Abstract
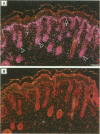
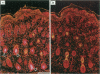
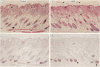
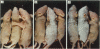
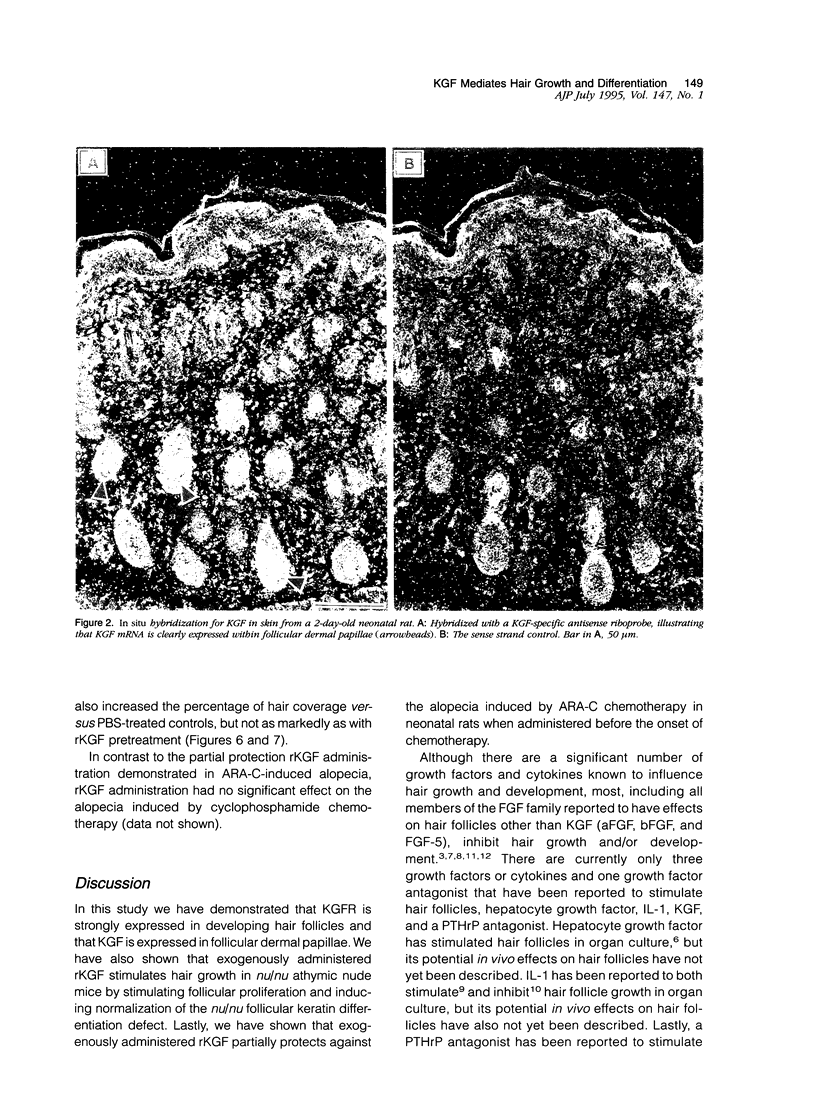
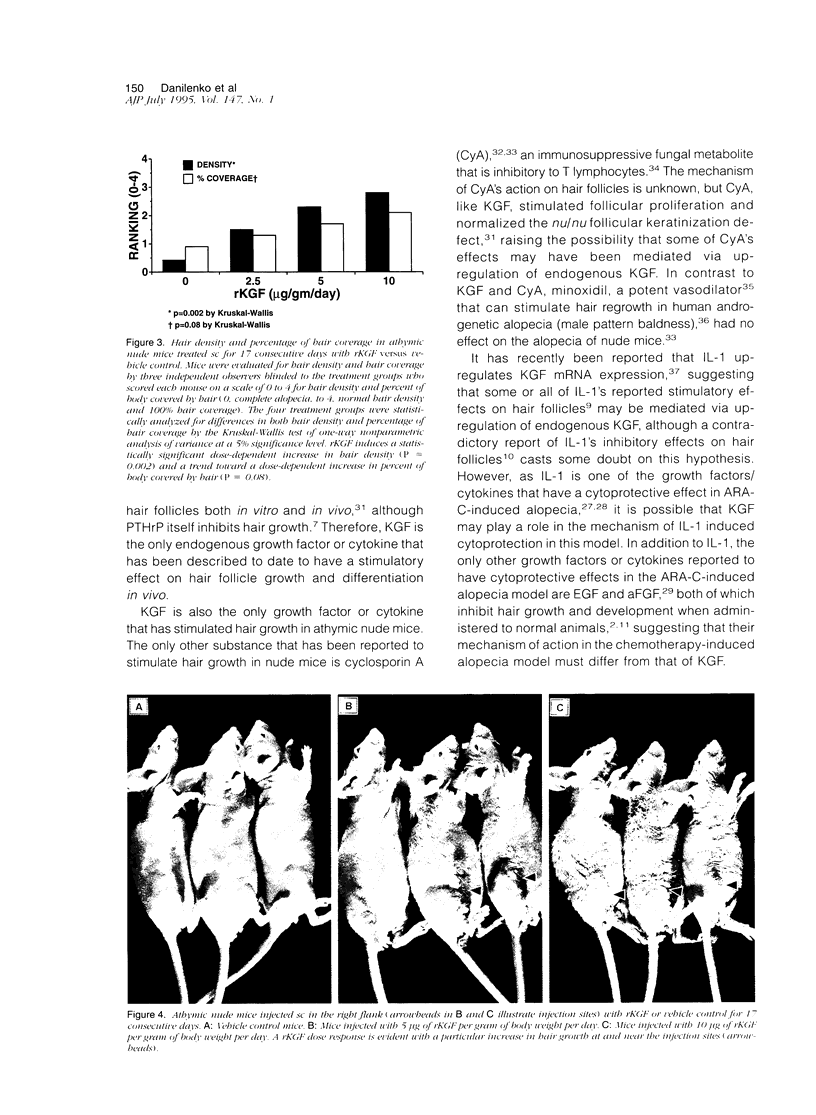
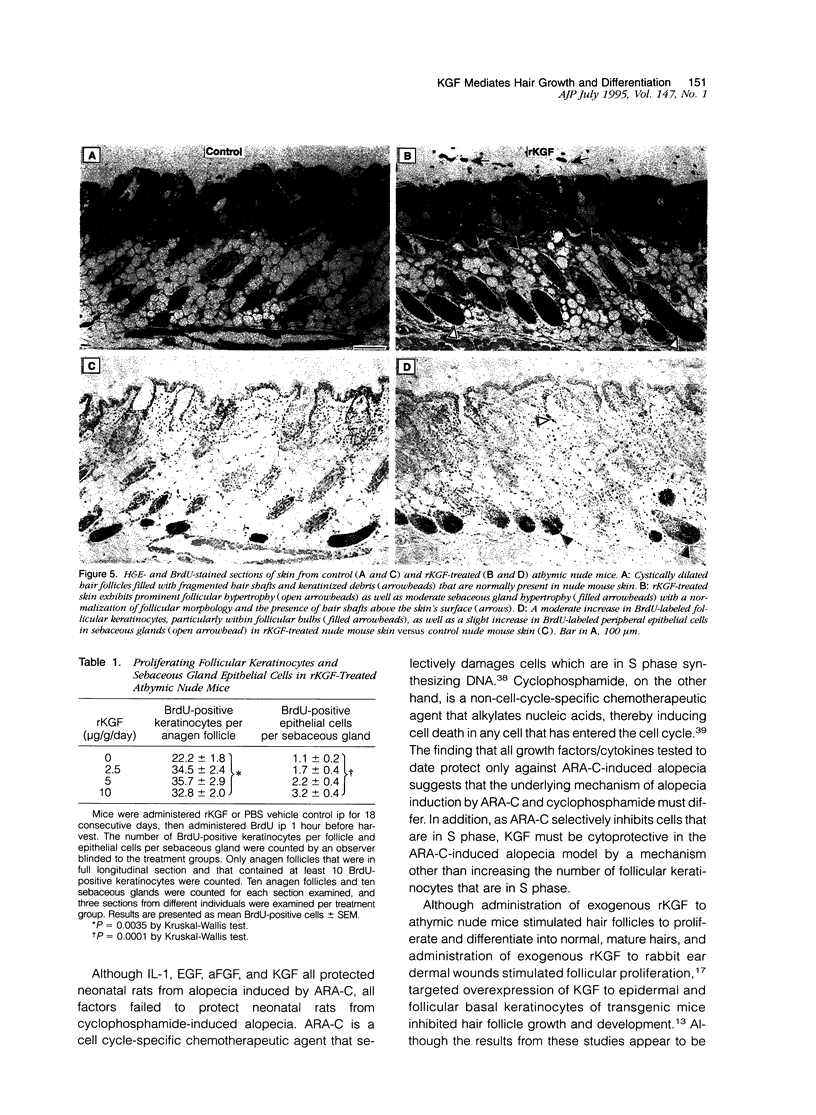
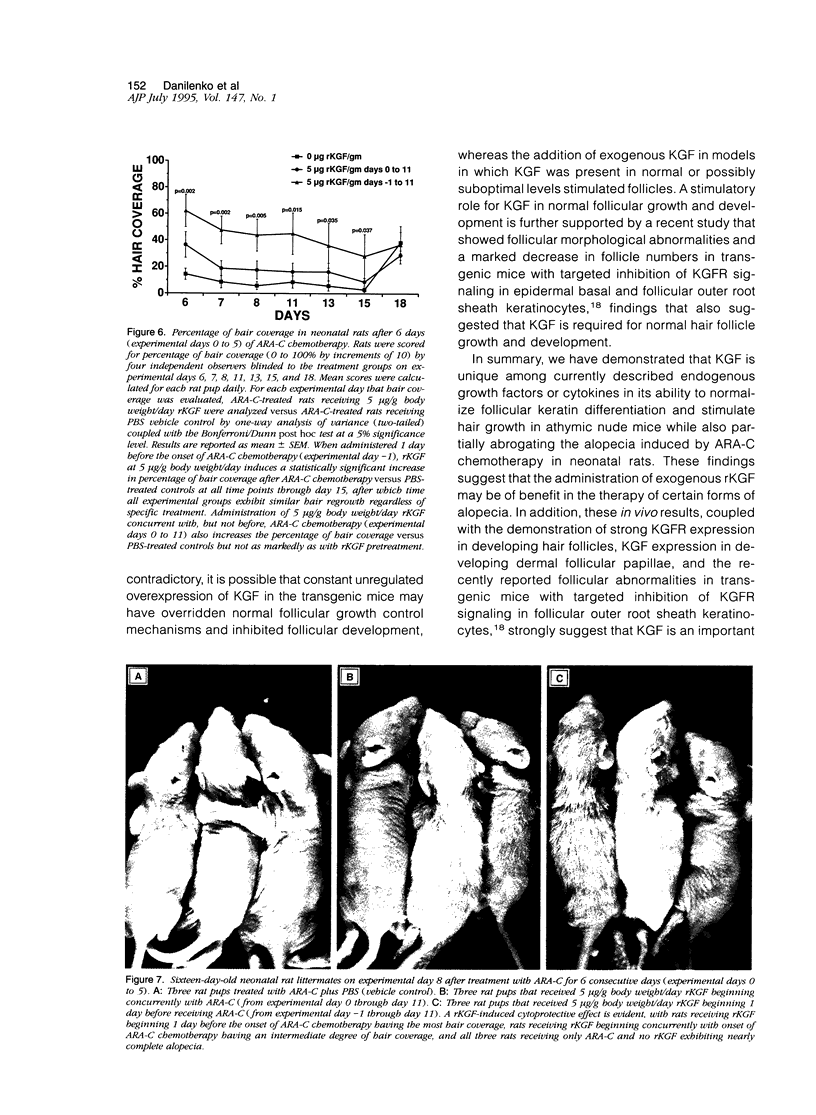
The growth and development of hair follicles is influenced by a number of different growth factors and cytokines, particularly members of the fibroblast growth factor (FGF) family. Keratinocyte growth factor (KGF or FGF-7) is a recently identified 28-kd member of the FGF family that induces proliferation of a wide variety of epithelial cells, including keratinocytes within the epidermis and dermal adnexa. Because KGF induces marked proliferation of keratinocytes, and both KGF and KGF receptor (KGFR) mRNA are expressed at high levels in skin, we sought to localize KGF and KGFR in skin by in situ hybridization. KGFR mRNA was relatively strongly expressed by keratinocytes in the basilar epidermis as well as throughout developing hair follicles of rat embryos and neonates. KGF mRNA was expressed at lower levels than was KGFR but could be localized to follicular dermal papillae in rat embryos and neonates. These results prompted us to investigate the effects of KGF on hair follicles in two distinct murine models of alopecia. In the first model, recombinant KGF (rKGF) induced dose-dependent hair growth over most of the body in nu/nu athymic nude mice when administered intraperitoneally or subcutaneously over 17 to 18 days. When administered subcutaneously, rKGF induced the most extensive hair growth at the sites of injection. Histologically, rKGF induced marked follicular and sebaceous gland hypertrophy, a normalization of the nu/nu follicular keratinization defect, and an