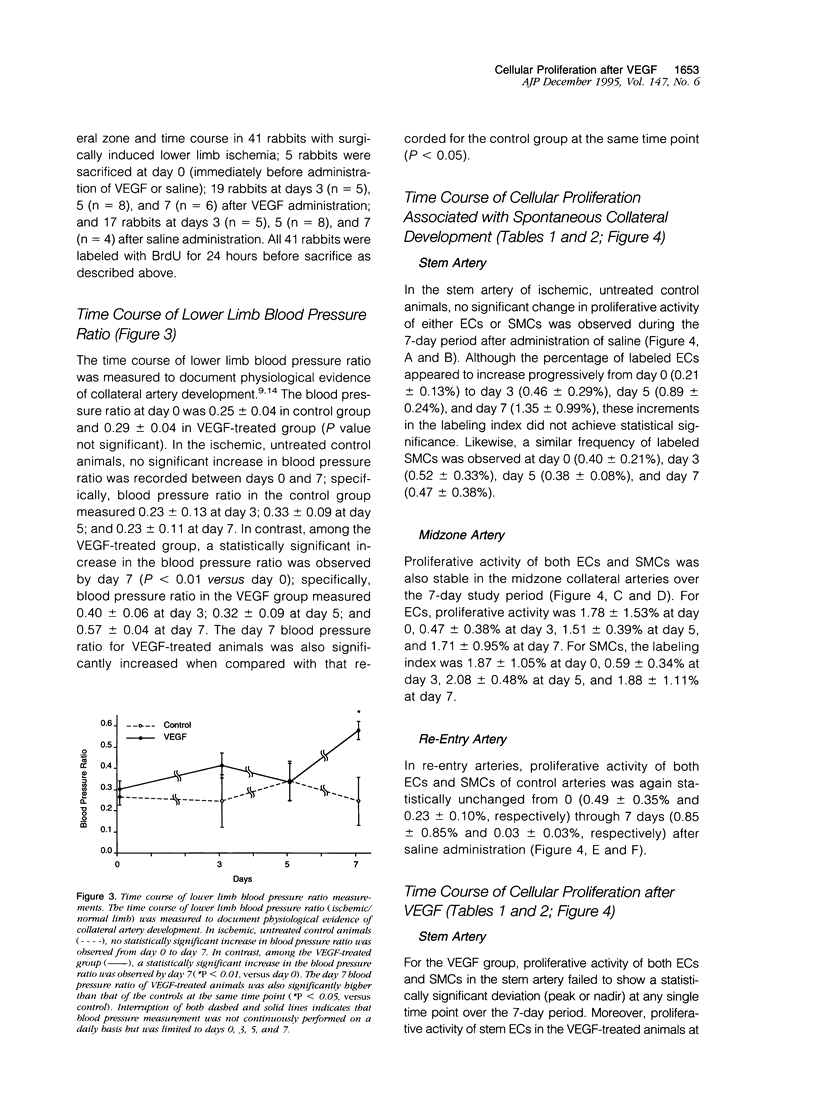
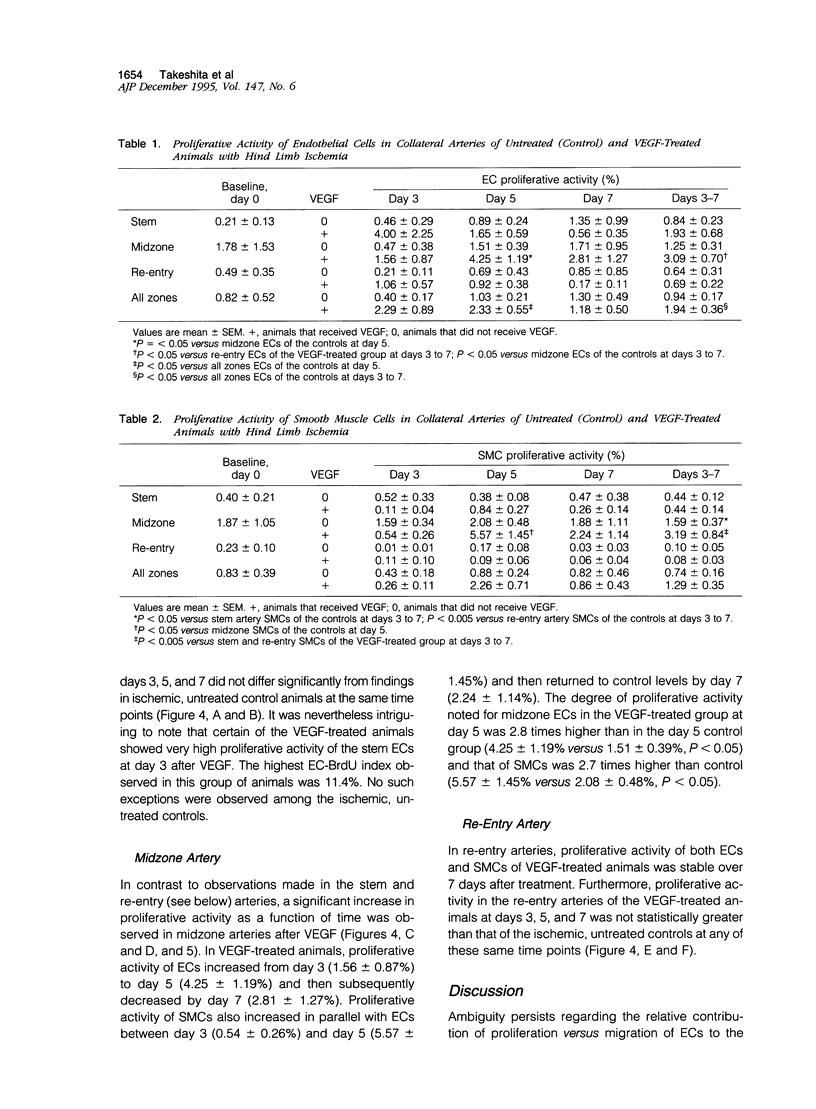
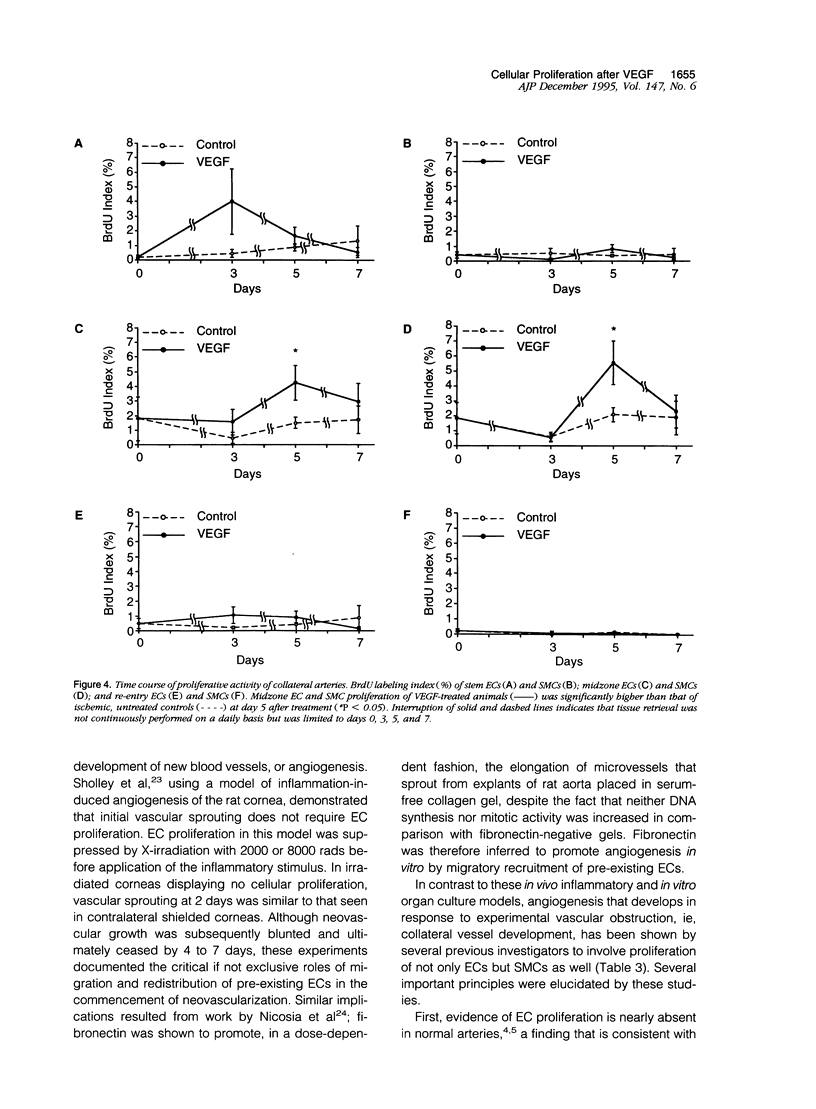
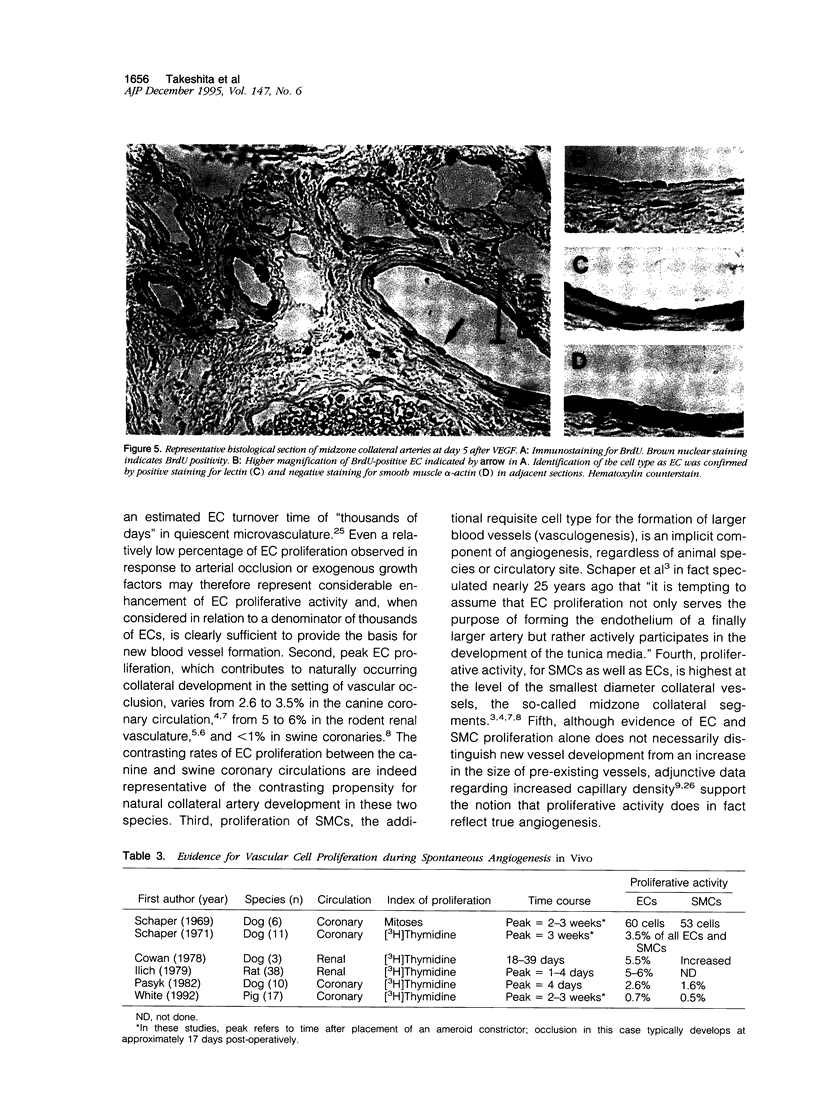
Abstract
Proliferation of vascular cells has been previously shown to contribute to spontaneous development of coronary collaterals. Recent studies from several laboratories have established that collateral artery growth in both the heart and limb can be enhanced by administration of angiogenic growth factors, or therapeutic angiogenesis. In this study, we sought (1) to define the extent and time course of endothelial cell (EC) and smooth muscle cell (SMC) proliferation accompanying spontaneous collateral development during limb ischemia and (2) to determine the extent to which proliferative activity of ECs and SMCs is augmented during therapeutic angiogenesis with vascular endothelial growth factor (VEGF), a heparin-binding EC-specific mitogen. Ten days after induction of limb ischemia by surgically excising the femoral artery of rabbits, either VEGF (500 to 1000 micrograms) or saline was administered as a bolus into the iliac artery of the ischemic limb. Cellular proliferation was evaluated by bromodeoxyuridine labeling for 24 hours at day 0 (immediately before VEGF administration) and at days 3, 5, and 7 after VEGF, EC proliferation in the midzone collaterals of VEGF-treated animals increased 2.8-fold at day 5 (P < 0.05 versus control), and returned to baseline levels by day 7. SMC proliferation in midzone collaterals also increased 2.7-fold in response to VEGF (P < 0.05). No significant increase in EC or SMC proliferation was observed in either the stem or re-entry collaterals of VEGF-treated animals compared with untreated ischemic control animals. Reduction of hemodynamic deficit in the ischemic limb measured by lower limb blood pressure was documented at day 7 after VEGF (P < 0.01 versus untreated, ischemic control). These data thus (1) establish the contribution of cellular proliferation to collateral vessel development in limb ischemia and (2) support the concept that augmented cellular proliferation contributes to the enhanced formation of collateral vessels after therapeutic angiogenesis with VEGF.