Abstract
The priming of tumor-antigen-specific T cells is critical for the initiation of successful anti-tumor immune responses, yet the fate of such cells during tumor progression is unknown. Naive CD4+ T cells specific for an antigen expressed by tumor cells were transferred into tumor-bearing mice. Transient clonal expansion occurred early after transfer, accompanied by phenotypic changes associated with antigen recognition. Nevertheless, these cells had a diminished response to peptide antigen in vitro and were unable to be primed in vivo. The development of antigen-specific T cell anergy is an early event in the tumor-bearing host, and it suggests that tolerance to tumor antigens may impose a significant barrier to therapeutic vaccination.
Significant progress has been made in the identification of antigens expressed by tumor cells that are recognized by the T cell arm of the immune system (1). These discoveries have led to the development of tumor-antigen-specific vaccine strategies, a number of which are currently undergoing clinical evaluation as therapy for patients with metastatic cancer. Although the existence of T cells having specificity for antigens preferentially expressed by cancer cells is a prerequisite for the generation of anti-tumor immune responses, little is known about the fate of such cells during tumor progression. As most cancer vaccine strategies are currently being examined as therapy for an existing tumor burden rather than as prophylaxis, the consequences of antigen-specific T cell interaction with tumor is a critical parameter likely to impact on the efficacy of this therapeutic approach.
In most murine models of tumor vaccines, a far greater tumor burden can be rejected when non-tumor-bearing animals are immunized first, followed some time later by the tumor challenge, than when immunization takes place after the establishment of the tumor. In the latter setting, extending the interval from tumor challenge to vaccination by even a few days often results in a profound decrease in the efficacy of the observed anti-tumor immune response. The discordant anti-tumor immune responses generated in tumor-free versus tumor-bearing vaccine recipients has been explained by such diverse factors as the rapid kinetics of tumor growth in mouse models, the generation of tumor-induced suppressor T cells (2, 3), alterations in T cell signal transduction in the tumor-bearing host (4–9), tumor induction of T cell apoptosis (10), and the development of peripheral tolerance to tumor antigens (11).
We wished to examine the fate of T cells that recognize an antigen expressed by tumor cells during tumor progression. We have designed a system in which an identifiable population of naive CD4+ T cells of a defined specificity is monitored in vivo during the progression of a tumor that has been engineered to express its antigen. This system graphically demonstrates that antigen-specific T cells undergo significant changes in phenotype and function shortly after exposure to nominal antigen in the tumor-bearing host, leading to a state of antigen-specific unresponsiveness. These changes occur early during the course of tumor progression and significantly precede the onset of the more generalized immunosuppression that frequently accompanies advanced tumor burdens. These results suggest that T cell anergy to tumor antigens may impose a significant barrier to therapeutic tumor vaccine strategies.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old male BALB/c mice were obtained from the National Institutes of Health (Frederick, Maryland). T cell antigen receptor (TCR) transgenic mice expressing an αβ TCR specific for peptide 110–120 from influenza hemagglutinin (HA) presented by I-Ed (12) were a generous gift of Harald von Boehmer. These mice were crossed to a BALB/c background for more than 10 generations. Transgenic mice used in these experiments were heterozygous for the transgene. All experiments involving the use of mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
Tumor Cells.
A20 cells were obtained from the American Type Culture Collection (ATCC) (Rockville, MD). Cells were cultured in vitro in RPMI 1640 medium, supplemented with 10% fetal calf serum (FCS), penicillin (50 units/ml), streptomycin (50 μg/ml), l-glutamine (2 mM), and 2-mercaptoethanol (50 mM) (complete medium), and grown as a suspension culture at 37°C in a 5% CO2 atmosphere. A20HAneo was selected and grown in complete medium supplemented with the neomycin analogue G418 (400 μg/ml). Electroporation of A20 cells was used for plasmid transfection in the creation of A20HA as previously reported (13).
Adoptive Transfer.
Single-cell suspensions were made from peripheral lymph nodes and spleen that were harvested from TCR-transgenic donors. The percentage of lymphocytes doubly positive for CD4 and the clonotypic TCR was determined by flow cytometry as described below. Cells were washed three times in sterile Hanks’ balanced salt solution (HBSS) and injected into the tail vein of male BALB/c recipients such that a total of 2.5 × 106 CD4+ anti-HA TCR+ T cells were transferred to each recipient. A20 or A20HA cells used for in vivo tumor challenge were washed three times in sterile HBSS and injected via the tail vein in a total volume of 0.5 ml, 1 × 106 tumor cells per mouse. Tumor-free survival was determined by twice weekly inspection, and mice were euthanized after the development of a tumor, which was evident as increasing abdominal girth and palpable abdominal mass. All euthanized animals had the presence of tumors confirmed at autopsy (hepatic and splenic nodules and mesenteric nodal enlargement).
