Abstract
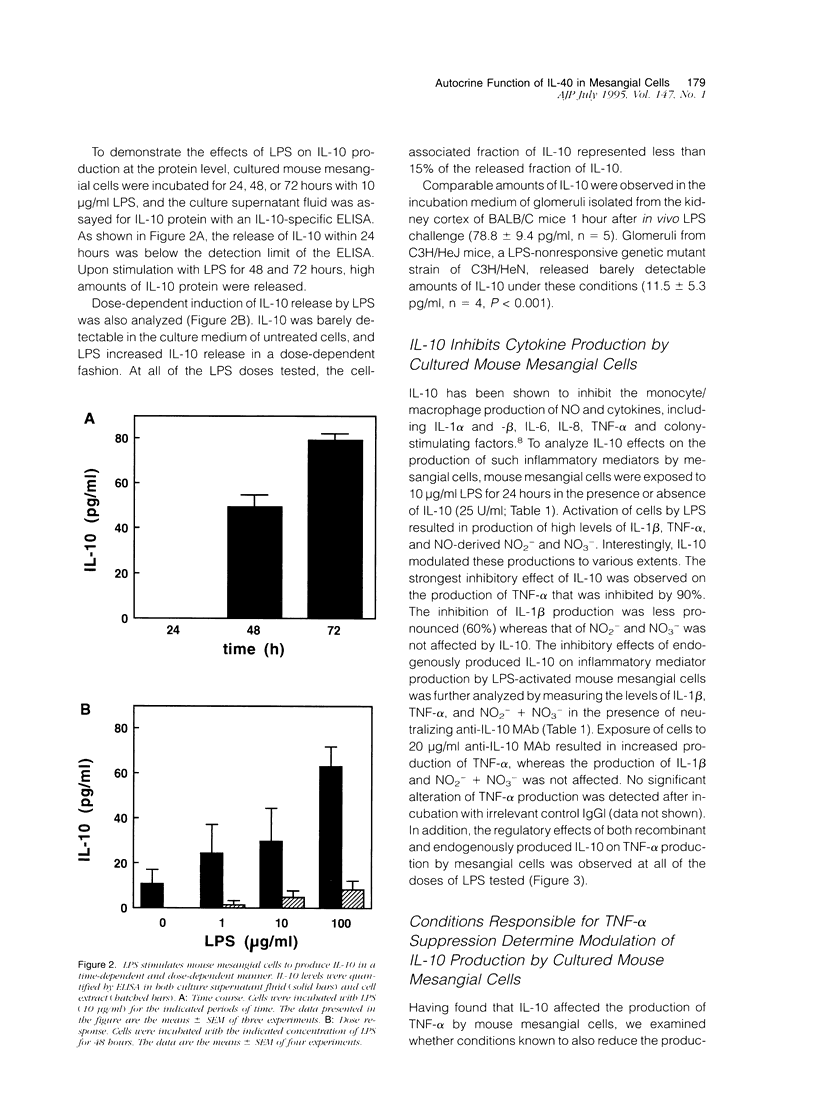
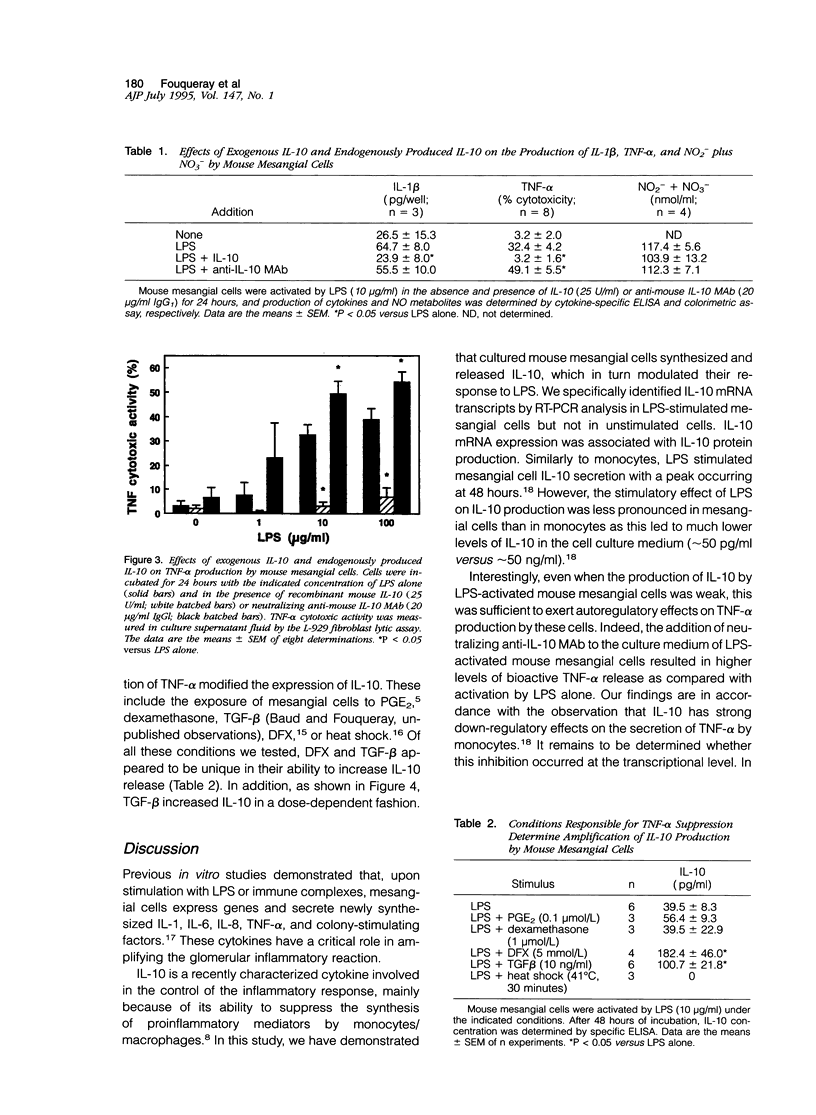
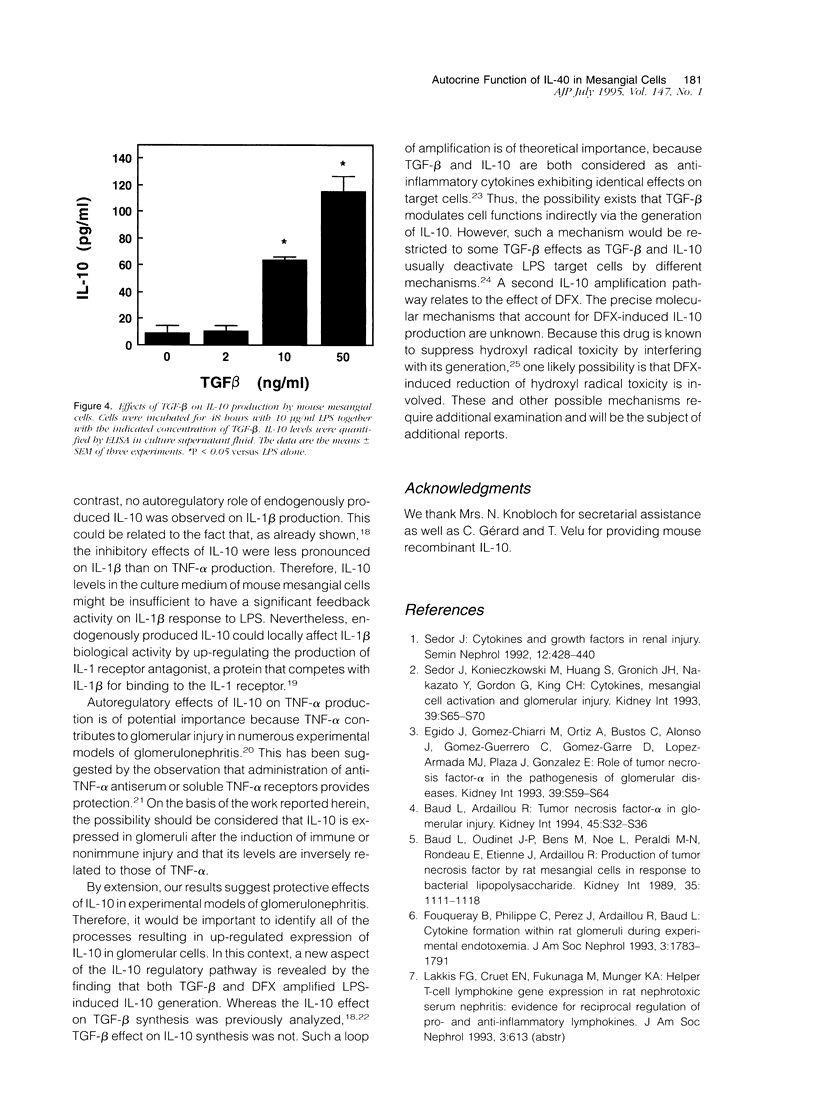
Interleukin (IL)-10 is a novel cytokine produced by a variety of cells, including monocytes/macrophages, upon exposure to lipopolysaccharide (LPS). Recent observations indicate that, in turn, IL-10 exerts suppressive effects on macrophage response to LPS. Because mesangial cells are also a target for LPS, we have examined the potential role of IL-10 in the regulation of mesangial cell response to LPS. To this aim, we have studied the synthesis and the autocrine/paracrine function of IL-10 in cultured mouse mesangial cells. IL-10 mRNA expression and IL-10 protein secretion were determined by a reverse transcription polymerase chain reaction technique and a specific enzyme-linked immunosorbent assay, respectively. No IL-10 mRNA expression was detectable in unactivated cells. LPS induced IL-10 mRNA expression in a dose-dependent fashion (1 to 100 micrograms/ml). In addition, LPS induced IL-10 protein release that was both dose dependent (1 to 100 micrograms/ml) and time dependent (24 to 72 hours). We have also studied the effect of IL-10 on the production of inflammatory mediators by LPS-activated mouse mesangial cells. Whereas recombinant IL-10 inhibited the generation of tumor necrosis factor-alpha (TNF-alpha) and IL-1 beta by 90 and 60%, respectively, it did not affect the formation of nitric oxide-derived nitrite (NO2-) and nitrate (NO3-). As shown by the use of anti-IL-10 monoclonal antibody, endogenously produced IL-10 affected the generation of TNF-alpha but neither that of IL-1 beta nor that of NO2- and NO3-. Finally, we have examined whether conditions known to also reduce the