Abstract
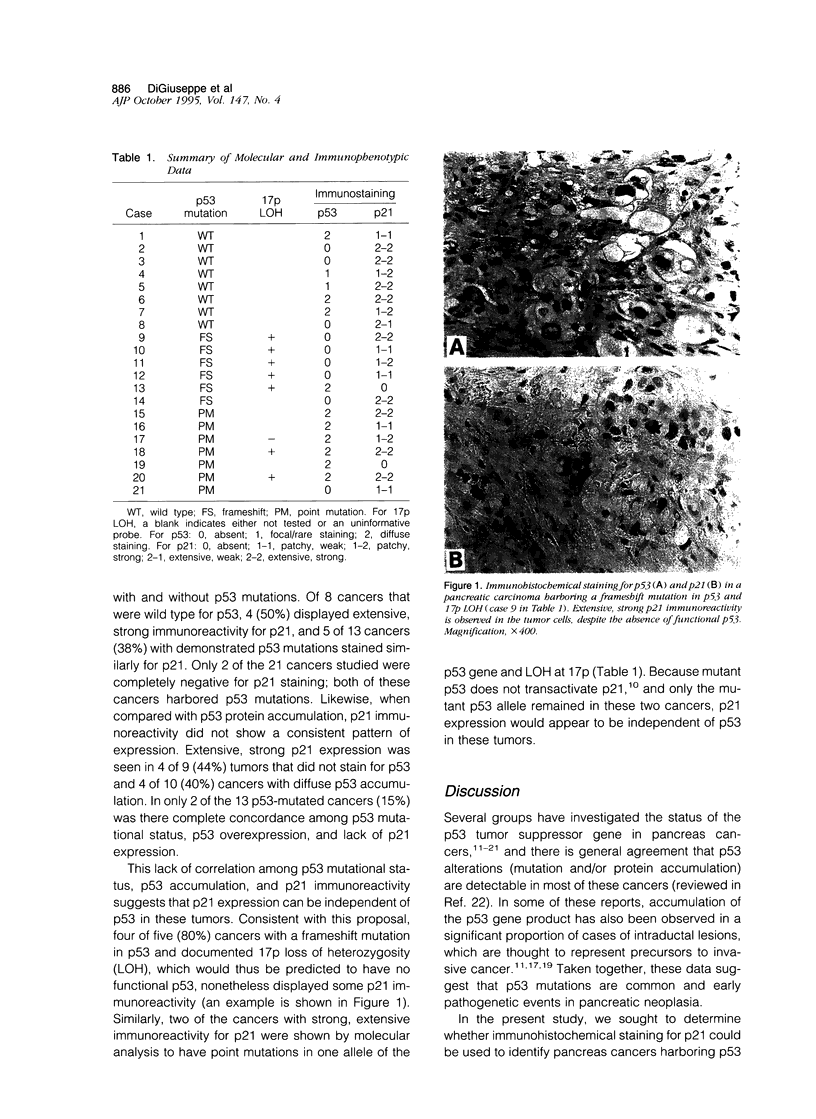
The p53 tumor suppressor gene is mutated in the majority of pancreatic adenocarcinomas, and several studies have suggested that loss of p53 function may contribute to the aggressive clinical behavior of pancreas cancer. Although immunocytochemical accumulation of the p53 gene product has previously been assessed as a marker for p53 mutations in cancers of the pancreas and other organ systems, the relationship between p53 mutations and p53 protein accumulation is variable. The cyclin-dependent kinase inhibitor, p21 (also known as WAF1 and CIP1), is induced by wild-type but not mutant p53, and recent work has implicated p21 as a downstream mediator of the growth-suppressing and apoptosis-promoting functions of wild-type p53. In the present work, we sought to determine whether loss of p21 expression could more precisely identify those tumors with p53 mutations and/or loss, compared with immunocytochemical assessment of p53 protein accumulation. We evaluated p53 and p21 expression immunohistochemically in a series of 21 ductal adenocarcinomas of the pancreas with known p53 mutational status. Diffuse overexpression of p53 was found in 3 of 8 cases (38%) with wild-type p53 and 7 of 13 cases (54%) with p53 mutations with or without loss of heterozygosity at 17p. Surprisingly, expression of p21 correlated neither with p53 mutational status nor with p53 protein expression. In particular, strong p21 expression was seen even in carcinomas in which molecular analysis revealed a frameshift mutation in one allele of p53 and loss of the second. These data suggest that p21 expression in pancreatic adenocarcinoma may also be induced by a p53-independent pathway and that p21 expression, as assessed immunocytochemically, does not reflect the functional status of p53 in these carcinomas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton C. M., Staddon S. L., Hughes C. M., Hall P. A., O'Sullivan C., Klöppel G., Theis B., Russell R. C., Neoptolemos J., Williamson R. C. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991 Dec;64(6):1076–1082. doi: 10.1038/bjc.1991.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey G., Yamanaka Y., Friess H., Kobrin M. S., Lopez M. E., Buchler M., Beger H. G., Korc M. p53 mutations are common in pancreatic cancer and are absent in chronic pancreatitis. Cancer Lett. 1993 May 14;69(3):151–160. doi: 10.1016/0304-3835(93)90168-9. [DOI] [PubMed] [Google Scholar]
- Ciechanover A., DiGiuseppe J. A., Bercovich B., Orian A., Richter J. D., Schwartz A. L., Brodeur G. M. Degradation of nuclear oncoproteins by the ubiquitin system in vitro. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):139–143. doi: 10.1073/pnas.88.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- DiGiuseppe J. A., Hruban R. H., Goodman S. N., Polak M., van den Berg F. M., Allison D. C., Cameron J. L., Offerhaus G. J. Overexpression of p53 protein in adenocarcinoma of the pancreas. Am J Clin Pathol. 1994 Jun;101(6):684–688. doi: 10.1093/ajcp/101.6.684. [DOI] [PubMed] [Google Scholar]