Flow Cytometric Analysis.
A20 cells were stained with one of the following: (i) rat anti-mouse CD80 (PharMingen), (ii) monoclonal antibody (mAb) GL1 (rat anti-mouse CD86), or (iii) rat anti-clonotypic TCR mAb 6.5 (as an irrelevant primary antibody control), followed by phycoerythrin (PE)-conjugated goat anti-rat IgG (Caltag, South San Francisco, CA). A total of 10,000 gated events were collected on a FACScan (Becton Dickinson) and analyzed by using CellQuest software (Becton Dickinson). Analysis of splenocytes was performed after depleting lymphoma cells and enriching for T cells by passage over nylon wool followed by complement lysis with the mAb J11.d.2, which is specific for heat stable antigen (HSA) expressed by A20 cells. Purified T cells were stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse CD4 (Caltag) and biotinylated rat anti-clonotypic TCR mAb 6.5 followed by PE-conjugated streptavidin (Caltag). A total of 100,000 gated events were collected per sample. Values represent the mean ± SE of the percentage of cells expressing the clonotypic TCR for four mice. Background staining was less than 0.05%. Expression of activation markers on clonotype-positive cells was determined by three-color flow cytometric analysis of splenocytes isolated as above. T cells were stained with Cy-Chrome-labeled anti-mouse CD4 (PharMingen), biotinylated anti-TCR clonotype mAb 6.5 followed by PE-labeled streptavidin, and FITC-conjugated anti-mouse CD44 (PharMingen), anti-mouse CD45RB (PharMingen), or anti-mouse CD62L (PharMingen). Live gating on CD4+ T cells was set, and 100,000 events were collected per sample. Mean fluorescence intensity ± SE is shown for each activation marker expressed by CD4+ TCR clonotype+ T cells (four mice per group).
Antigen-Specific Proliferation.
Purified T cells (4 × 104 per well) from the experimental groups were mixed with fresh splenocytes (8 × 104 per well) from naive BALB/c mice to which HA peptide (12.5 μg/ml) was added. The assay was pulsed with [3H]thymidine (1 μCi per well; Amersham; 1 μCi = 37 kBq) after 3 days in culture. Cells were harvested 18 hr later with a Packard Micromate cell harvester. Thymidine incorporation into DNA was measured as counts per minute (cpm) on a Packard Matrix96 direct β counter. Data are displayed as cpm from which values for medium alone were subtracted. Values represent the mean (± SE) cpm/absolute number of clonotypic T cells per well from four mice in each group.
Cytokine Release.
T cells purified and plated as above were cultured with media alone or HA peptide (12.5 μg/ml) plus fresh BALB/c splenocytes. Forty-eight hours later, supernatants were collected and assayed for interleukin (IL)-2, IL-4, and γ-interferon by ELISA (R & D Systems). Values are the mean ± SE of triplicate cultures from four animals in each group. Values for T cells cultured in media alone were less than 10% of the values for stimulated T cells.
In Vivo Priming with vacc-HA.
A recombinant vaccinia virus encoding HA from the 1934 PR8 strain of influenza virus was a generous gift of Frank Guarnieri (Johns Hopkins University). vacc-HA was expanded on Hu-TK− cells in the presence of 5-bromo-2′-deoxyuridine (Sigma), at 25 μg/ml. Virus was purified from the cellular lysate by sucrose banding and titered by plaque assay on BSC-1 cells. Mice were primed by inoculation with 1 × 107 plaque-forming units (pfu) of recombinant virus in the hind footpad in 50 μl.
Proliferative Response to Vaccinia Antigens.
Normal BALB/c splenocytes were infected with wild-type vaccinia virus (3 pfu/cell) for 6 hr. Infected cells were washed three times and then cultured with purified T cells from the experimental groups at a stimulator/responder ratio of 2:1. [3H]Thymidine incorporation was determined after 3 days in culture. Values represent mean ± SE of triplicate cultures.
