Abstract
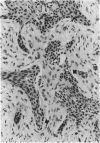
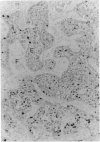
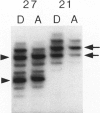
Adamantinoma of long bones is a rare malignant tumor composed of cells with epithelial characteristics in various differentiation patterns surrounded by fibrous cells. Evidence as to whether this neoplasm should be designated as an epithelial bone tumor or a biphasic sarcoma with both epithelial and mesenchymal features is lacking. In this study the nature of the mesenchymal and epithelial components of adamantinoma was investigated by DNA flow cytometry, DNA image cytometry, p53 immunohistochemistry, and polymerase chain reaction-based loss of heterozygosity detection at the p53 locus. Specimens from 6 of 15 patients (40%) analyzed by flow cytometry had an aneuploid DNA index. Image cytometry analysis of Feulgen-stained paraffin sections of 6 aneuploid and 2 diploid tumors revealed that aneuploid nuclei were detected in cells with an epithelial phenotype only, whereas all fibrous cells were diploid. Immunohistochemistry for p53 on specimens from 25 patients revealed moderate or strong immunoreactivity in 12 tumors (48%) restricted to the epithelial cells. Loss of heterozygosity at the p53 locus could be confirmed in the epithelial component of an immunohistochemically p53-positive tumor. Additionally, sections of 7 lung metastases were studied histologically. Only keratin-positive epithelial cells, predominantly in the spindle cell pattern, were present in these metastases, whereas the osteofibrous tissue present in the primary tumors was not detected. These results suggest that either adamantinoma consists of a malignant epithelial part with a reactive osteofibrous stroma or that the malignant epithelial cells develop next to a proliferating benign fibrous component. Additional analysis of common genetic abnormalities in the fibrous and epithelial cells of adamantinoma is therefore indicated.
Full text
PDF


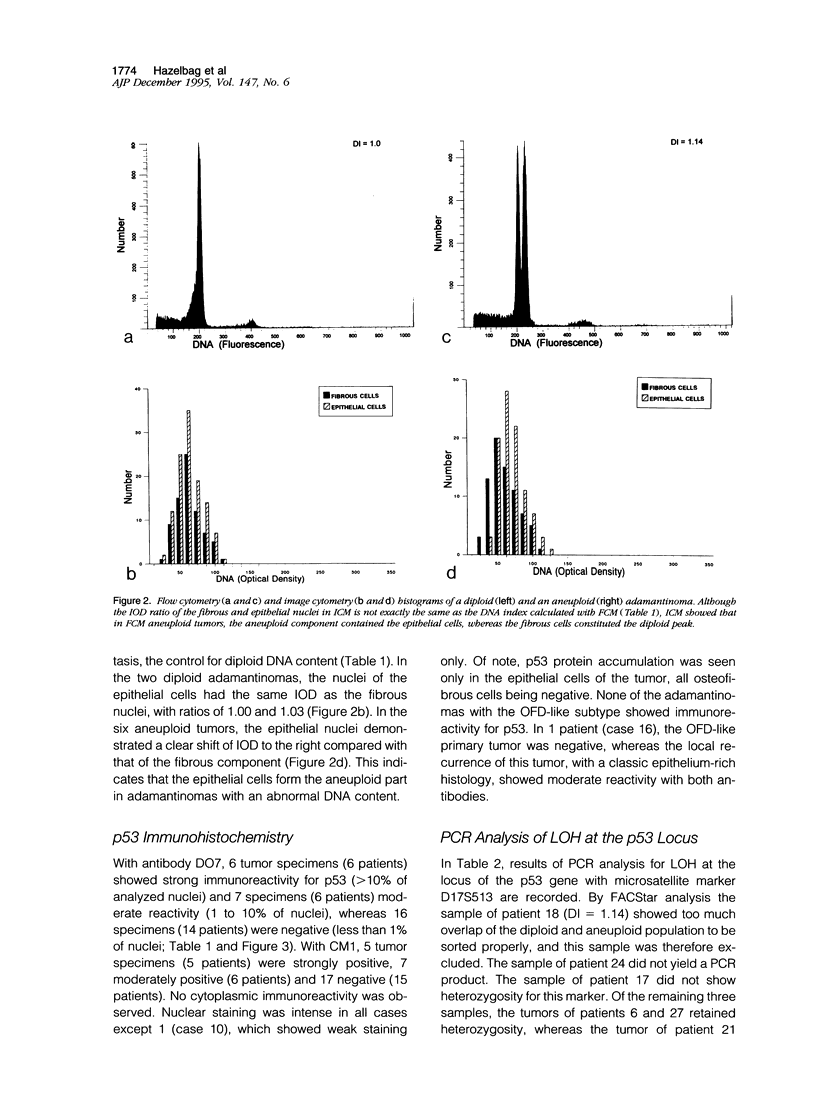





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeln E. C., Corver W. E., Kuipers-Dijkshoorn N. J., Fleuren G. J., Cornelisse C. J. Molecular genetic analysis of flow-sorted ovarian tumour cells: improved detection of loss of heterozygosity. Br J Cancer. 1994 Aug;70(2):255–262. doi: 10.1038/bjc.1994.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas I. O., Mulder J. W., Offerhaus G. J., Vogelstein B., Hamilton S. R. An evaluation of six antibodies for immunohistochemistry of mutant p53 gene product in archival colorectal neoplasms. J Pathol. 1994 Jan;172(1):5–12. doi: 10.1002/path.1711720104. [DOI] [PubMed] [Google Scholar]
- Bauer H. C., Kreicbergs A. Feulgen DNA stainability of bone tumors after demineralization. Cytometry. 1987 Nov;8(6):590–594. doi: 10.1002/cyto.990080610. [DOI] [PubMed] [Google Scholar]
- Bloem J. L., van der Heul R. O., Schuttevaer H. M., Kuipers D. Fibrous dysplasia vs adamantinoma of the tibia: differentiation based on discriminant analysis of clinical and plain film findings. AJR Am J Roentgenol. 1991 May;156(5):1017–1023. doi: 10.2214/ajr.156.5.2017924. [DOI] [PubMed] [Google Scholar]
- Bridge J. A., Dembinski A., DeBoer J., Travis J., Neff J. R. Clonal chromosomal abnormalities in osteofibrous dysplasia. Implications for histopathogenesis and its relationship with adamantinoma. Cancer. 1994 Mar 15;73(6):1746–1752. doi: 10.1002/1097-0142(19940315)73:6<1746::aid-cncr2820730632>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Carey F. A. Measurement of nuclear DNA content in histological and cytological specimens: principles and applications. J Pathol. 1994 Apr;172(4):307–312. doi: 10.1002/path.1711720404. [DOI] [PubMed] [Google Scholar]
