Abstract
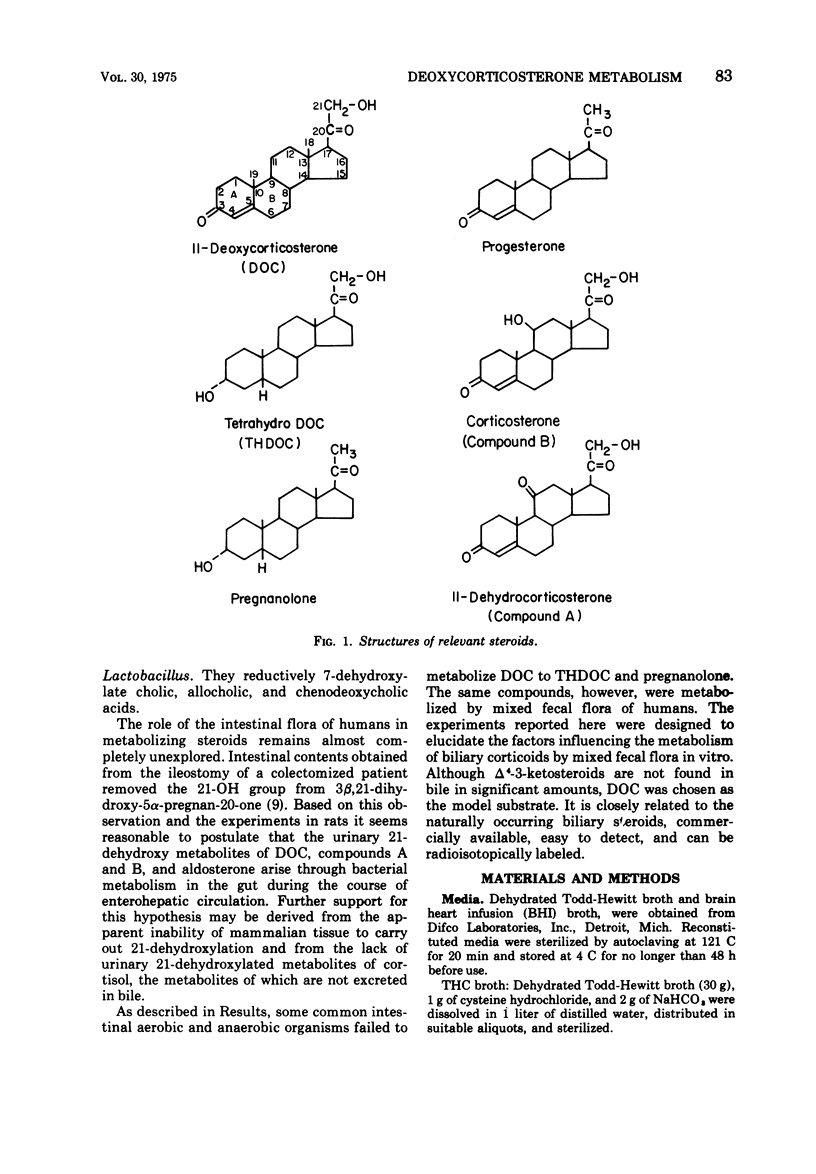
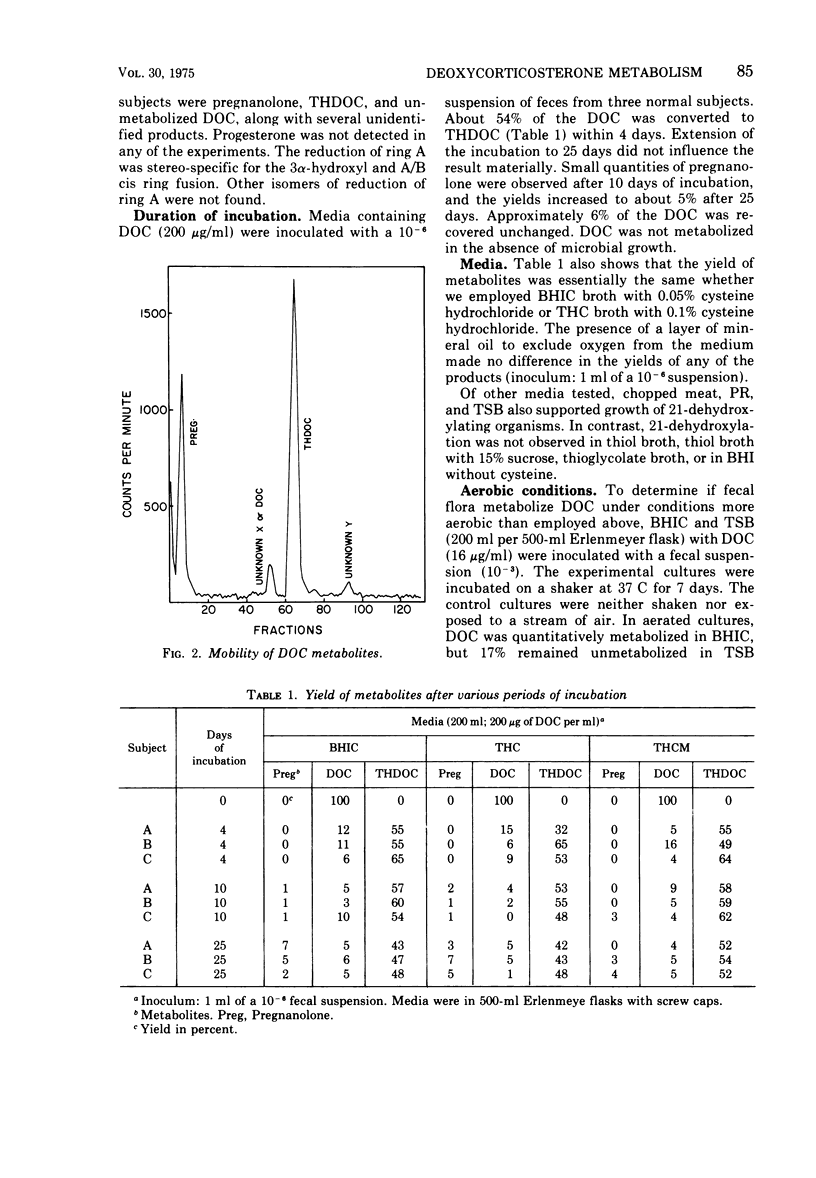
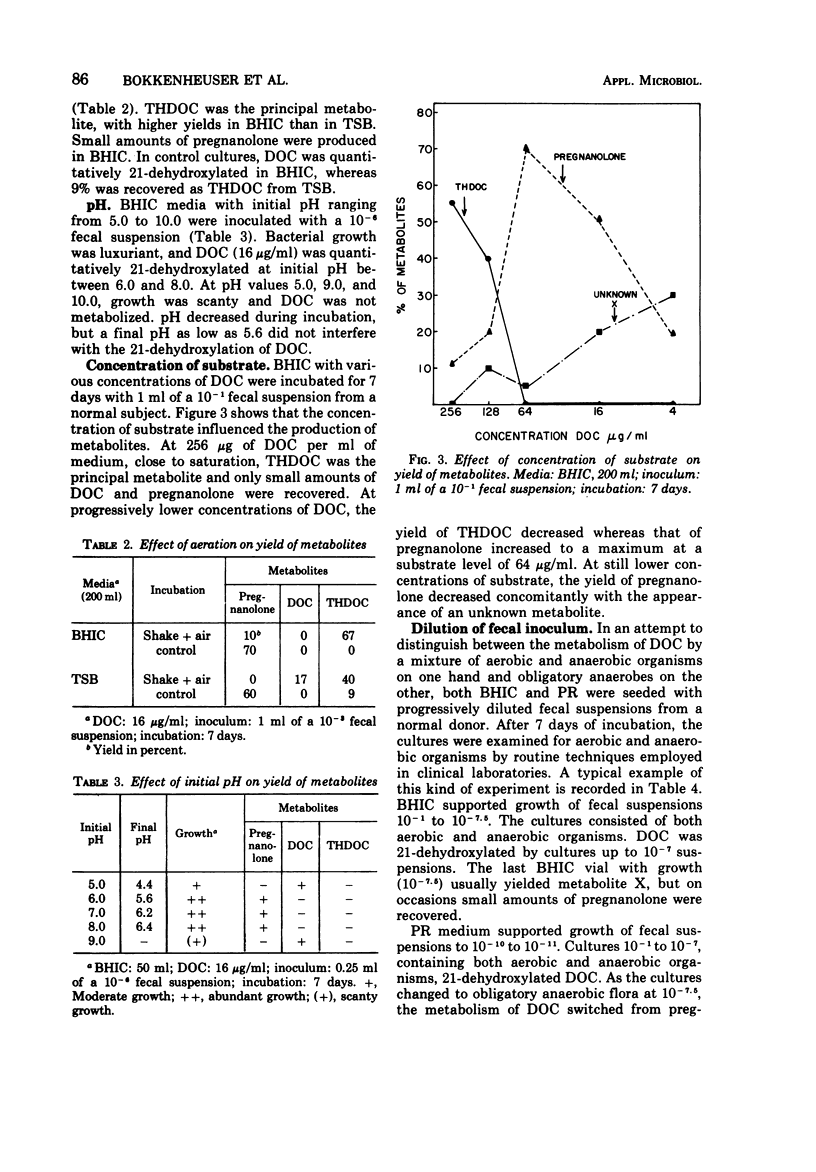
21-Dehydroxylation, a feature of metabolism of corticoids in humans, was observed in mixed cultures of fecal flora of normal individuals on a Western diet. The model substrate, 11-deoxycorticosterone (DOC), was metabolized to 3α-21-dihydroxy-5β-pregnan-20-one (THDOC), 3α-hydroxy-5β-pregnan-20-one (pregnanolone), and to two unidentified structures, metabolites X and Y. DOC was not metabolized in all media supporting growth of fecal flora. Conversion required an initial pH between 6.0 and 8.0. 21-Dehydroxylation occurred within 4 days of incubation in media inoculated with 10-1 to 10-7 fecal suspensions. In higher dilutions, containing obligatory anaerobes only, DOC was converted to metabolite X and sometimes also to metabolite Y. The yield of pregnanolone was related to the promptness with which the specimen was processed, to the presence of cysteine in the medium, and to the concentration of substrate (optimum, 16 to 64 μg of DOC per ml). The yield of THDOC was related to the delay in the processing of the specimen, the concentration of substrate (maximum at 256 μg/ml), and aeration of the culture. Pure cultures of aerobic organisms of fecal origin either failed to metabolize DOC or converted it to metabolite Y. Pure cultures of fecal anaerobes converted DOC to metabolite X and sometimes also to metabolite Y. Neither THDOC nor pregnanolone was produced by pure cultures.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADLOW H. L., FRAZELL E. L., GALLAGHER T. F., HELLMAN L. Tracer studies of the absorption and fate of steroid hormones in man. J Clin Invest. 1956 Sep;35(9):1033–1044. doi: 10.1172/JCI103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Eriksson H., Gustafsson J. A. Microbial formation of 17-alpha-C21 steroids. Stereochemistry of saturation of the delta-16-double bond. Eur J Biochem. 1971 Jun 11;20(3):340–343. doi: 10.1111/j.1432-1033.1971.tb01399.x. [DOI] [PubMed] [Google Scholar]