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baffour R., Berman J., Garb J. L., Rhee S. W., Kaufman J., Friedmann P. Enhanced angiogenesis and growth of collaterals by in vivo administration of recombinant basic fibroblast growth factor in a rabbit model of acute lower limb ischemia: dose-response effect of basic fibroblast growth factor. J Vasc Surg. 1992 Aug;16(2):181–191. [PubMed] [Google Scholar]
- Banai S., Jaklitsch M. T., Shou M., Lazarous D. F., Scheinowitz M., Biro S., Epstein S. E., Unger E. F. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994 May;89(5):2183–2189. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- Bauters C., Asahara T., Zheng L. P., Takeshita S., Bunting S., Ferrara N., Symes J. F., Isner J. M. Physiological assessment of augmented vascularity induced by VEGF in ischemic rabbit hindlimb. Am J Physiol. 1994 Oct;267(4 Pt 2):H1263–H1271. doi: 10.1152/ajpheart.1994.267.4.H1263. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Clauss M., Gerlach M., Gerlach H., Brett J., Wang F., Familletti P. C., Pan Y. C., Olander J. V., Connolly D. T., Stern D. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990 Dec 1;172(6):1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. D., Harrison J., Schwartz S., Heimark R. Angioblast differentiation and morphogenesis of the vascular endothelium in the mouse embryo. Dev Biol. 1991 Nov;148(1):51–62. doi: 10.1016/0012-1606(91)90316-u. [DOI] [PubMed] [Google Scholar]
- Collins T., Ginsburg D., Boss J. M., Orkin S. H., Pober J. S. Cultured human endothelial cells express platelet-derived growth factor B chain: cDNA cloning and structural analysis. Nature. 1985 Aug 22;316(6030):748–750. doi: 10.1038/316748a0. [DOI] [PubMed] [Google Scholar]
- Collins T., Pober J. S., Gimbrone M. A., Jr, Hammacher A., Betsholtz C., Westermark B., Heldin C. H. Cultured human endothelial cells express platelet-derived growth factor A chain. Am J Pathol. 1987 Jan;126(1):7–12. [PMC free article] [PubMed] [Google Scholar]
