Abstract
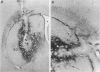
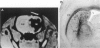
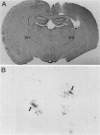
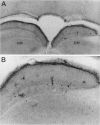
Delivery of adenovirus, herpes simplex virus (HSV), and paramagnetic monocrystalline iron oxide nanoparticles (MION) to rat brain (n = 64) was assessed after intracerebral inoculation or osmotic disruption of the blood-brain barrier (BBB). After intracerebral inoculation, the area of distribution was 7.93 +/- 0.43 mm2 (n = 9) for MION and 9.17 +/- 1.27 mm2 (n = 9) for replication-defective adenovirus. The replication-compromised HSV RH105 spread to 14.00 +/- 0.87 mm2 (n = 8), but also had a large necrotic center (3.54 +/- 0.47 mm2). No infection was detected when virus was administered intra-arterially without hyperosmotic mannitol. After osmotic BBB disruption, delivery of the viruses and MIONs was detected throughout the disrupted cerebral cortex. Positive staining was found in 4 to 845 cells/100 microns thick coronal brain section (n = 7) after adenovirus administration, and in 13 to 197 cells/section (n = 8) after HSV administration. Cells of glial morphology were more frequently stained after administration of adenovirus, whereas neuronal cells were preferentially stained after delivery of both HSV vectors and MION. In a preliminary test of vector delivery in the feline, MION was detected throughout the white matter tracts after inoculation into normal cat brain. Thus MION may be a tool for use in vivo, to monitor the delivery of virus to the central nervous system. Additionally, BBB disruption may be an effective method to globally deliver recombinant viruses to the CNS.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobo R. H., Laske D. W., Akbasak A., Morrison P. F., Dedrick R. L., Oldfield E. H. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boviatsis E. J., Chase M., Wei M. X., Tamiya T., Hurford R. K., Jr, Kowall N. W., Tepper R. I., Breakefield X. O., Chiocca E. A. Gene transfer into experimental brain tumors mediated by adenovirus, herpes simplex virus, and retrovirus vectors. Hum Gene Ther. 1994 Feb;5(2):183–191. doi: 10.1089/hum.1994.5.2-183. [DOI] [PubMed] [Google Scholar]
- Boviatsis E. J., Park J. S., Sena-Esteves M., Kramm C. M., Chase M., Efird J. T., Wei M. X., Breakefield X. O., Chiocca E. A. Long-term survival of rats harboring brain neoplasms treated with ganciclovir and a herpes simplex virus vector that retains an intact thymidine kinase gene. Cancer Res. 1994 Nov 15;54(22):5745–5751. [PubMed] [Google Scholar]
- Chen S. H., Shine H. D., Goodman J. C., Grossman R. G., Woo S. L. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3054–3057. doi: 10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiocca E. A., Choi B. B., Cai W. Z., DeLuca N. A., Schaffer P. A., DiFiglia M., Breakefield X. O., Martuza R. L. Transfer and expression of the lacZ gene in rat brain neurons mediated by herpes simplex virus mutants. New Biol. 1990 Aug;2(8):739–746. [PubMed] [Google Scholar]
- Coen D. M., Goldstein D. J., Weller S. K. Herpes simplex virus ribonucleotide reductase mutants are hypersensitive to acyclovir. Antimicrob Agents Chemother. 1989 Aug;33(8):1395–1399. doi: 10.1128/aac.33.8.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Kosz-Vnenchak M., Jacobson J. G., Leib D. A., Bogard C. L., Schaffer P. A., Tyler K. L., Knipe D. M. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4736–4740. doi: 10.1073/pnas.86.12.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B. L., Allen E. D., Kozarsky K. F., Wilson J. M., Roessler B. J. A model system for in vivo gene transfer into the central nervous system using an adenoviral vector. Nat Genet. 1993 Mar;3(3):219–223. doi: 10.1038/ng0393-219. [DOI] [PubMed] [Google Scholar]
- Davidson B. L., Doran S. E., Shewach D. S., Latta J. M., Hartman J. W., Roessler B. J. Expression of Escherichia coli beta-galactosidase and rat HPRT in the CNS of Macaca mulatta following adenoviral mediated gene transfer. Exp Neurol. 1994 Feb;125(2):258–267. doi: 10.1006/exnr.1994.1028. [DOI] [PubMed] [Google Scholar]
