogenetic and clinical implications. J Urol. 1994 Jun;151(6):1587–1592. doi: 10.1016/s0022-5347(17)35309-0. [DOI] [PubMed] [Google Scholar]
- Esrig D., Spruck C. H., 3rd, Nichols P. W., Chaiwun B., Steven K., Groshen S., Chen S. C., Skinner D. G., Jones P. A., Cote R. J. p53 nuclear protein accumulation correlates with mutations in the p53 gene, tumor grade, and stage in bladder cancer. Am J Pathol. 1993 Nov;143(5):1389–1397. [PMC free article] [PubMed] [Google Scholar]
- Gaidano G., Ballerini P., Gong J. Z., Inghirami G., Neri A., Newcomb E. W., Magrath I. T., Knowles D. M., Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Isaacs W. B., Carter B. S., Ewing C. M. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991 Sep 1;51(17):4716–4720. [PubMed] [Google Scholar]
- Ittmann M., Wieczorek R., Heller P., Dave A., Provet J., Krolewski J. Alterations in the p53 and MDM-2 genes are infrequent in clinically localized, stage B prostate adenocarcinomas. Am J Pathol. 1994 Aug;145(2):287–293. [PMC free article] [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Momand J., Finlay C. A. The p53 tumour suppressor gene. Nature. 1991 Jun 6;351(6326):453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- Li Z. H., Zheng J., Weiss L. M., Shibata D. c-k-ras and p53 mutations occur very early in adenocarcinoma of the lung. Am J Pathol. 1994 Feb;144(2):303–309. [PMC free article] [PubMed] [Google Scholar]
- Miller G. J., Cygan J. M. Diagnostic correlations with whole mounts of radical prostatectomy specimens. Monogr Pathol. 1992;(34):183–197. [PubMed] [Google Scholar]
- Miller G. J., Cygan J. M. Morphology of prostate cancer: the effects of multifocality on histological grade, tumor volume and capsule penetration. J Urol. 1994 Nov;152(5 Pt 2):1709–1713. doi: 10.1016/s0022-5347(17)32368-6. [DOI] [PubMed] [Google Scholar]
- Milner B. J., Allan L. A., Eccles D. M., Kitchener H. C., Leonard R. C., Kelly K. F., Parkin D. E., Haites N. E. p53 mutation is a common genetic event in ovarian carcinoma. Cancer Res. 1993 May 1;53(9):2128–2132. [PubMed] [Google Scholar]
- Navone N. M., Troncoso P., Pisters L. L., Goodrow T. L., Palmer J. L., Nichols W. W., von Eschenbach A. C., Conti C. J. p53 protein accumulation and gene mutation in the progression of human prostate carcinoma. J Natl Cancer Inst. 1993 Oct 20;85(20):1657–1669. doi: 10.1093/jnci/85.20.1657. [DOI] [PubMed] [Google Scholar]
- O'Malley F. P., Grignon D. J., Keeney M., Kerkvliet N., McLean C. DNA heterogeneity in prostatic adenocarcinoma. A DNA flow cytometric mapping study with whole organ sections of prostate. Cancer. 1993 May 1;71(9):2797–2802. doi: 10.1002/1097-0142(19930501)71:9<2797::aid-cncr2820710918>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Sakr W. A., Macoska J. A., Benson P., Grignon D. J., Wolman S. R., Pontes J. E., Crissman J. D. Allelic loss in locally metastatic, multisampled prostate cancer. Cancer Res. 1994 Jun 15;54(12):3273–3277. [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata D., Hawes D., Li Z. H., Hernandez A. M., Spruck C. H., Nichols P. W. Specific genetic analysis of microscopic tissue after selective ultraviolet radiation fractionation and the polymerase chain reaction. Am J Pathol. 1992 Sep;141(3):539–543. [PMC free article] [PubMed] [Google Scholar]
- Stamey T. A., McNeal J. E., Freiha F. S., Redwine E. Morphometric and clinical studies on 68 consecutive radical prostatectomies. J Urol. 1988 Jun;139(6):1235–1241. doi: 10.1016/s0022-5347(17)42876-x. [DOI] [PubMed] [Google Scholar]
- Strickler J. G., Zheng J., Shu Q., Burgart L. J., Alberts S. R., Shibata D. p53 mutations and microsatellite instability in sporadic gastric cancer: when guardians fail. Cancer Res. 1994 Sep 1;54(17):4750–4755. [PubMed] [Google Scholar]
- Thompson S. J., Mellon K., Charlton R. G., Marsh C., Robinson M., Neal D. E. P53 and Ki-67 immunoreactivity in human prostate cancer and benign hyperplasia. Br J Urol. 1992 Jun;69(6):609–613. doi: 10.1111/j.1464-410x.1992.tb15632.x. [DOI] [PubMed] [Google Scholar]
- Uchida T., Wada C., Shitara T., Egawa S., Koshiba K. Infrequent involvement of p53 gene mutations in the tumourigenesis of Japanese prostate cancer. Br J Cancer. 1993 Oct;68(4):751–755. doi: 10.1038/bjc.1993.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villers A., McNeal J. E., Freiha F. S., Stamey T. A. Multiple cancers in the prostate. Morphologic features of clinically recognized versus incidental tumors. Cancer. 1992 Nov 1;70(9):2313–2318. doi: 10.1002/1097-0142(19921101)70:9<2313::aid-cncr2820700917>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Visakorpi T., Kallioniemi O. P., Heikkinen A., Koivula T., Isola J. Small subgroup of aggressive, highly proliferative prostatic carcinomas defined by p53 accumulation. J Natl Cancer Inst. 1992 Jun 3;84(11):883–887. doi: 10.1093/jnci/84.11.883. [DOI] [PubMed] [Google Scholar]
- Voeller H. J., Sugars L. Y., Pretlow T., Gelmann E. P. p53 oncogene mutations in human prostate cancer specimens. J Urol. 1994 Feb;151(2):492–495. doi: 10.1016/s0022-5347(17)35000-0. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Hannon G. J., Zhang H., Casso D., Kobayashi R., Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993 Dec 16;366(6456):701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]