Abstract
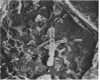
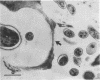
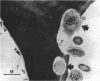
The association of rumen bacteria with specific leaf tissues of the forage grass Kentucky-31 tall fescue (Festuca arundinacea Schreb.) during in vitro degradation was investigated by transmission and scanning electron microscopy. Examination of degraded leaf cross-sections revealed differential rates of tissue degradation in that the cell walls of the mesophyll and pholem were degraded prior to those of the outer bundle sheath and epidermis. Rumen bacteria appeared to degrade the mesophyll, in some cases, and phloem without prior attachment to the plant cell walls. The degradation of bundle sheath and epidermal cell walls appeared to be preceded by attachment of bacteria to the plant cell wall. Ultrastructural features apparently involved in the adhesion of large cocci to plant cells were observed by transmission and scanning electron microscopy. The physical association between plant and rumen bacterial cells during degradation apparently varies with tissue types. Bacterial attachment, by extracellular features in some microorganisms, is required prior to degradation of the more resistant tissues.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akin D. E., Burdick D., Michaels G. E. Rumen bacterial interrelationships with plant tissue during degradation revealed by transmission electron microscopy. Appl Microbiol. 1974 Jun;27(6):1149–1156. doi: 10.1128/am.27.6.1149-1156.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg B., von Hofsten B., Pettersson G. Electronmicroscopie observations on the degradation of cellulose fibres by Cellvibrio fulvus and Sporocytophaga myxococcoides. J Appl Bacteriol. 1972 Jun;35(2):215–219. doi: 10.1111/j.1365-2672.1972.tb03692.x. [DOI] [PubMed] [Google Scholar]
- Bryant M. P. Nutritional requirements of the predominant rumen cellulolytic bacteria. Fed Proc. 1973 Jul;32(7):1809–1813. [PubMed] [Google Scholar]
- Cagle G. D. Critical-point drying: rapid method for the determination of bacterial extracellular polymer and surface structures. Appl Microbiol. 1974 Aug;28(2):312–316. doi: 10.1128/am.28.2.312-316.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen J. A., Dehority B. A. Degradation and utilization of hemicellulose from intact forages by pure cultures of rumen bacteria. Appl Microbiol. 1970 Sep;20(3):362–368. doi: 10.1128/am.20.3.362-368.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Damgaard H. N., Cheng K. J. Cell envelope morphology of rumen bacteria. J Bacteriol. 1974 Jun;118(3):1132–1143. doi: 10.1128/jb.118.3.1132-1143.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton J. W., Ingram J. M., Cheng K. J. Structure and function of the cell envelope of gram-negative bacteria. Bacteriol Rev. 1974 Mar;38(1):87–110. doi: 10.1128/br.38.1.87-110.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Hemicellulose degradation by rumen bacteria. Fed Proc. 1973 Jul;32(7):1819–1825. [PubMed] [Google Scholar]
- Dehority B. A. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968 May;16(5):781–786. doi: 10.1128/am.16.5.781-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leatherwood J. M. Cellulose degradation by Ruminococcus. Fed Proc. 1973 Jul;32(7):1814–1818. [PubMed] [Google Scholar]
- McDougall E. I. Studies on ruminant saliva. 1. The composition and output of sheep's saliva. Biochem J. 1948;43(1):99–109. [PMC free article] [PubMed] [Google Scholar]
- Shilo M. Lysis of blue-green algae by myxobacter. J Bacteriol. 1970 Oct;104(1):453–461. doi: 10.1128/jb.104.1.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R., Yu I., Hungate R. E. Factors affecting cellulolysis by Ruminococcus albus. J Bacteriol. 1973 May;114(2):729–737. doi: 10.1128/jb.114.2.729-737.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer E. L., Roth I. L. The ultrastructure of the capsules of Diplococcus pneumoniae and Klebsiella pneumoniae stained with ruthenium red. J Gen Microbiol. 1973 Jan;74(1):21–31. doi: 10.1099/00221287-74-1-21. [DOI] [PubMed] [Google Scholar]
- Tarakanov B. V. Elektronno-mikroskopicheskoe izuchenie mikroflory rubtsa severnykh olenei. Mikrobiologiia. 1972 Sep-Oct;41(5):862–870. [PubMed] [Google Scholar]