- Connolly D. T., Heuvelman D. M., Nelson R., Olander J. V., Eppley B. L., Delfino J. J., Siegel N. R., Leimgruber R. M., Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989 Nov;84(5):1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan D. F., Hollenberg N. K., Connelly C. M., Williams D. H., Abrams H. L. Increased collateral arterial and venous endothelial cell turnover after renal artery stenosis in the dog. Invest Radiol. 1978 Mar-Apr;13(2):143–149. doi: 10.1097/00004424-197803000-00008. [DOI] [PubMed] [Google Scholar]
- D'Amore P. A., Thompson R. W. Mechanisms of angiogenesis. Annu Rev Physiol. 1987;49:453–464. doi: 10.1146/annurev.ph.49.030187.002321. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986 Dec 25;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Henzel W. J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989 Jun 15;161(2):851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Leung D. W., Cachianes G., Winer J., Henzel W. J. Purification and cloning of vascular endothelial growth factor secreted by pituitary folliculostellate cells. Methods Enzymol. 1991;198:391–405. doi: 10.1016/0076-6879(91)98040-d. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Angiogenesis. J Biol Chem. 1992 Jun 5;267(16):10931–10934. [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. Heparin-binding growth factor-1 stimulation of human endothelial cells induces platelet-derived growth factor A-chain gene expression. J Biol Chem. 1990 Feb 25;265(6):3284–3292. [PubMed] [Google Scholar]
- Graham A. M., Baffour R., Burdon T., DeVarennes B., Ricci M. A., Common A., Lisbona R., Sniderman A. D., Symes J. F. A demonstration of vascular proliferation in response to arteriovenous reversal in the ischemic canine hind limb. J Surg Res. 1989 Oct;47(4):341–347. doi: 10.1016/0022-4804(89)90145-5. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Lamarche I., Prabonnaud V., Dol F., Gauthier T. Tissue-type plasminogen activator is a potent mitogen for human aortic smooth muscle cells. J Biol Chem. 1994 Jan 28;269(4):3076–3080. [PubMed] [Google Scholar]
