Abstract
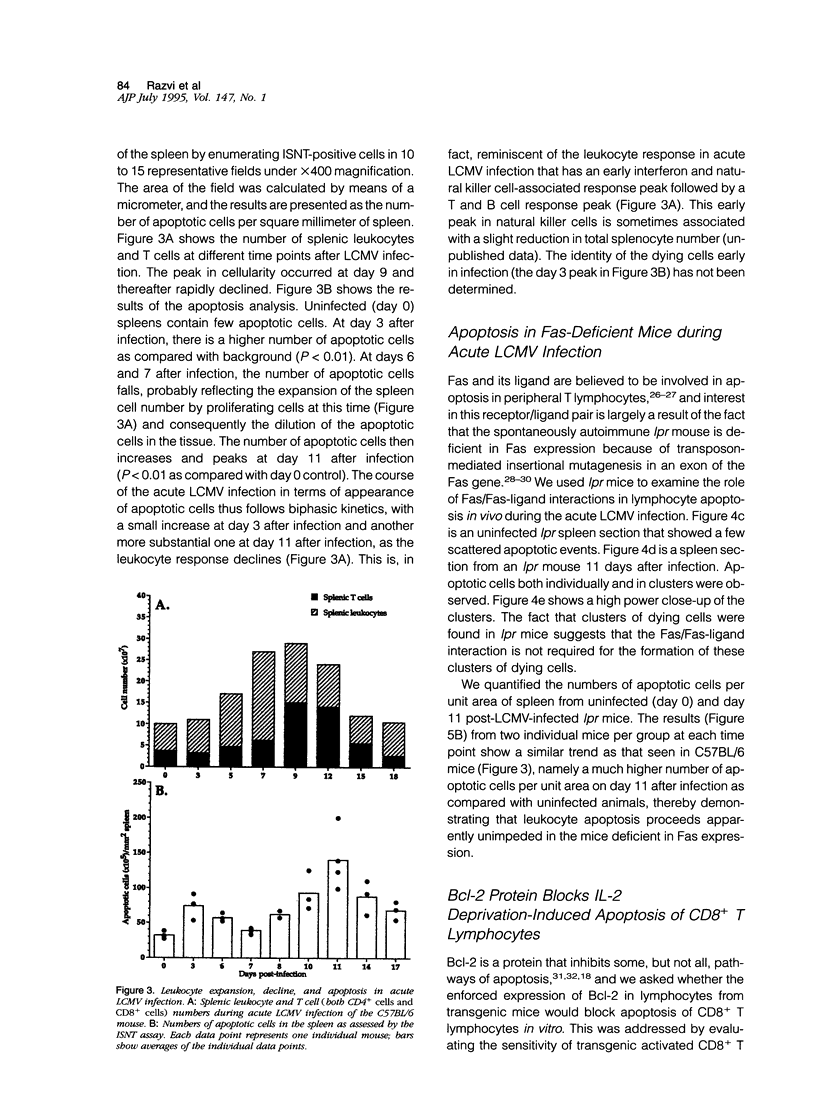
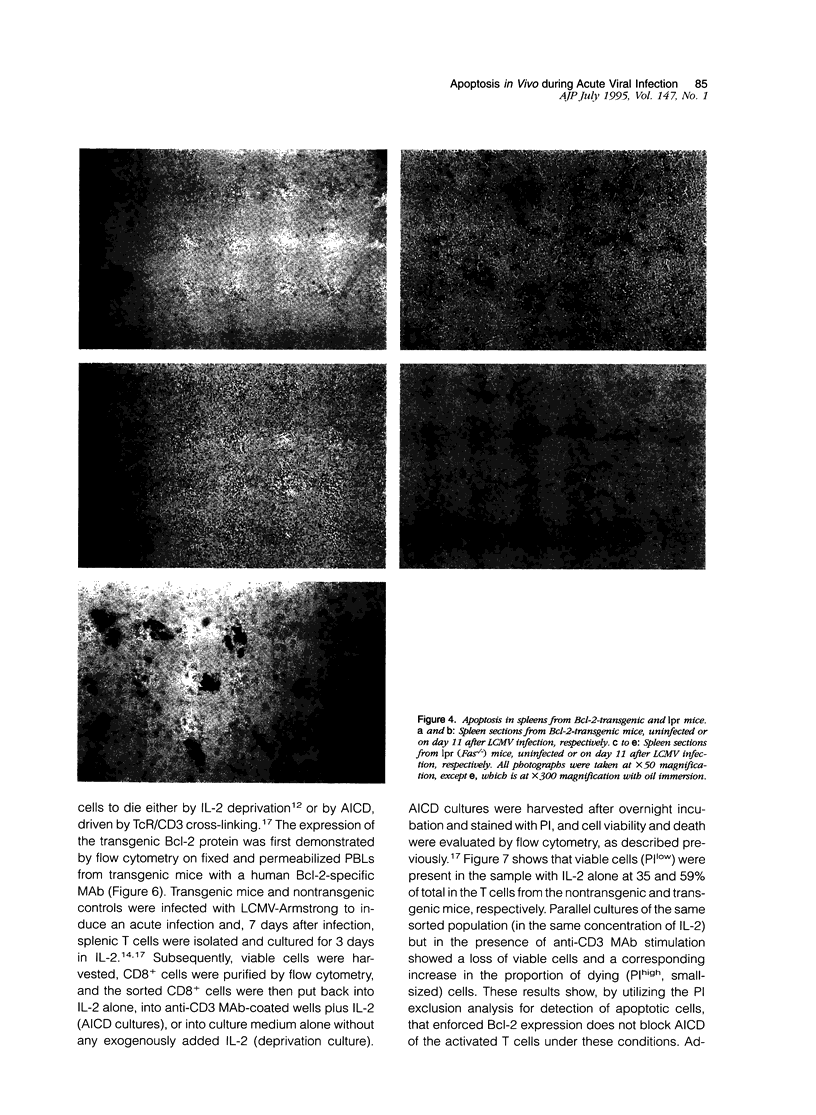
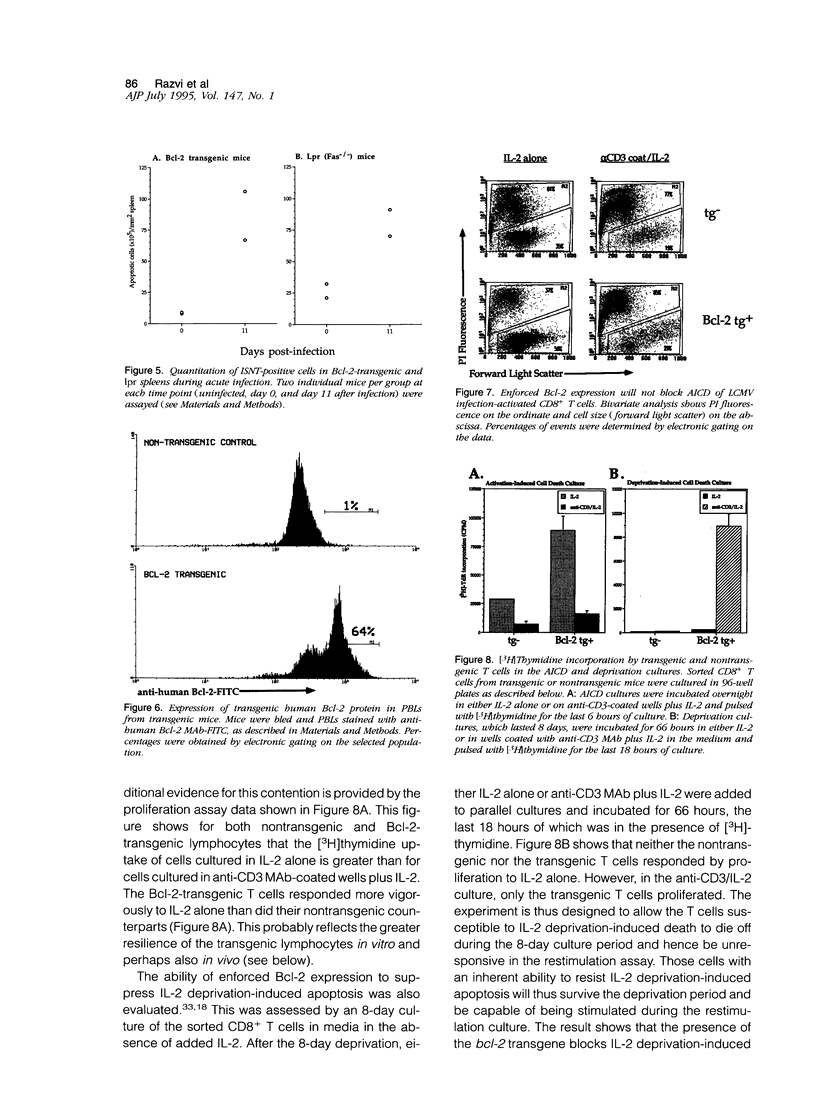
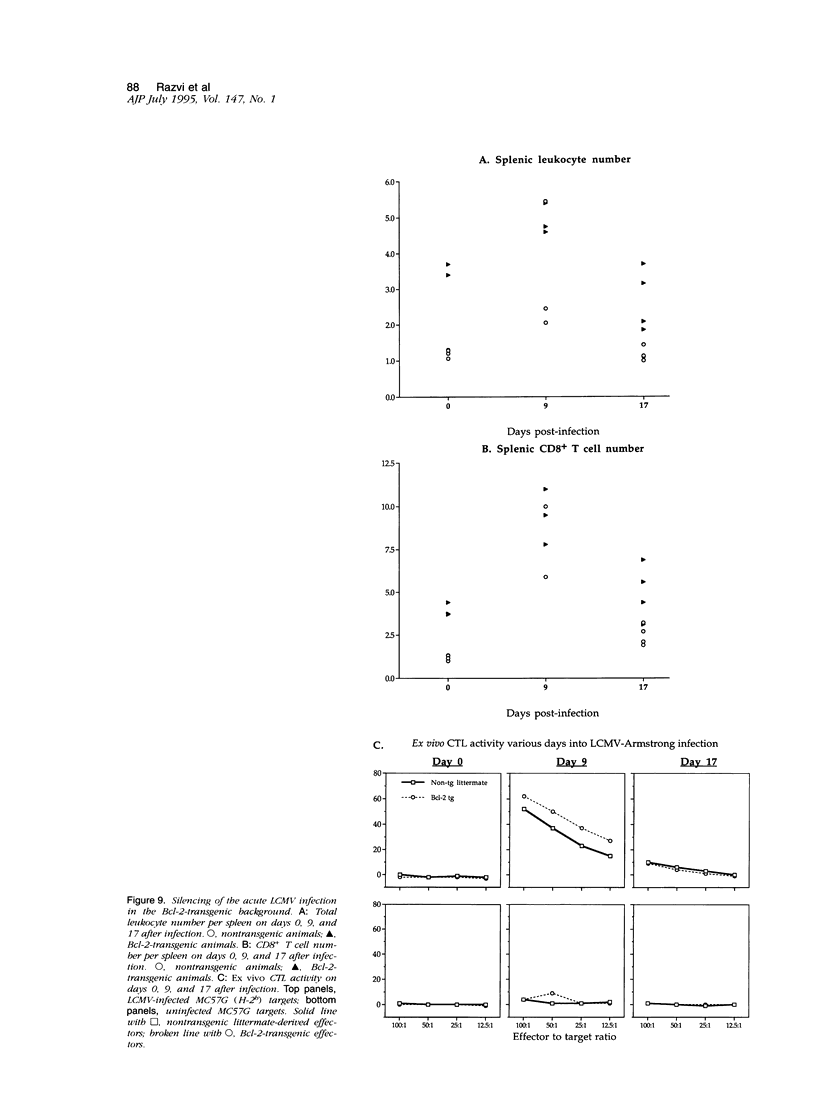
This report examines the mechanisms involved in the down-regulation of the immune response in acute viral infection and documents the presence of apoptotic lymphocytes in situ in the spleens of mice during the resolution of the immune response to acute lymphocytic choriomeningitis virus infection. Apoptotic cells were detected by an in situ nucleotidyl transferase assay. Both T and B lymphocytes were shown to be dying in vivo, the latter in clusters. A biphasic occurrence of apoptosis during the course of the acute infection was observed, with elevated levels occurring at day 3 after infection and a second more pronounced peak at day 11 after infection, coincident with the decline of the cytotoxic T lymphocyte response and with the decrease in total splenic leukocyte number. Apoptosis in vivo was detected in lpr mice, suggesting that Fas expression is not imperative for lymphocyte apoptosis in the context of an acute viral infection. Apoptosis in situ and the decline of the T lymphocyte response to acute lymphocytic choriomeningitis virus infection was unaffected by the enforced lymphocyte-directed expression of Bcl-2, a protein that blocks growth factor deprivation-induced apoptosis of lymphocytes in vitro. These results argue that the silencing of the T cell response to acute infection may not be a result simply of growth factor deprivation. The susceptibility of activated T cells to apoptotic death, which has previously been associated with virus-induced immune deficiency, may therefore also explain the en masse elimination of the expanded lymphocyte