RESULTS
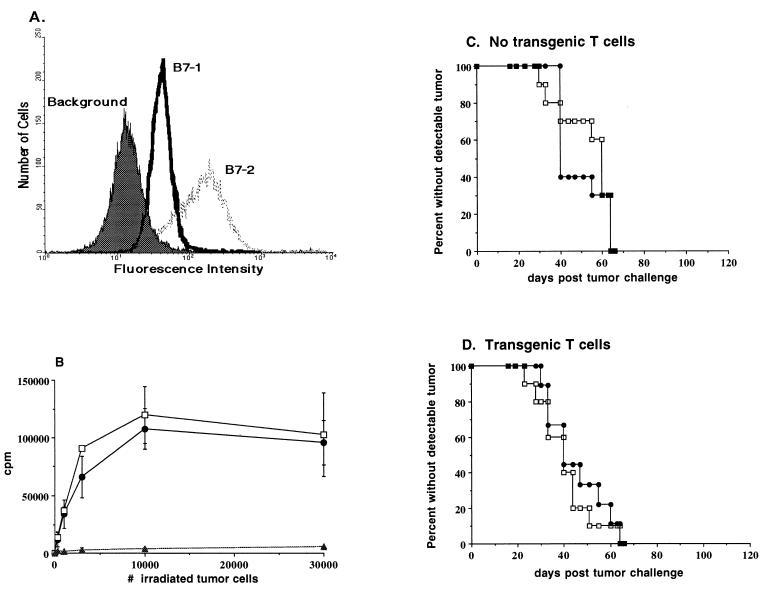
A20 is a BALB/c lymphoma expressing high levels of major histocompatibility complex (MHC) class I and II molecules as well as constitutively low levels of the T cell costimulatory molecules CD80 (B7–1) and CD86 (B7–2) (Fig. 1A). It is capable of presenting both exogenous and endogenous antigen and has been used extensively for in vitro studies of antigen processing (14). When injected i.v. into BALB/c mice, this tumor infiltrates the mesenteric lymph nodes, spleen, and liver, and it can be found in the bone marrow and peripheral blood. It therefore behaves similarly in vivo to many forms of human B cell lymphoma. As with the models described above, a modest systemic tumor burden can be rejected when immunization [with irradiated granulocyte/macrophage colony-stimulating factor (GM-CSF)-secreting A20 cells] occurs within 5 days of tumor challenge, but by 9 days, vaccination is without effect (13).
Figure 1.
Characteristics of A20 wild type and A20HA in vitro, and kinetics of tumor growth in vivo. (A) Flow cytometric analysis of CD80 (B7–1) and CD86 (B7–2) expression on A20 cells. (B) Recognition of A20HA by anti-HA/I-Ed TCR transgenic T cells in vitro. A20HA was explanted from tumor-bearing mice, and a single-cell suspension was made by mechanical dissociation and passage through nylon mesh. Explanted A20HA cells (•), A20HA cells passaged in vitro (□), or A20 wild-type cells passaged in vitro (▴) were irradiated with 10,000 centigrays and plated at the indicated cell number in 96-well microtiter plates together with 2 × 105 freshly isolated anti-HA/I-Ed TCR transgenic splenocytes per well. Incorporation of [3H]thymidine was determined after three days in culture. (C) BALB/c mice were injected intravenously (i.v.) with 1 × 106 A20WT (□) or A20HA (•) tumor cells on day zero and were inspected twice weekly for the development of tumors. Ten mice were included in each group. (D) Nine days after i.v. injection with 1 × 106 A20WT or A20HA tumor cells, BALB/c mice received 2.5 × 106 CD4+ TCR transgenic T cells specific for HA/I-Ed. Mice were inspected as above and euthanized when tumors were evident.
Using adoptively transferred T cells from a TCR-transgenic mouse (15), we followed the immune response to a model antigen, influenza virus HA, expressed either by A20 cells or in the context of a viral infection with recombinant vaccinia. A20 cells were transfected to express HA, and a stable transfectant was selected that expresses very low levels of HA. Although the level of HA expression by A20HA was below the limits of detection by flow cytometry, CD4+ T cells from TCR-transgenic mice specific for HA amino acids 110–120 restricted by I-Ed (12) proliferated vigorously when incubated with A20HA in vitro (Fig. 1B). Nonetheless, expression of HA did not measurably alter the immunogenicity of A20HA, as i.v. injection of BALB/c mice with either A20 wild type or A20HA resulted in tumor progression with similar kinetics (Fig. 1C). Interestingly, the kinetics of tumor growth was not affected by the adoptive transfer of anti-HA/I-Ed-transgenic T cells (Fig. 1D) given 9 days after tumor challenge. Nine days was chosen on the basis of our previous observation that A20 could not be cured by therapeutic vaccination after this interval. Subsequent experiments in which the transfer of transgenic T cells occurred prior to, or at the same time as, tumor cells gave similar results (data not shown). Outgrowth of A20HA was not accompanied by the loss of HA expression, as A20HA cells obtained from tumor explants stimulated anti-HA/I-Ed-transgenic T cells identically to A20HA passaged in vitro (Fig. 1B).
