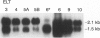Abstract
Uterine myometrial tumors are the most commonly found gynecological neoplasm in women. The underlying causes of uterine leiomyomata are poorly understood, a result in part of the absence of a good animal model system in which to study these tumors. This report describes a novel rat model (Eker rat) in which spontaneous gynecological smooth muscle tumors arise with a high frequency. Leiomyomas are the predominant reproductive tract tumor that arise in these animals, although leiomyosarcomas have also been observed. Cell lines have been established from both the benign and malignant lesions. All of the lines express smooth muscle-specific actin, and leiomyoma-derived cell lines express desmin. Two of the cell lines are tumorigenic in nude mice, and the lines are variable for expression of estrogen and progesterone receptors. These lines are the first rodent tumor-derived lines to be established from leiomyomata and are the only lines available from a hereditary form of these tumors. Together with Eker rats that spontaneously develop leiomyomata, they constitute an in vitro/in vivo model system for gaining insights into the mechanism of transformation of uterine smooth muscle cells and the role of steroid hormones and hormone receptors in myometrial tumorigenesis.
Full text
PDF



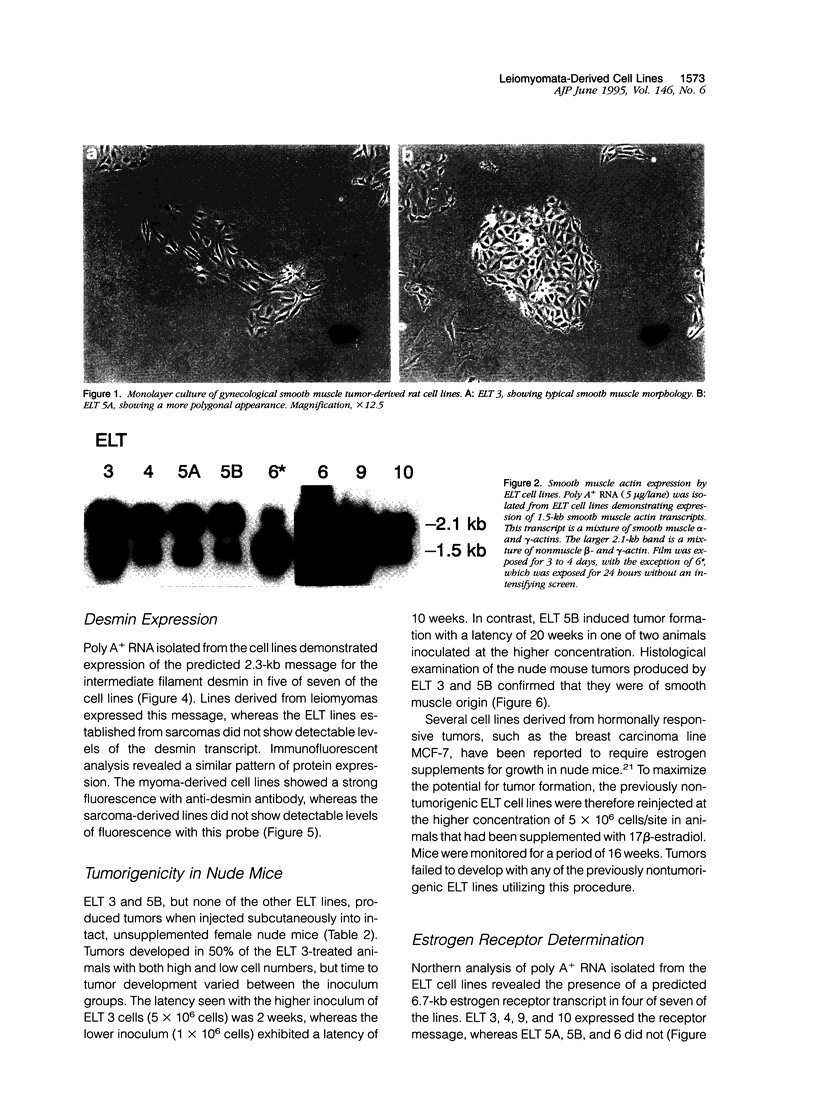

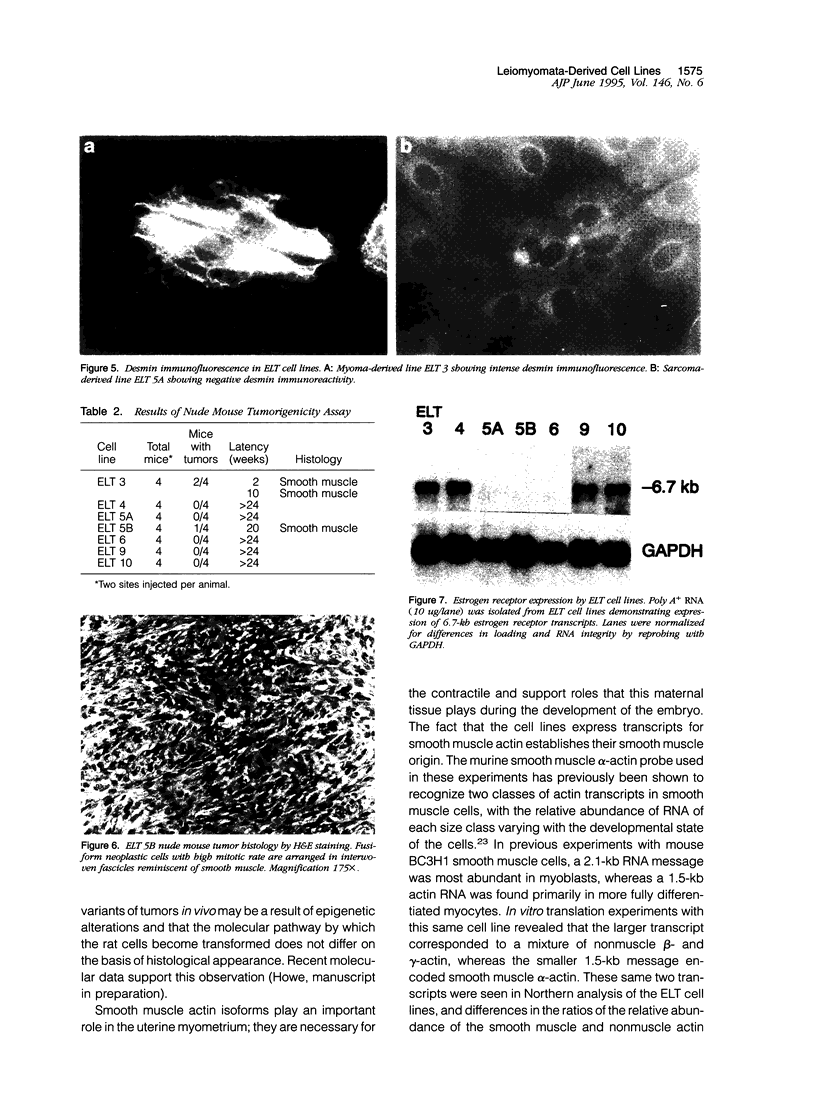




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkenstam A., Glaumann H., Martin M., Gustafsson J. A., Norstedt G. Hormonal regulation of estrogen receptor messenger ribonucleic acid in T47Dco and MCF-7 breast cancer cells. Mol Endocrinol. 1989 Jan;3(1):22–28. doi: 10.1210/mend-3-1-22. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brandon D. D., Bethea C. L., Strawn E. Y., Novy M. J., Burry K. A., Harrington M. S., Erickson T. E., Warner C., Keenan E. J., Clinton G. M. Progesterone receptor messenger ribonucleic acid and protein are overexpressed in human uterine leiomyomas. Am J Obstet Gynecol. 1993 Jul;169(1):78–85. doi: 10.1016/0002-9378(93)90135-6. [DOI] [PubMed] [Google Scholar]
- Buttram V. C., Jr, Reiter R. C. Uterine leiomyomata: etiology, symptomatology, and management. Fertil Steril. 1981 Oct;36(4):433–445. doi: 10.1016/s0015-0282(16)45789-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Deligdish L., Loewenthal M. Endometrial changes associated with myomata of the uterus. J Clin Pathol. 1970 Nov;23(8):676–680. doi: 10.1136/jcp.23.8.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dookwah H. D., Barhoumi R., Narasimhan T. R., Safe S. H., Burghardt R. C. Gap junctions in myometrial cell cultures: evidence for modulation by cyclic adenosine 3':5'-monophosphate. Biol Reprod. 1992 Sep;47(3):397–407. doi: 10.1095/biolreprod47.3.397. [DOI] [PubMed] [Google Scholar]