- Costa M. J., Vogelsan J., Young L. J. p53 gene mutation in female genital tract carcinosarcomas (malignant mixed müllerian tumors): a clinicopathologic study of 74 cases. Mod Pathol. 1994 Aug;7(6):619–627. [PubMed] [Google Scholar]
- Czerniak B., Rojas-Corona R. R., Dorfman H. D. Morphologic diversity of long bone adamantinoma. The concept of differentiated (regressing) adamantinoma and its relationship to osteofibrous dysplasia. Cancer. 1989 Dec 1;64(11):2319–2334. doi: 10.1002/1097-0142(19891201)64:11<2319::aid-cncr2820641123>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Gorstein F., Anderson T. L. Malignant mixed mesodermal tumors: carcinoma, sarcoma, or both? Hum Pathol. 1991 Mar;22(3):207–209. doi: 10.1016/0046-8177(91)90151-e. [DOI] [PubMed] [Google Scholar]
- Hauge X. Y., Litt M. A study of the origin of 'shadow bands' seen when typing dinucleotide repeat polymorphisms by the PCR. Hum Mol Genet. 1993 Apr;2(4):411–415. doi: 10.1093/hmg/2.4.411. [DOI] [PubMed] [Google Scholar]
- Hazelbag H. M., Fleuren G. J., vd Broek L. J., Taminiau A. H., Hogendoorn P. C. Adamantinoma of the long bones: keratin subclass immunoreactivity pattern with reference to its histogenesis. Am J Surg Pathol. 1993 Dec;17(12):1225–1233. doi: 10.1097/00000478-199312000-00003. [DOI] [PubMed] [Google Scholar]
- Hazelbag H. M., Taminiau A. H., Fleuren G. J., Hogendoorn P. C. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J Bone Joint Surg Am. 1994 Oct;76(10):1482–1499. doi: 10.2106/00004623-199410000-00008. [DOI] [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Hiddemann W., Schumann J., Andreef M., Barlogie B., Herman C. J., Leif R. C., Mayall B. H., Murphy R. F., Sandberg A. A. Convention on nomenclature for DNA cytometry. Committee on Nomenclature, Society for Analytical Cytology. Cancer Genet Cytogenet. 1984 Oct;13(2):181–183. doi: 10.1016/0165-4608(84)90059-1. [DOI] [PubMed] [Google Scholar]
- Huvos A. G., Marcove R. C. Adamantinoma of long bones. A clinicopathological study of fourteen cases with vascular origin suggested. J Bone Joint Surg Am. 1975 Mar;57(2):148–154. [PubMed] [Google Scholar]
- Ishida T., Iijima T., Kikuchi F., Kitagawa T., Tanida T., Imamura T., Machinami R. A clinicopathological and immunohistochemical study of osteofibrous dysplasia, differentiated adamantinoma, and adamantinoma of long bones. Skeletal Radiol. 1992;21(8):493–502. doi: 10.1007/BF00195230. [DOI] [PubMed] [Google Scholar]
- Knapp R. H., Wick M. R., Scheithauer B. W., Unni K. K. Adamantinoma of bone. An electron microscopic and immunohistochemical study. Virchows Arch A Pathol Anat Histopathol. 1982;398(1):75–86. doi: 10.1007/BF00585615. [DOI] [PubMed] [Google Scholar]
- Kreicbergs A., Silvferswärd C., Tribukait B. Flow DNA analysis of primary bone tumors. Relationship between cellular DNA content and histopathologic classification. Cancer. 1984 Jan 1;53(1):129–136. doi: 10.1002/1097-0142(19840101)53:1<129::aid-cncr2820530123>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- LEDERER H., SINCLAIR A. J. Malignant synovioma simulating "adamantinoma of the tibia". J Pathol Bacteriol. 1954 Jan;67(1):163–168. doi: 10.1002/path.1700670120. [DOI] [PubMed] [Google Scholar]