pool subsequent to an acute viral infection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderson M. R., Armitage R. J., Maraskovsky E., Tough T. W., Roux E., Schooley K., Ramsdell F., Lynch D. H. Fas transduces activation signals in normal human T lymphocytes. J Exp Med. 1993 Dec 1;178(6):2231–2235. doi: 10.1084/jem.178.6.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando K., Guidotti L. G., Wirth S., Ishikawa T., Missale G., Moriyama T., Schreiber R. D., Schlicht H. J., Huang S. N., Chisari F. V. Class I-restricted cytotoxic T lymphocytes are directly cytopathic for their target cells in vivo. J Immunol. 1994 Apr 1;152(7):3245–3253. [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Crispe I. N. Fatal interactions: Fas-induced apoptosis of mature T cells. Immunity. 1994 Aug;1(5):347–349. doi: 10.1016/1074-7613(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Dancescu M., Rubio-Trujillo M., Biron G., Bron D., Delespesse G., Sarfati M. Interleukin 4 protects chronic lymphocytic leukemic B cells from death by apoptosis and upregulates Bcl-2 expression. J Exp Med. 1992 Nov 1;176(5):1319–1326. doi: 10.1084/jem.176.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G., Podack E. R. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2189–2193. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Allan W., Eichelberger M., Carding S. R. Roles of alpha beta and gamma delta T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19(0):89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H., Torpier G., Monté D., Mouton Y., Capron A., Ameisen J. C. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992 Feb 1;175(2):331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Janssen O., Wesselborg S., Heckl-Ostreicher B., Pechhold K., Bender A., Schondelmaier S., Moldenhauer G., Kabelitz D. T cell receptor/CD3-signaling induces death by apoptosis in human T cell receptor gamma delta + T cells. J Immunol. 1991 Jan 1;146(1):35–39. [PubMed] [Google Scholar]