- Dugaiczyk A., Haron J. A., Stone E. M., Dennison O. E., Rothblum K. N., Schwartz R. J. Cloning and sequencing of a deoxyribonucleic acid copy of glyceraldehyde-3-phosphate dehydrogenase messenger ribonucleic acid isolated from chicken muscle. Biochemistry. 1983 Mar 29;22(7):1605–1613. doi: 10.1021/bi00276a013. [DOI] [PubMed] [Google Scholar]
- Eker R., Mossige J., Johannessen J. V., Aars H. Hereditary renal adenomas and adenocarcinomas in rats. Diagn Histopathol. 1981 Jan-Mar;4(1):99–110. [PubMed] [Google Scholar]
- Everitt J. I., Goldsworthy T. L., Wolf D. C., Walker C. L. Hereditary renal cell carcinoma in the Eker rat: a rodent familial cancer syndrome. J Urol. 1992 Dec;148(6):1932–1936. doi: 10.1016/s0022-5347(17)37087-8. [DOI] [PubMed] [Google Scholar]
- Eyden B. P., Hale R. J., Richmond I., Buckley C. H. Cytoskeletal filaments in the smooth muscle cells of uterine leiomyomata and myometrium: an ultrastructural and immunohistochemical analysis. Virchows Arch A Pathol Anat Histopathol. 1992;420(1):51–58. doi: 10.1007/BF01605984. [DOI] [PubMed] [Google Scholar]
- Farrer-Brown G., Beilby J. O., Tarbit M. H. The vascular patterns in myomatous uteri. J Obstet Gynaecol Br Commonw. 1970 Nov;77(11):967–975. doi: 10.1111/j.1471-0528.1970.tb03439.x. [DOI] [PubMed] [Google Scholar]
- Green S., Walter P., Kumar V., Krust A., Bornert J. M., Argos P., Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986 Mar 13;320(6058):134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- Han Z., Kokkonen G. C., Roth G. S. Effect of aging on populations of estrogen receptor-containing cells in the rat uterus. Exp Cell Res. 1989 Jan;180(1):234–242. doi: 10.1016/0014-4827(89)90227-9. [DOI] [PubMed] [Google Scholar]
- Hino O., Klein-Szanto A. J., Freed J. J., Testa J. R., Brown D. Q., Vilensky M., Yeung R. S., Tartof K. D., Knudson A. G. Spontaneous and radiation-induced renal tumors in the Eker rat model of dominantly inherited cancer. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):327–331. doi: 10.1073/pnas.90.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi K., Fujii S., Konishi I., Nanbu Y., Nonogaki H., Mori T. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989 Mar;160(3):637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K., Fujii S., Konishi I., Okamura H., Mori T. Ultrastructural study of cultured smooth muscle cells from uterine leiomyoma and myometrium under the influence of sex steroids. Gynecol Oncol. 1985 May;21(1):32–41. doi: 10.1016/0090-8258(85)90229-x. [DOI] [PubMed] [Google Scholar]