increase in follicular keratinocyte proliferation as assessed by bromodeoxyuridine labeling. In the second model, a neonatal rat model of cytosine arabinoside chemotherapy-induced alopecia in which interleukin-1, epidermal growth factor, and acidic FGF have all demonstrated some degree of alopecia cytoprotection, rKGF induced a dose-dependent cytoprotective effect, abrogating as much as 50% of the alopecia in this model when administered beginning 1 day before the onset of chemotherapy. Taken together, these data suggest that KGF is an important endogenous mediator of normal hair follicle growth, development, and differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blessing M., Nanney L. B., King L. E., Jones C. M., Hogan B. L. Transgenic mice as a model to study the role of TGF-beta-related molecules in hair follicles. Genes Dev. 1993 Feb;7(2):204–215. doi: 10.1101/gad.7.2.204. [DOI] [PubMed] [Google Scholar]
- Buhl A. E., Waldon D. J., Miller B. F., Brunden M. N. Differences in activity of minoxidil and cyclosporin A on hair growth in nude and normal mice. Comparisons of in vivo and in vitro studies. Lab Invest. 1990 Jan;62(1):104–107. [PubMed] [Google Scholar]
- Chedid M., Rubin J. S., Csaky K. G., Aaronson S. A. Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem. 1994 Apr 8;269(14):10753–10757. [PubMed] [Google Scholar]
- Colombani P. M., Hess A. D. T-lymphocyte inhibition by cyclosporine. Potential mechanisms of action. Biochem Pharmacol. 1987 Nov 15;36(22):3789–3793. doi: 10.1016/0006-2952(87)90438-2. [DOI] [PubMed] [Google Scholar]
- Dargie H. J., Dollery C. T., Daniel J. Minoxidil in resistant hypertension. Lancet. 1977 Sep 10;2(8037):515–518. doi: 10.1016/s0140-6736(77)90660-2. [DOI] [PubMed] [Google Scholar]