- Kobayashi S., Hirano T., Kakinuma M., Uede T. Transcriptional repression and differential splicing of Fas mRNA by early transposon (ETn) insertion in autoimmune lpr mice. Biochem Biophys Res Commun. 1993 Mar 15;191(2):617–624. doi: 10.1006/bbrc.1993.1262. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J. Interleukin-2 programs mouse alpha beta T lymphocytes for apoptosis. Nature. 1991 Oct 31;353(6347):858–861. doi: 10.1038/353858a0. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Janeway C. A., Jr Interferon gamma plays a critical role in induced cell death of effector T cell: a possible third mechanism of self-tolerance. J Exp Med. 1990 Dec 1;172(6):1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland H. I., Nahill S. R., Maciaszek J. W., Welsh R. M. CD11b (Mac-1): a marker for CD8+ cytotoxic T cell activation and memory in virus infection. J Immunol. 1992 Aug 15;149(4):1326–1333. [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Miyawaki T., Uehara T., Nibu R., Tsuji T., Yachie A., Yonehara S., Taniguchi N. Differential expression of apoptosis-related Fas antigen on lymphocyte subpopulations in human peripheral blood. J Immunol. 1992 Dec 1;149(11):3753–3758. [PubMed] [Google Scholar]
- Moss D. J., Bishop C. J., Burrows S. R., Ryan J. M. T lymphocytes in infectious mononucleosis. I. T cell death in vitro. Clin Exp Immunol. 1985 Apr;60(1):61–69. [PMC free article] [PubMed] [Google Scholar]