- Llombart-Bosch A., Ortuño-Pacheco G. Ultrastructural findings supporting the angioblastic nature of the so-called adamantinoma of the tibia. Histopathology. 1978 May;2(3):189–200. doi: 10.1111/j.1365-2559.1978.tb01709.x. [DOI] [PubMed] [Google Scholar]
- Louis D. N., von Deimling A., Seizinger B. R. A (CA)n dinucleotide repeat assay for evaluating loss of allelic heterozygosity in small and archival human brain tumor specimens. Am J Pathol. 1992 Oct;141(4):777–782. [PMC free article] [PubMed] [Google Scholar]
- Mankin H. J., Connor J. F., Schiller A. L., Perlmutter N., Alho A., McGuire M. Grading of bone tumors by analysis of nuclear DNA content using flow cytometry. J Bone Joint Surg Am. 1985 Mar;67(3):404–413. [PubMed] [Google Scholar]
- Mori H., Yamamoto S., Hiramatsu K., Miura T., Moon N. F. Adamantinoma of the tibia. Ultrastructural and immunohistochemic study with reference to histogenesis. Clin Orthop Relat Res. 1984 Nov;(190):299–310. [PubMed] [Google Scholar]
- Oliphant A. R., Wright E. C., Swensen J., Gruis N. A., Goldgar D., Skolnick M. H. Dinucleotide repeat polymorphism at the D17S513 locus. Nucleic Acids Res. 1991 Sep 11;19(17):4794–4794. [PMC free article] [PubMed] [Google Scholar]
- Perez-Atayde A. R., Kozakewich H. P., Vawter G. F. Adamantinoma of the tibia. An ultrastructural and immunohistochemical study. Cancer. 1985 Mar 1;55(5):1015–1023. doi: 10.1002/1097-0142(19850301)55:5<1015::aid-cncr2820550516>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Povýsil C., Matejovský Z. Ultrastructure of adamantinoma of long bones. Virchows Arch A Pathol Anat Histol. 1981;393(2):233–244. doi: 10.1007/BF00431079. [DOI] [PubMed] [Google Scholar]
- Rosai J. Adamantinoma of the tibia. Electron microscopic evidence of its epithelial origin. Am J Clin Pathol. 1969 Jun;51(6):786–792. doi: 10.1093/ajcp/51.6.786. [DOI] [PubMed] [Google Scholar]
- Rosai J., Pinkus G. S. Immunohistochemical demonstration of epithelial differentiation in adamantinoma of the tibia. Am J Surg Pathol. 1982 Jul;6(5):427–434. doi: 10.1097/00000478-198207000-00004. [DOI] [PubMed] [Google Scholar]
- Shankey T. V., Rabinovitch P. S., Bagwell B., Bauer K. D., Duque R. E., Hedley D. W., Mayall B. H., Wheeless L., Cox C. Guidelines for implementation of clinical DNA cytometry. International Society for Analytical Cytology. Cytometry. 1993;14(5):472–477. doi: 10.1002/cyto.990140503. [DOI] [PubMed] [Google Scholar]
- Springfield D. S., Rosenberg A. E., Mankin H. J., Mindell E. R. Relationship between osteofibrous dysplasia and adamantinoma. Clin Orthop Relat Res. 1994 Dec;(309):234–244. [PubMed] [Google Scholar]
- Sweet D. E., Vinh T. N., Devaney K. Cortical osteofibrous dysplasia of long bone and its relationship to adamantinoma. A clinicopathologic study of 30 cases. Am J Surg Pathol. 1992 Mar;16(3):282–290. doi: 10.1097/00000478-199203000-00009. [DOI] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J., Nissen N. I. A detergent-trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry. 1983 Mar;3(5):323–327. doi: 10.1002/cyto.990030503. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Kinzler K. W. p53 function and dysfunction. Cell. 1992 Aug 21;70(4):523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- van den Berg F. M., Baas I. O., Polak M. M., Offerhaus G. J. Detection of p53 overexpression in routinely paraffin-embedded tissue of human carcinomas using a novel target unmasking fluid. Am J Pathol. 1993 Feb;142(2):381–385. [PMC free article] [PubMed] [Google Scholar]