generation of TNF-alpha modified the expression of IL-10. Of all the conditions tested, only the addition of desferrioxamine and transforming growth factor-beta were found to increase IL-10 release. Together, these data demonstrate that mesangial cell-derived IL-10 has important regulatory effects on the inflammatory response of these cells to LPS because of its capacity to blunt TNF-alpha generation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affres H., Perez J., Hagege J., Fouqueray B., Kornprobst M., Ardaillou R., Baud L. Desferrioxamine regulates tumor necrosis factor release in mesangial cells. Kidney Int. 1991 May;39(5):822–830. doi: 10.1038/ki.1991.103. [DOI] [PubMed] [Google Scholar]
- Baud L., Ardaillou R. Tumor necrosis factor alpha in glomerular injury. Kidney Int Suppl. 1994 Feb;45:S32–S36. [PubMed] [Google Scholar]
- Baud L., Fouqueray B., Philipp C. Involvement of tumor necrosis factor-alpha in glomerular injury. Springer Semin Immunopathol. 1994;16(1):53–61. doi: 10.1007/BF00196713. [DOI] [PubMed] [Google Scholar]
- Baud L., Oudinet J. P., Bens M., Noe L., Peraldi M. N., Rondeau E., Etienne J., Ardaillou R. Production of tumor necrosis factor by rat mesangial cells in response to bacterial lipopolysaccharide. Kidney Int. 1989 May;35(5):1111–1118. doi: 10.1038/ki.1989.98. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Nathan C. Modulation of macrophage function by transforming growth factor beta, interleukin-4, and interleukin-10. Ann N Y Acad Sci. 1993 Jun 23;685:713–739. doi: 10.1111/j.1749-6632.1993.tb35934.x. [DOI] [PubMed] [Google Scholar]
- Bogdan C., Paik J., Vodovotz Y., Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992 Nov 15;267(32):23301–23308. [PubMed] [Google Scholar]
