Abstract
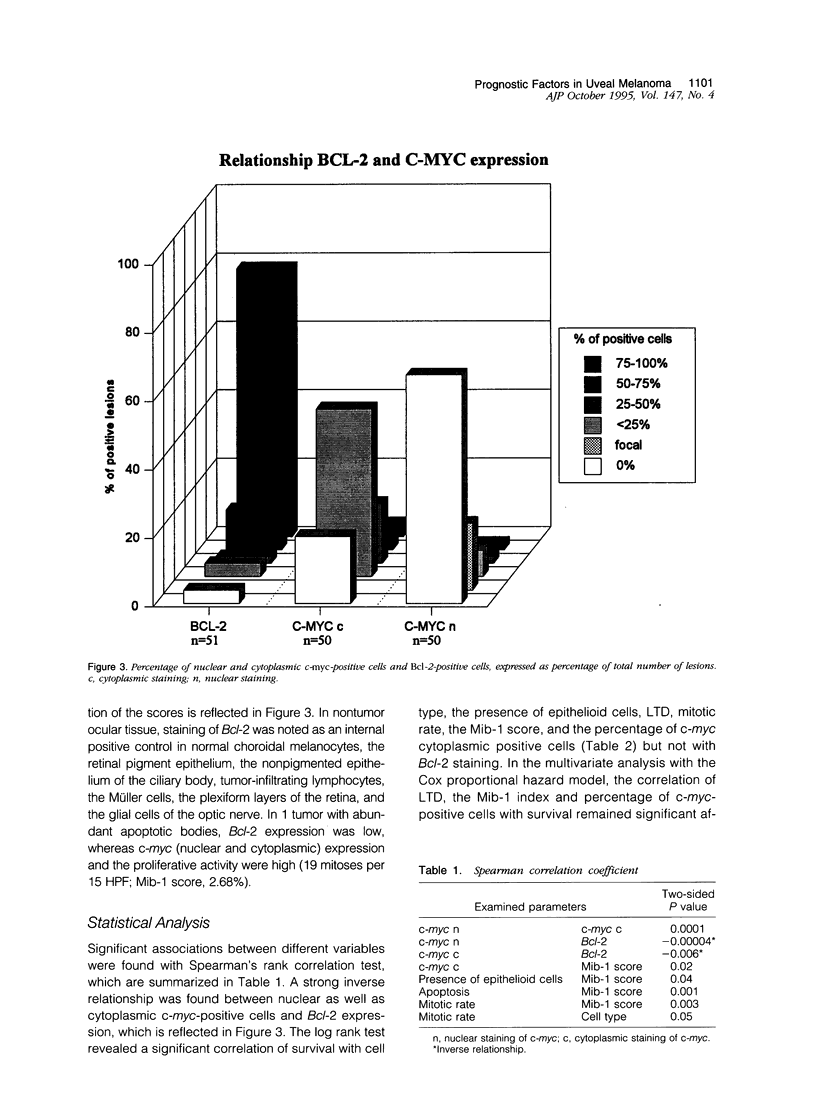
Neoplasia can be defined as deregulated tissue homeostasis caused by an imbalance between proliferation and apoptosis. Many genes are involved in the maintenance of tissue homeostasis, eg, the c-myc oncoprotein, which is an important regulator of cell proliferation and Bcl-2 protein, which is involved in the regulation of apoptosis. We studied retrospectively indices of proliferation, such as mitotic count and the Mib-1 index, on 51 uveal melanomas and compared their prognostic significance with established indicators of prognosis such as cell type and tumor size. Along the same line we investigated the expression of the regulating proteins c-myc and Bcl-2. Of all parameters tested, the largest tumor diameter and mitotic count were most strongly associated with tumor-related death (P < 0.001 and P = 0.005, respectively). In addition, cell type, the presence of epithelioid cells, the Mib-1 index, and the percentage of cytoplasmic c-myc-positive cells were significant predictive factors. Multivariate analysis showed that the Mib-1 index, largest tumor diameter, and the percentage of cytoplasmic c-myc-positive cells were independent prognostic parameters. Bcl-2 expression did not correlate with clinical outcome. The Mib-1 index correlated with the presence of epithelioid cells (P < 0.03) and the presence of apoptotic bodies (P < 0.001) and c-myc. A strong inverse relationship was found between (nuclear and cytoplasmic) c-myc and Bcl-2 (P < 0.00004 and P < 0.006, respectively), suggesting that Bcl-2 cooperates with c-myc to immortalize uveal melanoma cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arends M. J., Morris R. G., Wyllie A. H. Apoptosis. The role of the endonuclease. Am J Pathol. 1990 Mar;136(3):593–608. [PMC free article] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Bagg A., Cossman J. BCL-2: physiology and role in neoplasia. Cancer Treat Res. 1992;63:141–166. doi: 10.1007/978-1-4615-3088-6_7. [DOI] [PubMed] [Google Scholar]
- Bhargava V., Kell D. L., van de Rijn M., Warnke R. A. Bcl-2 immunoreactivity in breast carcinoma correlates with hormone receptor positivity. Am J Pathol. 1994 Sep;145(3):535–540. [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Bissonnette R. P., Echeverri F., Mahboubi A., Green D. R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992 Oct 8;359(6395):552–554. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Becker M. H., Key G., Duchrow M., Schlüter C., Galle J., Gerdes J. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol. 1992 Dec;168(4):357–363. doi: 10.1002/path.1711680404. [DOI] [PubMed] [Google Scholar]