- De Villez R. L. Topical minoxidil therapy in hereditary androgenetic alopecia. Arch Dermatol. 1985 Feb;121(2):197–202. doi: 10.1001/archderm.121.2.197. [DOI] [PubMed] [Google Scholar]
- DeWys W. D., Kight N. Kinetics of cyclophosphamide damage--sublethal damage repair and cell-cycle-related sensitivity. Mod Hosp. 1969 Jan;112(1):155–163. [PubMed] [Google Scholar]
- Finch P. W., Rubin J. S., Miki T., Ron D., Aaronson S. A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989 Aug 18;245(4919):752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Flanagan S. P. 'Nude', a new hairless gene with pleiotropic effects in the mouse. Genet Res. 1966 Dec;8(3):295–309. doi: 10.1017/s0016672300010168. [DOI] [PubMed] [Google Scholar]
- Guo L., Yu Q. C., Fuchs E. Targeting expression of keratinocyte growth factor to keratinocytes elicits striking changes in epithelial differentiation in transgenic mice. EMBO J. 1993 Mar;12(3):973–986. doi: 10.1002/j.1460-2075.1993.tb05738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon C. S., Nevins T. D. IL-1 alpha inhibits human hair follicle growth and hair fiber production in whole-organ cultures. Lymphokine Cytokine Res. 1993 Aug;12(4):197–203. [PubMed] [Google Scholar]
- Holick M. F., Ray S., Chen T. C., Tian X., Persons K. S. A parathyroid hormone antagonist stimulates epidermal proliferation and hair growth in mice. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8014–8016. doi: 10.1073/pnas.91.17.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley R. M., Morris C. F., Boyle W., Ring B., Biltz R., Tarpley J. E., Aukerman S. L., Devine P. L., Whitehead R. H., Pierce G. F. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994 Nov;94(5):1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein A. M., Jimenez J. J., McCall C. A., Yunis A. A. Protection from chemotherapy-induced alopecia in a rat model. Science. 1990 Sep 28;249(4976):1564–1566. doi: 10.1126/science.2218498. [DOI] [PubMed] [Google Scholar]