- Bokkenheuser V., Hoshita T., Mosbach E. H. Bacterial 7-dehydroxylation of cholic acid and allocholic acid. J Lipid Res. 1969 Jul;10(4):421–426. [PubMed] [Google Scholar]
- Campagnoli C., De Martinis C., Doglio R. Dimostrazione della conversione in vivo del desossicorticosterone in pregnandiolo. Folia Endocrinol. 1965 Oct;18(5):550–555. [PubMed] [Google Scholar]
- DECKER H. A., MIGEON C. J., PAUL A. C., SAMUELS L. T., SANDBERG A. A., SMITH D. F. Metabolism of 4-C 14-cortisol in man: body distribution and rates of conjugation. J Clin Endocrinol Metab. 1956 Sep;16(9):1137–1150. doi: 10.1210/jcem-16-9-1137b. [DOI] [PubMed] [Google Scholar]
- ENGEL L. L., CARTER P., FIELDING L. L. Urinary metabolites of administered corticosterone. I. Steroids liberated by glucuronidase hydrolysis. J Biol Chem. 1955 Mar;213(1):99–106. [PubMed] [Google Scholar]
- Eriksson H., Gustafsson J. A. Excretion of steroid hormones in adults. Steroids in faeces from adults. Eur J Biochem. 1971 Jan 1;18(1):146–150. doi: 10.1111/j.1432-1033.1971.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Gustafsson J. A., Sjövall J. Steroids in germfree and conventional rats. 21-dehydroxylation by intestinal microorganisms. Eur J Biochem. 1969 Jul;9(4):550–554. doi: 10.1111/j.1432-1033.1969.tb00644.x. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Gustafsson J. A., Sjövall J. Steroids in germfree and conventional rats. 4. Identification and bacterial formation of 17 alpha-pregnane derivatives. Eur J Biochem. 1968 Nov;6(2):219–226. doi: 10.1111/j.1432-1033.1968.tb00441.x. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Gustafsson J. A., Sjövall J. Studies on the structure, biosynthesis and bacterial metabolism of 15-hydroxylated steroids in the female rat. Eur J Biochem. 1971 Apr;19(3):433–441. doi: 10.1111/j.1432-1033.1971.tb01333.x. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Gustafsson J. A. Steroids in germfree and conventional rats. Distribution and excretion of labelled pregnenolone and corticosterone in male and female rats. Eur J Biochem. 1970 Jul;15(1):132–139. doi: 10.1111/j.1432-1033.1970.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Eriksson H., Gustafsson J. A. Steroids in germfree and conventional rats. Steroids in the mono- and disulphate fractions of faeces from female rats. Eur J Biochem. 1970 Oct;16(2):252–260. doi: 10.1111/j.1432-1033.1970.tb01079.x. [DOI] [PubMed] [Google Scholar]
- FUKUSHIMA D. K., GALLAGER T. F. Absence of 21-dehydroxylation in congenital adrenal hyperplasia. J Clin Endocrinol Metab. 1958 Jul;18(7):694–698. doi: 10.1210/jcem-18-7-694. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Sjövall J. Steroids in germfree and conventional rats. 5. Identification of C19 steroids in faeces from germfree rats. Eur J Biochem. 1968 Nov;6(2):227–235. doi: 10.1111/j.1432-1033.1968.tb00442.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. A., Sjövall J. Steroids in germfree and conventional rats. 6. Identification of 15 alpha- and 21-hydroxylated C21 steroids in faeces from germfree rats. Eur J Biochem. 1968 Nov;6(2):236–247. doi: 10.1111/j.1432-1033.1968.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Harris J. J., Hoegel C., Crane M. G. Deoxycorticosterone secretion rates before and during metyrapone administration in normal subjects. J Clin Endocrinol Metab. 