- Colombel M., Symmans F., Gil S., O'Toole K. M., Chopin D., Benson M., Olsson C. A., Korsmeyer S., Buttyan R. Detection of the apoptosis-suppressing oncoprotein bc1-2 in hormone-refractory human prostate cancers. Am J Pathol. 1993 Aug;143(2):390–400. [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Lewis G. K., Ramsay G., Bishop J. M. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985 Dec;5(12):3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fanidi A., Harrington E. A., Evan G. I. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992 Oct 8;359(6395):554–556. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- Folberg R., Rummelt V., Parys-Van Ginderdeuren R., Hwang T., Woolson R. F., Pe'er J., Gruman L. M. The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology. 1993 Sep;100(9):1389–1398. doi: 10.1016/s0161-6420(93)31470-3. [DOI] [PubMed] [Google Scholar]
- Gazin C., Rigolet M., Briand J. P., Van Regenmortel M. H., Galibert F. Immunochemical detection of proteins related to the human c-myc exon 1. EMBO J. 1986 Sep;5(9):2241–2250. doi: 10.1002/j.1460-2075.1986.tb04491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A. Review: assessment of cell proliferation in histological material. J Clin Pathol. 1990 Mar;43(3):184–192. doi: 10.1136/jcp.43.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi-Ariga S. M., Itani T., Kiji Y., Ariga H. Possible function of the c-myc product: promotion of cellular DNA replication. EMBO J. 1987 Aug;6(8):2365–2371. doi: 10.1002/j.1460-2075.1987.tb02513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrun D. P., Warnke R. A., Cleary M. L. Expression of bcl-2 in fetal tissues suggests a role in morphogenesis. Am J Pathol. 1993 Mar;142(3):743–753. [PMC free article] [PubMed] [Google Scholar]
- Leoncini L., Del Vecchio M. T., Megha T., Barbini P., Galieni P., Pileri S., Sabattini E., Gherlinzoni F., Tosi P., Kraft R. Correlations between apoptotic and proliferative indices in malignant non-Hodgkin's lymphomas. Am J Pathol. 1993 Mar;142(3):755–763. [PMC free article] [PubMed] [Google Scholar]
- Lu Q. L., Elia G., Lucas S., Thomas J. A. Bcl-2 proto-oncogene expression in Epstein-Barr-virus-associated nasopharyngeal carcinoma. Int J Cancer. 1993 Jan 2;53(1):29–35. doi: 10.1002/ijc.2910530107. [DOI] [PubMed] [Google Scholar]
- Luyten G. P., Mooy C. M., De Jong P. T., Hoogeveen A. T., Luider T. M. A chicken embryo model to study the growth of human uveal melanoma. Biochem Biophys Res Commun. 1993 Apr 15;192(1):22–29. doi: 10.1006/bbrc.1993.1376. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Troncoso P., Brisbay S. M., Logothetis C., Chung L. W., Hsieh J. T., Tu S. M., Campbell M. L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992 Dec 15;52(24):6940–6944. [PubMed] [Google Scholar]