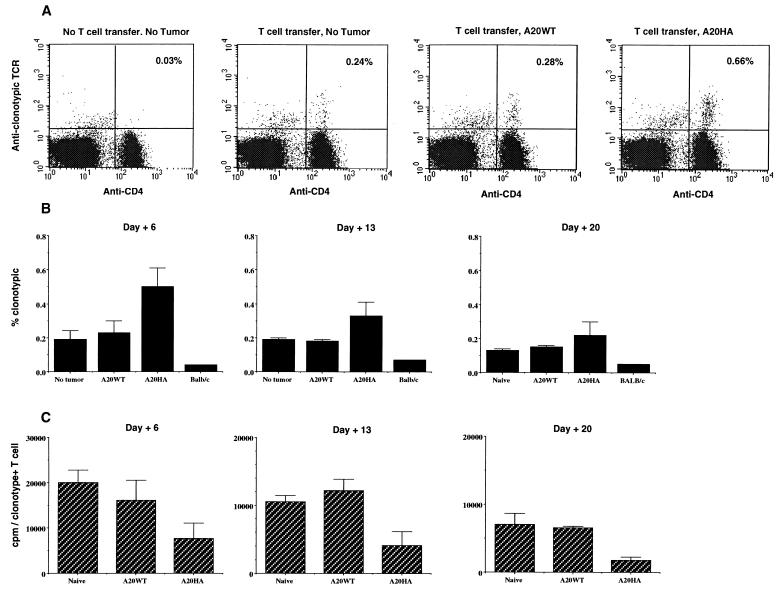
Anti-HA/I-Ed transgenic T cells were adoptively transferred into tumor-free BALB/c mice, or mice with established A20 wild type or A20HA tumors, and the percentage of CD4+ splenocytes expressing the clonotypic TCR was determined at weekly intervals after T cell transfer. Analysis of the clonotype frequency 6 days after T cell transfer revealed an initial expansion of clonotype-positive T cells in A20HA bearing mice relative to mice bearing A20 wild type or no tumor (Fig. 2A). After the first week, this percentage declined, and by 20 days after T cell transfer (when 3- to 5-mm lymphoma nodules were visible in the liver and spleen), the percentage of clonotype-positive cells in A20HA-bearing mice approached the baseline level present in the other groups (Fig. 2B). Although the percentage of clonotype-positive T cells in mice bearing A20HA declined after an initial expansion, their complete elimination was never observed, even at later time points in the face of an extensive tumor burden.
Figure 2.
Changes in clonotype-positive T cells after transfer into tumor-bearing mice. BALB/c mice were injected (i.v.) with 1 × 106 A20WT or A20HA tumor cells. Nine days later all mice, including a group not challenged with tumor, received 2.5 × 106 anti-HA/I-Ed TCR+ transgenic T cells i.v. Four mice per group were sacrificed on days +6, +13, and +20 after the adoptive transfer of T cells. Flow cytometric analysis was performed on purified splenic T cells stained with FITC-conjugated goat anti-mouse CD4 and biotinylated rat anti-clonotypic TCR antibody (mAb 6.5) followed by PE-conjugated streptavidin. For each sample 100,000 gated events were collected. (A) Representative two-color FACS analysis of splenocytes obtained 6 days after T cell transfer. (B) Change in the percentage of CD4+ anti-HA TCR+ T cells over time after T cell transfer. Values represent the mean ± SE of the percentage of cells expressing the clonotypic TCR for four mice. Background staining was less than 0.05%. (C) In vitro proliferative response of clonotype+ CD4+ T cells to stimulation with HA 110–120 peptide. Purified T cells (4 × 104 per well) from the mice in A were mixed with fresh splenocytes (8 × 104 per well) from naive BALB/c mice to which HA peptide (12.5 μg/ml) was added. 3H incorporation was assayed after 3 days of incubation and is shown as cpm from which values for medium alone were subtracted. Values represent the mean (±SE) cpm/absolute number of clonotypic T cells per well from four mice in each group.
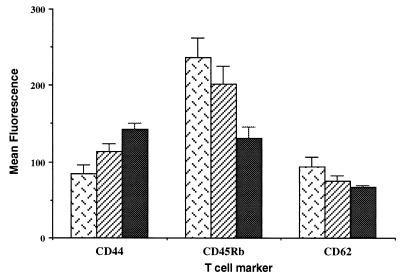
Three-color flow cytometric analysis of clonotype-positive CD4+ T cells was performed to address whether phenotypic changes associated with antigen recognition occur in A20HA-bearing mice (Fig. 3). An increase in the expression of CD44 and decreased expression of CD45RB and CD62L was observed on HA-specific transgenic T cells in A20HA-bearing mice relative to non-tumor-bearing mice, although intermediate changes were seen in some mice bearing A20 wild-type tumors as well.