- Kraus W. L., Katzenellenbogen B. S. Regulation of progesterone receptor gene expression and growth in the rat uterus: modulation of estrogen actions by progesterone and sex steroid hormone antagonists. Endocrinology. 1993 Jun;132(6):2371–2379. doi: 10.1210/endo.132.6.8504742. [DOI] [PubMed] [Google Scholar]
- Lemire J. M., Covin C. W., White S., Giachelli C. M., Schwartz S. M. Characterization of cloned aortic smooth muscle cells from young rats. Am J Pathol. 1994 May;144(5):1068–1081. [PMC free article] [PubMed] [Google Scholar]
- Leoni P., Carli F., Halliday D. Intermediate filaments in smooth muscle from pregnant and non-pregnant human uterus. Biochem J. 1990 Jul 1;269(1):31–34. doi: 10.1042/bj2690031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner L. J., Jordan V. C. Development of antiestrogens and their use in breast cancer: eighth Cain memorial award lecture. Cancer Res. 1990 Jul 15;50(14):4177–4189. [PubMed] [Google Scholar]
- Li H., Choudhary S. K., Milner D. J., Munir M. I., Kuisk I. R., Capetanaki Y. Inhibition of desmin expression blocks myoblast fusion and interferes with the myogenic regulators MyoD and myogenin. J Cell Biol. 1994 Mar;124(5):827–841. doi: 10.1083/jcb.124.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B. H., Strauch A. R., Foster D. N. Nucleotide sequence of a mouse vascular smooth muscle alpha-actin cDNA. Nucleic Acids Res. 1988 Nov 11;16(21):10374–10374. doi: 10.1093/nar/16.21.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misrahi M., Atger M., d'Auriol L., Loosfelt H., Meriel C., Fridlansky F., Guiochon-Mantel A., Galibert F., Milgrom E. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun. 1987 Mar 13;143(2):740–748. doi: 10.1016/0006-291x(87)91416-1. [DOI] [PubMed] [Google Scholar]
- Olea-Serrano N., Devleeschouwer N., Leclercq G., Heuson J. C. Assay for estrogen and progesterone receptors of breast cancer cell lines in monolayer culture. Eur J Cancer Clin Oncol. 1985 Aug;21(8):965–973. doi: 10.1016/0277-5379(85)90116-6. [DOI] [PubMed] [Google Scholar]
- Palmberg L., Thyberg J. Uterine smooth muscle cells in primary culture. Alterations in fine structure, cytoskeletal organization and growth characteristics. Cell Tissue Res. 1986;246(2):253–262. doi: 10.1007/BF00215887. [DOI] [PubMed] [Google Scholar]
- Park O. K., Mayo K. E. Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol. 1991 Jul;5(7):967–978. doi: 10.1210/mend-5-7-967. [DOI] [PubMed] [Google Scholar]
- Riemer R. K., Sadovsky Y., Roberts J. M. Myometrial characteristics of the Syrian hamster uterine smooth muscle cell line, SHM. In Vitro Cell Dev Biol Anim. 1993 Jun;29A(6):478–480. [PubMed] [Google Scholar]