- Doran S. E., Ren X. D., Betz A. L., Pagel M. A., Neuwelt E. A., Roessler B. J., Davidson B. L. Gene expression from recombinant viral vectors in the central nervous system after blood-brain barrier disruption. Neurosurgery. 1995 May;36(5):965–970. doi: 10.1227/00006123-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Fink D. J., Sternberg L. R., Weber P. C., Mata M., Goins W. F., Glorioso J. C. In vivo expression of beta-galactosidase in hippocampal neurons by HSV-mediated gene transfer. Hum Gene Ther. 1992 Feb;3(1):11–19. doi: 10.1089/hum.1992.3.1-11. [DOI] [PubMed] [Google Scholar]
- Ho D. Y., Mocarski E. S. Beta-galactosidase as a marker in the peripheral and neural tissues of the herpes simplex virus-infected mouse. Virology. 1988 Nov;167(1):279–283. doi: 10.1016/0042-6822(88)90079-7. [DOI] [PubMed] [Google Scholar]
- Huang Q., Vonsattel J. P., Schaffer P. A., Martuza R. L., Breakefield X. O., DiFiglia M. Introduction of a foreign gene (Escherichia coli lacZ) into rat neostriatal neurons using herpes simplex virus mutants: a light and electron microscopic study. Exp Neurol. 1992 Mar;115(3):303–316. doi: 10.1016/0014-4886(92)90196-w. [DOI] [PubMed] [Google Scholar]
- Koeppen A. H., Borke R. C. Experimental superficial siderosis of the central nervous system. I. Morphological observations. J Neuropathol Exp Neurol. 1991 Sep;50(5):579–594. doi: 10.1097/00005072-199109000-00005. [DOI] [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Muldoon L. L., Neuwelt E. A., Pagel M. A., Weiss D. L. Characterization of the molecular defect in a feline model for type II GM2-gangliosidosis (Sandhoff disease). Am J Pathol. 1994 May;144(5):1109–1118. [PMC free article] [PubMed] [Google Scholar]
- Neuwelt E. A., Goldman D. L., Dahlborg S. A., Crossen J., Ramsey F., Roman-Goldstein S., Braziel R., Dana B. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991 Sep;9(9):1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Pagel M. A., Dix R. D. Delivery of ultraviolet-inactivated 35S-herpesvirus across an osmotically modified blood-brain barrier. J Neurosurg. 1991 Mar;74(3):475–479. doi: 10.3171/jns.1991.74.3.0475. [DOI] [PubMed] [Google Scholar]
- Neuwelt E. A., Weissleder R., Nilaver G., Kroll R. A., Roman-Goldstein S., Szumowski J., Pagel M. A., Jones R. S., Remsen L. G., McCormick C. I. Delivery of virus-sized iron oxide particles to rodent CNS neurons. Neurosurgery. 1994 Apr;34(4):777–784. doi: 10.1227/00006123-199404000-00048. [DOI] [PubMed] [Google Scholar]
- Nilaver G., Muldoon L. L., Kroll R. A., Pagel M. A., Breakefield X. O., Davidson B. L., Neuwelt E. A. Delivery of herpesvirus and adenovirus to nude rat intracerebral tumors after osmotic blood-brain barrier disruption. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9829–9833. doi: 10.1073/pnas.92.21.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan R., Fink D. J., Jiang G., Desai P., Glorioso J. C., Levine M. Competitive quantitative PCR analysis of herpes simplex virus type 1 DNA and latency-associated transcript RNA in latently infected cells of the rat brain. J Virol. 1994 Mar;68(3):1864–1873. doi: 10.1128/jvi.68.3.1864-1873.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport S. I., Robinson P. J. Tight-junctional modification as the basis of osmotic opening of the blood-brain barrier. Ann N Y Acad Sci. 1986;481:250–267. doi: 10.1111/j.1749-6632.1986.tb27155.x. [DOI] [PubMed] [Google Scholar]
- Sengstock G. J., Olanow C. W., Menzies R. A., Dunn A. J., Arendash G. W. Infusion of iron into the rat substantia nigra: nigral pathology and dose-dependent loss of striatal dopaminergic markers. J Neurosci Res. 1993 May 1;35(1):67–82. doi: 10.1002/jnr.490350109. [DOI] [PubMed] [Google Scholar]
- Shen T., Weissleder R., Papisov M., Bogdanov A., Jr, Brady T. J. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn Reson Med. 1993 May;29(5):599–604. doi: 10.1002/mrm.1910290504. [DOI] [PubMed] [Google Scholar]
- Weissleder R., Elizondo G., Wittenberg J., Lee A. S., Josephson L., Brady T. J. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology. 1990 May;175(2):494–498. doi: 10.1148/radiology.175.2.2326475. [DOI] [PubMed] [Google Scholar]
- Wolfe J. H., Deshmane S. L., Fraser N. W. Herpesvirus vector gene transfer and expression of beta-glucuronidase in the central nervous system of MPS VII mice. Nat Genet. 1992 Aug;1(5):379–384. doi: 10.1038/ng0892-379. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tatsuno T., Carney J. M., Mattson M. P. Basic FGF, NGF, and IGFs protect hippocampal and cortical neurons against iron-induced degeneration. J Cereb Blood Flow Metab. 1993 May;13(3):378–388. doi: 10.1038/jcbfm.1993.51. [DOI] [PubMed] [Google Scholar]