Figure 3.
Phenotypic changes associated with antigen recognition on CD4+ TCR clonotype+ T cells after adoptive transfer into tumor-bearing mice. BALB/c mice were injected (i.v.) with 1 × 106 A20WT or A20HA tumor cells. Nine days later all mice received 2.5 × 106 anti-HA/I-Ed TCR+ transgenic T cells i.v. Fifteen days after transfer, T cells from non-tumor-bearing mice (patterned bars), mice bearing A20WT (hatched bars), or A20HA (solid bars) were isolated as in Fig. 2 and stained with Cy-Chrome-labeled anti-mouse CD4, biotinylated anti-TCR clonotype mAb 6.5 followed by PE-labeled streptavidin and FITC-conjugated anti-mouse CD44, anti-mouse CD45RB, or anti-mouse CD62L. Live gating on CD4+ T cells was set, and 100,000 events were collected per sample. Mean fluorescence intensity + SE is shown for each activation marker expressed by CD4+TCR clonotype+ T cells (four mice per group).
In spite of the initial expansion of HA-specific CD4+ T cells in A20HA-bearing mice and loss of the naive phenotype, T cells from this group had a diminished proliferative response to HA peptide in vitro (Fig. 2C). This blunted response in A20HA-bearing mice was evident as early as 6 days after the transfer of HA-specific T cells, and it remained impaired for the duration of the experiment. In contrast, transgenic T cells from mice with a comparable burden of A20 wild-type tumor responded equivalently to non-tumor-bearing mice for at least 22 days after T cell transfer, even in the presence of macroscopic tumor nodules infiltrating the spleen and liver at this late time point.
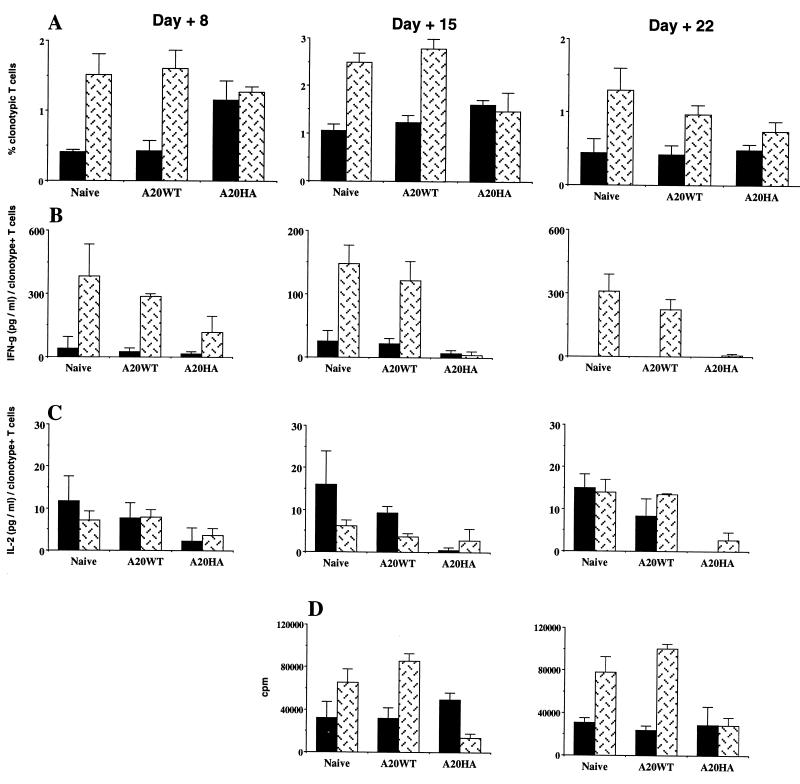
In contrast to the decreased responsiveness of HA-specific T cells when the antigen is expressed by A20HA cells, exposure to HA in the context of a viral infection enhanced T cell responsiveness. A recombinant vaccinia virus construct encoding influenza virus HA (vacc-HA) was used to immunize mice at varied intervals after T cell transfer (Fig. 4). Immunization resulted in a significant expansion of the clonotype-positive T cells in non-tumor-bearing mice (Fig. 4A). The response to vacc-HA generated in mice bearing A20 wild-type tumor was equivalent to that seen in non-tumor-bearing mice, even when immunization occurred late in the course of tumor progression. As was seen before, unimmunized A20HA-bearing mice had an increased percentage of clonotype-positive T cells 8 days after T cell transfer, followed at later time points by a decline in this percentage toward the baseline levels seen in the other groups. Strikingly, immunization of A20HA-bearing mice with vacc-HA failed to result in clonal expansion of HA-specific T cells. This impaired response to vacc-HA was not due to early clearance of the recombinant virus by circulating anti-HA antibodies, because such antibodies were undetectable in the serum of A20HA-bearing mice (data not shown).