- Ilich N., Hollenberg N. K., Williams D. H., Abrams H. L. Time course of increased collateral arterial and venous endothelial cell turnover after renal artery stenosis in the rat. Circ Res. 1979 Nov;45(5):579–582. doi: 10.1161/01.res.45.5.579. [DOI] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- LONGLAND C. J. The collateral circulation of the limb; Arris and Gale lecture delivered at the Royal College of Surgeons of England on 4th February, 1953. Ann R Coll Surg Engl. 1953 Sep;13(3):161–176. [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Lindner V., Lappi D. A., Baird A., Majack R. A., Reidy M. A. Role of basic fibroblast growth factor in vascular lesion formation. Circ Res. 1991 Jan;68(1):106–113. doi: 10.1161/01.res.68.1.106. [DOI] [PubMed] [Google Scholar]
- Nicosia R. F., Bonanno E., Smith M. Fibronectin promotes the elongation of microvessels during angiogenesis in vitro. J Cell Physiol. 1993 Mar;154(3):654–661. doi: 10.1002/jcp.1041540325. [DOI] [PubMed] [Google Scholar]
- Pasyk S., Schaper W., Schaper J., Pasyk K., Miskiewicz G., Steinseifer B. DNA synthesis in coronary collaterals after coronary artery occlusion in conscious dog. Am J Physiol. 1982 Jun;242(6):H1031–H1037. doi: 10.1152/ajpheart.1982.242.6.H1031. [DOI] [PubMed] [Google Scholar]
- Pepper M. S., Ferrara N., Orci L., Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992 Dec 15;189(2):824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- Pu L. Q., Sniderman A. D., Brassard R., Lachapelle K. J., Graham A. M., Lisbona R., Symes J. F. Enhanced revascularization of the ischemic limb by angiogenic therapy. Circulation. 1993 Jul;88(1):208–215. doi: 10.1161/01.cir.88.1.208. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J., Kariya B., Harker L. A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1207–1210. doi: 10.1073/pnas.71.4.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper W., De Brabander M., Lewi P. DNA synthesis and mitoses in coronary collateral vessels of the dog. Circ Res. 1971 Jun;28(6):671–679. doi: 10.1161/01.res.28.6.671. [DOI] [PubMed] [Google Scholar]
- Schaper W., Schaper J., Xhonneux R., Vandesteene R. The morphology of intercoronary anastomoses in chronic coronary artery occlusion. Cardiovasc Res. 1969 Jul;3(3):315–323. doi: 10.1093/cvr/3.3.315. [DOI] [PubMed] [Google Scholar]