- Hébert J. M., Rosenquist T., Götz J., Martin G. R. FGF5 as a regulator of the hair growth cycle: evidence from targeted and spontaneous mutations. Cell. 1994 Sep 23;78(6):1017–1025. doi: 10.1016/0092-8674(94)90276-3. [DOI] [PubMed] [Google Scholar]
- Jimenez J. J., Wong G. H., Yunis A. A. Interleukin 1 protects from cytosine arabinoside-induced alopecia in the rat model. FASEB J. 1991 Jul;5(10):2456–2458. doi: 10.1096/fasebj.5.10.2065892. [DOI] [PubMed] [Google Scholar]
- Jimenez J. J., Yunis A. A. Protection from 1-beta-D-arabinofuranosylcytosine-induced alopecia by epidermal growth factor and fibroblast growth factor in the rat model. Cancer Res. 1992 Jan 15;52(2):413–415. [PubMed] [Google Scholar]
- Jindo T., Tsuboi R., Imai R., Takamori K., Rubin J. S., Ogawa H. Hepatocyte growth factor/scatter factor stimulates hair growth of mouse vibrissae in organ culture. J Invest Dermatol. 1994 Sep;103(3):306–309. doi: 10.1111/1523-1747.ep12394731. [DOI] [PubMed] [Google Scholar]
- Lenaz L., Sternberg S. S., Philips F. S. Cytotoxic effects of 1-beta-D-arabinofuranosyl-5-fluorocytosine and of 1-beta-D-arabinofuranosylcytosine in proliferating tissues in mice. Cancer Res. 1969 Oct;29(10):1790–1798. [PubMed] [Google Scholar]
- Luetteke N. C., Qiu T. H., Peiffer R. L., Oliver P., Smithies O., Lee D. C. TGF alpha deficiency results in hair follicle and eye abnormalities in targeted and waved-1 mice. Cell. 1993 Apr 23;73(2):263–278. doi: 10.1016/0092-8674(93)90228-i. [DOI] [PubMed] [Google Scholar]
- Mann G. B., Fowler K. J., Gabriel A., Nice E. C., Williams R. L., Dunn A. R. Mice with a null mutation of the TGF alpha gene have abnormal skin architecture, wavy hair, and curly whiskers and often develop corneal inflammation. Cell. 1993 Apr 23;73(2):249–261. doi: 10.1016/0092-8674(93)90227-h. [DOI] [PubMed] [Google Scholar]