Figure 4.
HA-specific T cells fail to respond to in vivo priming in mice bearing A20HA but not A20WT tumors. BALB/c mice were given 1 × 106 A20WT or A20HA tumor cells i.v. Nine days later, all mice, including a group not challenged with tumor, received 2.5 × 106 anti-HA/I-Ed TCR+ transgenic T cells. Half the mice in each group were immunized with 1 × 107 pfu of vacc-HA subcutaneously (s.c.) on day 2, 9, or 16 after T cell transfer and were sacrificed for analysis six days after immunization (days 8, 15, or 22). Unimmunized controls received 0.1 ml of HBSS s.c. (A) Purified T cells from unimmunized (solid bars) and vacc-HA-immunized animals (patterned bars) were analyzed by two-color flow cytometry staining for CD4 versus anti-HA TCR clonotype as in Fig. 2. Values represent mean + SE of percentage of T cells expressing the clonotypic TCR. T cells were cultured with media alone or HA peptide (12.5 μg/ml) plus fresh BALB/c splenocytes. Forty-eight hours later, supernatants were collected and assayed for γ-interferon (B) and IL-2 (C) by ELISA (R & D Systems). Values are the mean + SE of triplicate cultures from four animals in each group. Data are expressed as the amount of cytokine produced per 100 clonotype-positive cells. Values for T cells cultured in media alone were less than 10% of the values for stimulated T cells. (D) HA-specific proliferative response of clonotype-positive T cells from immunized versus unimmunized animals. Values represent the mean proliferation of T cells from which values for medium alone were subtracted. Four mice per group were analyzed.
T cells primed with vacc-HA in vivo released γ-interferon on in vitro culture with HA peptide (Fig. 4B). This response was preserved in mice bearing wild-type A20 but was significantly impaired in mice bearing A20HA, even when immunization with vacc-HA occurred as early as 2 days after T cell transfer and was assayed 6 days later. No detectable IL-4 release was observed in any group (data not shown). Although incubation with HA peptide resulted in measurable IL-2 release even in the absence of in vivo priming with vacc-HA, this response was also blunted in the A20HA-bearing mice (Fig. 4C). Finally, the proliferative response to HA peptide was enhanced by vacc-HA priming in non-tumor-bearing mice as well as those bearing A20 wild-type tumor, but not in mice with A20HA (Fig. 4D). Although the overall proliferative response of unvaccinated A20HA-bearing mice was equivalent to unvaccinated non-tumor-bearing or A20 wild-type-bearing mice, on a per cell basis, this response was again diminished, as seen in Fig. 2C (data not shown).
We wished to confirm further that the T cell unresponsiveness induced by A20HA was specific for HA and did not simply reflect tumor-induced global immunosuppression. Non-tumor-bearing mice or mice with A20HA were primed with vacc-HA, and splenocytes were assayed for proliferation in response to either HA peptide or vaccinia antigens expressed by BALB/c splenocytes that were infected with wild-type vaccinia in vitro (Table 1). While the T cell proliferative response to HA peptide was again diminished in A20HA bearing mice, these same mice had a proliferative response to vaccinia antigens that was equivalent to non-tumor-bearing mice primed with vacc-HA. Furthermore, the response to the pan-T cell mitogen Con A was similar in the two groups, suggesting that other elements of the T cell repertoire were functionally intact. Interestingly, the proliferative response of transgenic T cells from A20HA-bearing mice to HA peptide was partially restored in the presence of exogenous IL-2, reminiscent of the findings observed in in vitro models of T cell anergy (16).
Table 1.