- Halevy O., Novitch B. G., Spicer D. B., Skapek S. X., Rhee J., Hannon G. J., Beach D., Lassar A. B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995 Feb 17;267(5200):1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Adami G. R., Wei N., Keyomarsi K., Elledge S. J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993 Nov 19;75(4):805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Kalthoff H., Schmiegel W., Roeder C., Kasche D., Schmidt A., Lauer G., Thiele H. G., Honold G., Pantel K., Riethmüller G. p53 and K-RAS alterations in pancreatic epithelial cell lesions. Oncogene. 1993 Feb;8(2):289–298. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Kuerbitz S. J., Plunkett B. S., Walsh W. V., Kastan M. B. Wild-type p53 is a cell cycle checkpoint determinant following irradiation. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7491–7495. doi: 10.1073/pnas.89.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. S., Rush M., Charalambous D., Rode J. Immunohistochemical demonstration of the p53 tumour suppressor gene product in cancer of the pancreas and chronic pancreatitis. J Gastroenterol Hepatol. 1993 Sep-Oct;8(5):465–469. doi: 10.1111/j.1440-1746.1993.tb01550.x. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Parker S. B., Eichele G., Zhang P., Rawls A., Sands A. T., Bradley A., Olson E. N., Harper J. W., Elledge S. J. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science. 1995 Feb 17;267(5200):1024–1027. doi: 10.1126/science.7863329. [DOI] [PubMed] [Google Scholar]
- Pellegata N. S., Sessa F., Renault B., Bonato M., Leone B. E., Solcia E., Ranzani G. N. K-ras and p53 gene mutations in pancreatic cancer: ductal and nonductal tumors progress through different genetic lesions. Cancer Res. 1994 Mar 15;54(6):1556–1560. [PubMed] [Google Scholar]
- Redston M. S., Caldas C., Seymour A. B., Hruban R. H., da Costa L., Yeo C. J., Kern S. E. p53 mutations in pancreatic carcinoma and evidence of common involvement of homocopolymer tracts in DNA microdeletions. Cancer Res. 1994 Jun 1;54(11):3025–3033. [PubMed] [Google Scholar]
- Ruggeri B., Zhang S. Y., Caamano J., DiRado M., Flynn S. D., Klein-Szanto A. J. Human pancreatic carcinomas and cell lines reveal frequent and multiple alterations in the p53 and Rb-1 tumor-suppressor genes. Oncogene. 1992 Aug;7(8):1503–1511. [PubMed] [Google Scholar]
- Scarpa A., Capelli P., Mukai K., Zamboni G., Oda T., Iacono C., Hirohashi S. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993 May;142(5):1534–1543. [PMC free article] [PubMed] [Google Scholar]
- Scheffner M., Huibregtse J. M., Vierstra R. D., Howley P. M. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993 Nov 5;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- Schwaller J., Koeffler H. P., Niklaus G., Loetscher P., Nagel S., Fey M. F., Tobler A. Posttranscriptional stabilization underlies p53-independent induction of p21WAF1/CIP1/SDI1 in differentiating human leukemic cells. J Clin Invest. 1995 Mar;95(3):973–979. doi: 10.1172/JCI117806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohara M., el-Deiry W. S., Wada M., Nakamaki T., Takeuchi S., Yang R., Chen D. L., Vogelstein B., Koeffler H. P. Absence of WAF1 mutations in a variety of human malignancies. Blood. 1994 Dec 1;84(11):3781–3784. [PubMed] [Google Scholar]
- Simon B., Weinel R., Höhne M., Watz J., Schmidt J., Körtner G., Arnold R. Frequent alterations of the tumor suppressor genes p53 and DCC in human pancreatic carcinoma. Gastroenterology. 1994 Jun;106(6):1645–1651. doi: 10.1016/0016-5085(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Zhang S. Y., Ruggeri B., Agarwal P., Sorling A. F., Obara T., Ura H., Namiki M., Klein-Szanto A. J. Immunohistochemical analysis of p53 expression in human pancreatic carcinomas. Arch Pathol Lab Med. 1994 Feb;118(2):150–154. [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]