- McLean I. W., Foster W. D., Zimmerman L. E., Gamel J. W. Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983 Oct;96(4):502–509. doi: 10.1016/s0002-9394(14)77914-0. [DOI] [PubMed] [Google Scholar]
- Mooy C. M., de Jong P. T., Van der Kwast T. H., Mulder P. G., Jager M. J., Ruiter D. J. Ki-67 immunostaining in uveal melanoma. The effect of pre-enucleation radiotherapy. Ophthalmology. 1990 Oct;97(10):1275–1280. doi: 10.1016/s0161-6420(90)32420-x. [DOI] [PubMed] [Google Scholar]
- Pezzella F., Turley H., Kuzu I., Tungekar M. F., Dunnill M. S., Pierce C. B., Harris A., Gatter K. C., Mason D. Y. bcl-2 protein in non-small-cell lung carcinoma. N Engl J Med. 1993 Sep 2;329(10):690–694. doi: 10.1056/NEJM199309023291003. [DOI] [PubMed] [Google Scholar]
- Reed J. C., Meister L., Tanaka S., Cuddy M., Yum S., Geyer C., Pleasure D. Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991 Dec 15;51(24):6529–6538. [PubMed] [Google Scholar]
- Royds J. A., Sharrard R. M., Parsons M. A., Lawry J., Rees R., Cottam D., Wagner B., Rennie I. G. C-myc oncogene expression in ocular melanomas. Graefes Arch Clin Exp Ophthalmol. 1992;230(4):366–371. doi: 10.1007/BF00165947. [DOI] [PubMed] [Google Scholar]
- Sisley K., Rennie I. G., Cottam D. W., Potter A. M., Potter C. W., Rees R. C. Cytogenetic findings in six posterior uveal melanomas: involvement of chromosomes 3, 6, and 8. Genes Chromosomes Cancer. 1990 Sep;2(3):205–209. doi: 10.1002/gcc.2870020307. [DOI] [PubMed] [Google Scholar]
- Skouteris G. G., Michalopoulos G. K. Synthesis and phosphorylation of an extracellular polypeptide reacting with anti-MYC antisera in primary rat hepatocyte cultures. Biochem Biophys Res Commun. 1991 Oct 31;180(2):631–637. doi: 10.1016/s0006-291x(05)81112-x. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wagner A. J., Small M. B., Hay N. Myc-mediated apoptosis is blocked by ectopic expression of Bcl-2. Mol Cell Biol. 1993 Apr;13(4):2432–2440. doi: 10.1128/mcb.13.4.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitz W., Loidl P. Cell cycle dependent association of c-myc protein with the nuclear matrix. Oncogene. 1991 Jan;6(1):29–35. [PubMed] [Google Scholar]
- Williams G. T., Smith C. A. Molecular regulation of apoptosis: genetic controls on cell death. Cell. 1993 Sep 10;74(5):777–779. doi: 10.1016/0092-8674(93)90457-2. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br J Cancer. 1993 Feb;67(2):205–208. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oord J. J., Vandeghinste N., De Ley M., De Wolf-Peeters C. Bcl-2 expression in human melanocytes and melanocytic tumors. Am J Pathol. 1994 Aug;145(2):294–300. [PMC free article] [PubMed] [Google Scholar]