- Ross R. K., Pike M. C., Vessey M. P., Bull D., Yeates D., Casagrande J. T. Risk factors for uterine fibroids: reduced risk associated with oral contraceptives. Br Med J (Clin Res Ed) 1986 Aug 9;293(6543):359–362. doi: 10.1136/bmj.293.6543.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
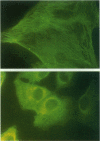
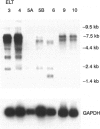
- Schürch W., Skalli O., Seemayer T. A., Gabbiani G. Intermediate filament proteins and actin isoforms as markers for soft tissue tumor differentiation and origin. I. Smooth muscle tumors. Am J Pathol. 1987 Jul;128(1):91–103. [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soule H. D., McGrath C. M. Estrogen responsive proliferation of clonal human breast carcinoma cells in athymic mice. Cancer Lett. 1980 Aug;10(2):177–189. doi: 10.1016/0304-3835(80)90042-7. [DOI] [PubMed] [Google Scholar]
- Strauch A. R., Offord J. D., Chalkley R., Rubenstein P. A. Characterization of actin mRNA levels during BC3H1 cell differentiation. J Biol Chem. 1986 Jan 15;261(2):849–855. [PubMed] [Google Scholar]
- Suda A., Sato T., Watanabe Y., Yamakawa M., Imai Y. Immunohistochemical study of steroid hormones and an estrogen binding assay in malignant soft tissue tumors. Nihon Seikeigeka Gakkai Zasshi. 1990 Sep;64(9):814–823. [PubMed] [Google Scholar]
- Sumida C., Lecerf F., Pasqualini J. R. Control of progesterone receptors in fetal uterine cells in culture: effects of estradiol, progestins, antiestrogens, and growth factors. Endocrinology. 1988 Jan;122(1):3–11. doi: 10.1210/endo-122-1-3. [DOI] [PubMed] [Google Scholar]
- VAN VELSEN R. J., CROWLEY N. C. Centrosema mosaic: a plant virus disease transmitted by both aphids and plant bugs. Nature. 1961 Mar 11;189:858–858. doi: 10.1038/189858a0. [DOI] [PubMed] [Google Scholar]
- Walker C., Ginsler J. Development of a quantitative in vitro transformation assay for kidney epithelial cells. Carcinogenesis. 1992 Jan;13(1):25–32. doi: 10.1093/carcin/13.1.25. [DOI] [PubMed] [Google Scholar]
- Wei L. L., Krett N. L., Francis M. D., Gordon D. F., Wood W. M., O'Malley B. W., Horwitz K. B. Multiple human progesterone receptor messenger ribonucleic acids and their autoregulation by progestin agonists and antagonists in breast cancer cells. Mol Endocrinol. 1988 Jan;2(1):62–72. doi: 10.1210/mend-2-1-62. [DOI] [PubMed] [Google Scholar]
- Yeung R. S., Xiao G. H., Jin F., Lee W. C., Testa J. R., Knudson A. G. Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11413–11416. doi: 10.1073/pnas.91.24.11413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Ashry D., Oñate S. A., Nordeen S. K., Edwards D. P. Human progesterone receptor complexed with the antagonist RU 486 binds to hormone response elements in a structurally altered form. Mol Endocrinol. 1989 Oct;3(10):1545–1558. doi: 10.1210/mend-3-10-1545. [DOI] [PubMed] [Google Scholar]