1967 Jan;27(1):105–113. doi: 10.1210/jcem-27-1-106. [DOI] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S., Hawksworth G., Aries V., Crowther J. S., Williams R. E. Bacteria and aetiology of cancer of large bowel. Lancet. 1971 Jan 16;1(7690):95–100. doi: 10.1016/s0140-6736(71)90837-3. [DOI] [PubMed] [Google Scholar]
- KELLY W. G., BANDI L., LIEBERMAN S. ISOLATION AND CHARACTERIZATION OF HUMAN URINARY METABOLITES OF ALDOSTERONE. IV. THE SYNTHESIS AND STEREOCHEMISTRY OF TWO BICYCLIC ACETAL METABOLITES. Biochemistry. 1963 Nov-Dec;2:1243–1249. doi: 10.1021/bi00906a012. [DOI] [PubMed] [Google Scholar]
- KELLY W. G., BANDI L., SHOOLERY J. N., LIEBERMAN S. Isolation and characterization of aldosterone metabolites from human urine; two metabolites bearing a bicyclic acetal structure. Biochemistry. 1962 Jan;1:172–181. doi: 10.1021/bi00907a026. [DOI] [PubMed] [Google Scholar]
- Kelyy W. G. Questions concerning the validity of one of the assumptions underlying the determination of the secretory rate of cortisol. Steroids. 1970 Nov;16(5):579–602. doi: 10.1016/s0039-128x(70)80139-8. [DOI] [PubMed] [Google Scholar]
- Laatikainen T. Excretion of neutral steroid hormones in human bile. Ann Clin Res. 1970;2(Suppl):1–28. [PubMed] [Google Scholar]
- Laatikainen T., Vihko R. Unconjugated C19 and C21 steroids in human faeces. Ann Clin Res. 1970 Dec;2(4):350–353. [PubMed] [Google Scholar]
- MIGEON C. J., PAUL A. C., SAMUELS L. T., SANDBERG A. A. Metabolism of 4-C14-corticosterone in man. J Clin Endocrinol Metab. 1956 Oct;16(10):1291–1298. doi: 10.1210/jcem-16-10-1291. [DOI] [PubMed] [Google Scholar]
- Midtvedt T., Norman A. Parameters in 7-alpha-dehydroxylation of bile acids by anaerobic lactobacilli. Acta Pathol Microbiol Scand. 1968;72(2):313–329. doi: 10.1111/j.1699-0463.1968.tb01345.x. [DOI] [PubMed] [Google Scholar]