- Shen H., Clauss M., Ryan J., Schmidt A. M., Tijburg P., Borden L., Connolly D., Stern D., Kao J. Characterization of vascular permeability factor/vascular endothelial growth factor receptors on mononuclear phagocytes. Blood. 1993 May 15;81(10):2767–2773. [PubMed] [Google Scholar]
- Sholley M. M., Ferguson G. P., Seibel H. R., Montour J. L., Wilson J. D. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab Invest. 1984 Dec;51(6):624–634. [PubMed] [Google Scholar]
- Symes J. F., Graham A. M., Stein L., Sniderman A. D. Salvage of a severely ischemic limb by arteriovenous revascularization: a case report. Can J Surg. 1984 May;27(3):274–277. [PubMed] [Google Scholar]
- Takeshita S., Gal D., Leclerc G., Pickering J. G., Riessen R., Weir L., Isner J. M. Increased gene expression after liposome-mediated arterial gene transfer associated with intimal smooth muscle cell proliferation. In vitro and in vivo findings in a rabbit model of vascular injury. J Clin Invest. 1994 Feb;93(2):652–661. doi: 10.1172/JCI117017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S., Zheng L. P., Brogi E., Kearney M., Pu L. Q., Bunting S., Ferrara N., Symes J. F., Isner J. M. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994 Feb;93(2):662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White F. C., Carroll S. M., Magnet A., Bloor C. M. Coronary collateral development in swine after coronary artery occlusion. Circ Res. 1992 Dec;71(6):1490–1500. doi: 10.1161/01.res.71.6.1490. [DOI] [PubMed] [Google Scholar]
- Yanagisawa-Miwa A., Uchida Y., Nakamura F., Tomaru T., Kido H., Kamijo T., Sugimoto T., Kaji K., Utsuyama M., Kurashima C. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science. 1992 Sep 4;257(5075):1401–1403. doi: 10.1126/science.1382313. [DOI] [PubMed] [Google Scholar]
- Zerwes H. G., Risau W. Polarized secretion of a platelet-derived growth factor-like chemotactic factor by endothelial cells in vitro. J Cell Biol. 1987 Nov;105(5):2037–2041. doi: 10.1083/jcb.105.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]