Response of T cells from vaccHA-primed mice
| In vitro stimulation | [3H]Thymidine incorporation, cpm
|
|
|---|---|---|
| Normal mice | A20HA-bearing mice | |
| None | 3,026 ± 635 | 3,268 ± 1,203 |
| HA peptide* | 44,864 ± 9,168 | 11,155 ± 3,746 |
| Vaccinia-infected splenocytes† | 16,133 ± 2,307 | 17,359 ± 3,532 |
| Con A‡ | 72,511 ± 10,204 | 59,115 ± 15,786 |
| HA + IL-2§ | 64,114 ± 10,891 | 41,800 ± 4,177 |
BALB/c mice were given 1 × 106 A20HA tumor cells intravenously or received no tumor. Nine days later, all mice received 2.5 × 106 anti-HA/I-Ed TCR+ transgenic T cells. Nine days after T cell transfer, all mice were immunized with vacc-HA as in Fig. 4. On day +22 after T cell transfer, the mice were sacrificed and T cells were purified as before. Purified cells (4 × 104 per well) were added to BALB/c splenocytes (8 × 104 per well) and cultured with media alone or with the indicated stimulation. Con A stimulation was done without the addition of BALB/c splenocytes. [3H]Thymidine incorporation was determined after 3 days in culture. Values represent mean ± SE of triplicate cultures.
HA peptide was 12.5 μg/ml.
Normal BALB/c splenocytes were infected with vaccinia virus (3 pfu per cell) for 6 hr. Infected cells were washed three times and then cultured with purified T cells at different stimulator/responder ratios. Values represent proliferation at a ratio of 2:1.
Con A was 50 μg/ml.
HA peptide at 12.5 μg/ml + IL-2 at 20 units/ml.
DISCUSSION
These findings demonstrate that induction of antigen-specific T cell unresponsiveness can occur early in the course of tumor–T cell interaction and significantly precede the development of a more generalized state of immunosuppression. A number of studies have supported the hypothesis that tumors evade immunologic rejection by inducing a state of global immunosuppression. This state has been clearly demonstrated in animals or patients harboring advanced tumor burdens and is characterized by hyporesponsiveness to challenge with common recall antigens in vivo, and diminished T cell function in vitro that correlates with specific alterations in the T cell signal transduction pathways (4–9). The factor or factors that mediate these changes have yet to be identified, but the alterations are found in a large percentage of the T cell pool, affecting a wide range of antigen specificities. While this form of immunosuppression is likely to have significant impact on vaccine efficacy in the setting of advanced malignancies, it is not clear that these changes can account for the early events that limit the generation of anti-tumor immunity in response to vaccination.
An alternate explanation for the impaired vaccine responses seen in the setting of an established tumor burden is that tumor-antigen-specific T cells become tolerized upon encountering antigen in vivo. Full activation of resting T cells not only requires an antigen-specific signal provided by engagement of the TCR with the appropriate peptide/MHC complexes but also requires a second “costimulatory” signal delivered by specialized antigen-presenting cells (APCs). One source of this second signal is engagement of CD28 on the T cell by B7–1 (CD80) and B7–2 (CD86) expressed by activated APCs. T CR engagement in the absence of a costimulatory signal results in T cells that fail to develop full effector function and are rendered “anergic,” even if both signals are provided in a subsequent encounter with antigen (17). This requirement for T cell costimulation is thought to maintain tolerance to normal self-antigens expressed in tissues that cannot deliver the second signal. Because most tumor cells are poor APCs that are incapable of expressing costimulatory molecules (but usually express MHC class I molecules and can be induced to express MHC class II), the above paradigm predicts that tumor-specific T cells would be rendered anergic upon encountering tumor antigen on the MHC molecules of the cancer cell. In support of this hypothesis, several studies have demonstrated an enhanced ability to prime T cell-mediated anti-tumor immunity through vaccination with tumor cells transfected to express B7–1 (18–20).
The above reasoning, however, does not completely account for the failed immune responses against cancers that are derived from APCs (21). Tumors such as B cell lymphomas express high levels of MHC class I and class II molecules and have inducible expression of B7–1, B7–2, and intercellular adhesion molecule 1 (ICAM-1). Furthermore, lymphoma cell lines have been shown to be capable of processing and presenting antigen to T cells in vitro, leading to T cell activation (22–24). In spite of these features, lymphoma is often a highly aggressive cancer that progresses in the very compartment where primary T cell responses are normally generated.
One explanation for these findings is that the level of B7–1 and/or B7–2 expressed by lymphoma cells may favor the high-affinity interaction with CTLA4 over the lower-affinity interaction with CD28 on activated T cells (25, 26). Engagement of CTLA4 is thought to exert a counter-regulatory inhibition of T cell activation, with CTLA4 expression peaking 24–48 hr after T cell activation. The observation that T cell unresponsiveness in our model was accompanied by an initial clonal expansion and the loss of a naive phenotype by the transgenic T cells is consistent with this hypothesis. Indeed, in other systems, in vivo blockade of CTLA4 engagement has been shown to prevent the induction of anergy to peptide antigen (27), to exacerbate autoimmune reactions (28, 29), and to enhance anti-tumor immune responses (30).