- Razvi E. S., Welsh R. M., McFarland H. I. In vivo state of antiviral CTL precursors. Characterization of a cycling cell population containing CTL precursors in immune mice. J Immunol. 1995 Jan 15;154(2):620–632. [PubMed] [Google Scholar]
- Razvi E. S., Welsh R. M. Programmed cell death of T lymphocytes during acute viral infection: a mechanism for virus-induced immune deficiency. J Virol. 1993 Oct;67(10):5754–5765. doi: 10.1128/jvi.67.10.5754-5765.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. H., White C. L., Loh D. Y., Meleedy-Rey P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2151–2155. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saron M. F., Shidani B., Nahori M. A., Guillon J. C., Truffa-Bachi P. Lymphocytic choriomeningitis virus-induced immunodepression: inherent defect of B and T lymphocytes. J Virol. 1990 Sep;64(9):4076–4083. doi: 10.1128/jvi.64.9.4076-4083.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Fadok V., Henson P., Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993 Mar;14(3):131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Shi Y. F., Sahai B. M., Green D. R. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989 Jun 22;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Singer G. G., Abbas A. K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994 Aug;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Swat W., Ignatowicz L., Kisielow P. Detection of apoptosis of immature CD4+8+ thymocytes by flow cytometry. J Immunol Methods. 1991 Mar 1;137(1):79–87. doi: 10.1016/0022-1759(91)90396-w. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Tanaka M., Brannan C. I., Jenkins N. A., Copeland N. G., Suda T., Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994 Mar 25;76(6):969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]