- Miki T., Bottaro D. P., Fleming T. P., Smith C. L., Burgess W. H., Chan A. M., Aaronson S. A. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore G. P., Panaretto B. A., Robertson D. Epidermal growth factor delays the development of the epidermis and hair follicles of mice during growth of the first coat. Anat Rec. 1983 Jan;205(1):47–55. doi: 10.1002/ar.1092050107. [DOI] [PubMed] [Google Scholar]
- Panos R. J., Rubin J. S., Csaky K. G., Aaronson S. A., Mason R. J. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993 Aug;92(2):969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce G. F., Yanagihara D., Klopchin K., Danilenko D. M., Hsu E., Kenney W. C., Morris C. F. Stimulation of all epithelial elements during skin regeneration by keratinocyte growth factor. J Exp Med. 1994 Mar 1;179(3):831–840. doi: 10.1084/jem.179.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada M., Terada N., Taniguchi H., Tateishi R., Mori Y. Cyclosporin A stimulates hair growth in nude mice. Lab Invest. 1987 Jun;56(6):684–686. [PubMed] [Google Scholar]
- Tam J. P. Physiological effects of transforming growth factor in the newborn mouse. Science. 1985 Aug 16;229(4714):673–675. doi: 10.1126/science.3860952. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Cardiff R., Yin S., Bikhazi N., Biltz R., Morris C. F., Pierce G. F. Keratinocyte growth factor is a growth factor for mammary epithelium in vivo. The mammary epithelium of lactating rats is resistant to the proliferative action of keratinocyte growth factor. Am J Pathol. 1994 May;144(5):862–868. [PMC free article] [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Longmuir K., Yin S., Biltz R., Morris C. F., Housley R. M., Pierce G. F. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994 Mar;93(3):1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Peters K. G., Longaker M. T., Fuller-Pace F., Banda M. J., Williams L. T. Large induction of keratinocyte growth factor expression in the dermis during wound healing. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6896–6900. doi: 10.1073/pnas.89.15.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Smola H., Liao X., Longaker M. T., Krieg T., Hofschneider P. H., Williams L. T. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994 Nov 4;266(5186):819–822. doi: 10.1126/science.7973639. [DOI] [PubMed] [Google Scholar]
- Wysolmerski J. J., Broadus A. E., Zhou J., Fuchs E., Milstone L. M., Philbrick W. M. Overexpression of parathyroid hormone-related protein in the skin of transgenic mice interferes with hair follicle development. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1133–1137. doi: 10.1073/pnas.91.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Elliott J. L., Matheson C., Sun J., Zhang L., Mu X., Rex K. L., Snider W. D. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993 Dec;24(12):1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]
- du Cros D. L. Fibroblast growth factor and epidermal growth factor in hair development. J Invest Dermatol. 1993 Jul;101(1 Suppl):106S–113S. doi: 10.1111/1523-1747.ep12363020. [DOI] [PubMed] [Google Scholar]
- du Cros D. L. Fibroblast growth factor influences the development and cycling of murine hair follicles. Dev Biol. 1993 Apr;156(2):444–453. doi: 10.1006/dbio.1993.1091. [DOI] [PubMed] [Google Scholar]
- du Cros D. L., Isaacs K., Moore G. P. Distribution of acidic and basic fibroblast growth factors in ovine skin during follicle morphogenesis. J Cell Sci. 1993 Jul;105(Pt 3):667–674. doi: 10.1242/jcs.105.3.667. [DOI] [PubMed] [Google Scholar]