- Boutard V., Fouqueray B., Philippe C., Perez J., Baud L. Fish oil supplementation and essential fatty acid deficiency reduce nitric oxide synthesis by rat macrophages. Kidney Int. 1994 Nov;46(5):1280–1286. doi: 10.1038/ki.1994.395. [DOI] [PubMed] [Google Scholar]
- Boyce N. W., Holdsworth S. R. Hydroxyl radical mediation of immune renal injury by desferrioxamine. Kidney Int. 1986 Dec;30(6):813–817. doi: 10.1038/ki.1986.260. [DOI] [PubMed] [Google Scholar]
- Cassatella M. A., Meda L., Gasperini S., Calzetti F., Bonora S. Interleukin 10 (IL-10) upregulates IL-1 receptor antagonist production from lipopolysaccharide-stimulated human polymorphonuclear leukocytes by delaying mRNA degradation. J Exp Med. 1994 May 1;179(5):1695–1699. doi: 10.1084/jem.179.5.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. The mesangial cell: a tissue culture view. Kidney Int. 1994 Feb;45(2):320–327. doi: 10.1038/ki.1994.41. [DOI] [PubMed] [Google Scholar]
- Egido J., Gómez-Chiarri M., Ortíz A., Bustos C., Alonso J., Gómez-Guerrero C., Gómez-Garre D., López-Armada M. J., Plaza J., Gonzalez E. Role of tumor necrosis factor-alpha in the pathogenesis of glomerular diseases. Kidney Int Suppl. 1993 Jan;39:S59–S64. [PubMed] [Google Scholar]
- Fouqueray B., Philippe C., Amrani A., Perez J., Baud L. Heat shock prevents lipopolysaccharide-induced tumor necrosis factor-alpha synthesis by rat mononuclear phagocytes. Eur J Immunol. 1992 Nov;22(11):2983–2987. doi: 10.1002/eji.1830221133. [DOI] [PubMed] [Google Scholar]
- Fouqueray B., Philippe C., Herbelin A., Perez J., Ardaillou R., Baud L. Cytokine formation within rat glomeruli during experimental endotoxemia. J Am Soc Nephrol. 1993 May;3(11):1783–1791. doi: 10.1681/ASN.V3111783. [DOI] [PubMed] [Google Scholar]
- Gérard C., Bruyns C., Marchant A., Abramowicz D., Vandenabeele P., Delvaux A., Fiers W., Goldman M., Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993 Feb 1;177(2):547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Johnson K. J., Todd R. F., 3rd, Issekutz T. B., Miyasaka M., Tamatani T., Smith C. W., Anderson D. C., Ward P. A. Requirements for leukocyte adhesion molecules in nephrotoxic nephritis. J Clin Invest. 1993 Feb;91(2):577–587. doi: 10.1172/JCI116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe C., Philippe B., Fouqueray B., Perez J., Lebret M., Baud L. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am J Pathol. 1993 Dec;143(6):1713–1723. [PMC free article] [PubMed] [Google Scholar]
- Satriano J. A., Hora K., Shan Z., Stanley E. R., Mori T., Schlondorff D. Regulation of monocyte chemoattractant protein-1 and macrophage colony-stimulating factor-1 by IFN-gamma, tumor necrosis factor-alpha, IgG aggregates, and cAMP in mouse mesangial cells. J Immunol. 1993 Mar 1;150(5):1971–1978. [PubMed] [Google Scholar]
- Sedor J. R. Cytokines and growth factors in renal injury. Semin Nephrol. 1992 Sep;12(5):428–440. [PubMed] [Google Scholar]
- Sedor J. R., Konieczkowski M., Huang S., Gronich J. H., Nakazato Y., Gordon G., King C. H. Cytokines, mesangial cell activation and glomerular injury. Kidney Int Suppl. 1993 Jan;39:S65–S70. [PubMed] [Google Scholar]
- Van Vlasselaer P., Borremans B., van Gorp U., Dasch J. R., De Waal-Malefyt R. Interleukin 10 inhibits transforming growth factor-beta (TGF-beta) synthesis required for osteogenic commitment of mouse bone marrow cells. J Cell Biol. 1994 Feb;124(4):569–577. doi: 10.1083/jcb.124.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]