Alternatively, additional costimulatory signals besides CD28 engagement may be necessary to initiate an activated immune response in vivo. The inability of B cell tumors to provide these undefined signals may reflect the general ability of nontransformed B cells to activate versus tolerize naive T cells in vivo (31–34). It is interesting to note that similar changes in the phenotype and function of antigen-specific T cells were seen in a model of peripheral tolerance in which the self antigen was under the regulation of an Igκ promoter and enhancer, and was therefore largely expressed by B cells (35).
It is unclear whether the development of antigen-specific CD4+ T cell anergy is a unique feature of MHC class II-positive tumors such as B cell lymphoma. Although we have shown that A20HA can be recognized by purified HA-specific transgenic CD4+ T cells in vitro (13), the changes in T cell function observed in vivo may have resulted either from the direct encounter with HA peptide presented by MHC class II+ lymphoma cells or from uptake and presentation of antigen by host APCs. If the latter mechanism is operative, this form of tumor tolerance may be seen with nonhematopoietic cancers as well. Of note, in a model of plasmacytoma, which is a B cell lineage tumor unable to express MHC class II antigens, CD4+ T cell tolerance to the tumor Ig idiotype protein was demonstrated (36). In that setting, however, tolerance was largely mediated by clonal deletion of idiotype-specific T cells, which was induced in a dose-dependent fashion by the tumor idiotype protein that was abundantly secreted in the serum. In the present model, we failed to detect circulating antigen in the serum, even from mice with an extensive tumor burden.
The identification of antigen-specific T cell anergy as an early event in tumor progression has clear implications for the continued development of cancer immunotherapy. The persistence of this state after a reduction in tumor burden with other treatment modalities remains to be determined, but in their current form, such strategies are likely to be most effective in the treatment of minimal residual disease. The identification of the molecular basis for tumor-induced T cell anergy and of strategies to restore T cell responsiveness, will be critical to the ultimate success of active anti-tumor immunotherapy.
Acknowledgments
We thank Wendy Pavlat and Mojgan Ahmadzadeh for expert technical assistance and Percy Smith for animal care and breeding. This work was supported by a grant from the American Cancer Society (ACS IM-73551). H.L. is a Scholar of the Leukemia Society of America.
ABBREVIATIONS
- TCR
T cell antigen receptor
- HA
hemagglutinin
- PE
phycoerythrin
- FITC
fluorescein isothiocyanate
- IL
interleukin
- pfu
plaque-forming units
- MHC
major histocompatibility complex
- APCs
antigen-presenting cells
References
- 1.Boon T, van der Bruggen P. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.North R J. J Exp Med. 1982;55:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awwad M, North R J. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- 4.Mizoguchi H, O’Shea J J, Longo D L, Loeffler C M, McVicar D W, Ochoa A C. Science. 1992;258:1795–1798. doi: 10.1126/science.1465616. [DOI] [PubMed] [Google Scholar]
- 5.Finke J H, Zea A H, Stanley J, Longo D L, Mizoguchi H, Tubbs R R, Wiltrout R H, O’Shea J J, Kudoh S, Klein E, Bukowski R M, Ochoa A C. Cancer Res. 1993;53:5613–5616. [PubMed] [Google Scholar]
- 6.Nakagomi H, Petersson M, Magnusson I, Juhlin C, Matsuda M, Mellstedt H, Taupin J L, Vivier E, Anderson P, Kiessling R. Cancer Res. 1993;53:5610–5612. [PubMed] [Google Scholar]
- 7.Tartour E, Latour S, Mathiot C, Thiounn N, Mosseri V, Joyeux I, D’Enghien C D, Lee R, Debre B, Fridman W H. Int J Cancer. 1995;63:205–212. doi: 10.1002/ijc.2910630210. [DOI] [PubMed] [Google Scholar]
- 8.Renner C, Ohnesorge S, Held G, Bauer S, Jung W, Pfitzenmeier J P, Pfreundschuh M. Blood. 1996;88:236–241. [PubMed] [Google Scholar]
- 9.Alexander J P, Kudoh S, Melsop K A, Hamilton T A, Edinger M G, Tubbs R R, Sica D, Tuason L, Klein E, Bukowski R M, Finke J H. Cancer Res. 1993;53:1380–1387. [PubMed] [Google Scholar]
- 10.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J-C, Tschopp J. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 11.Ye X, McCarrick J, Jewett L, Knowles B B. Proc Natl Acad Sci USA. 1994;91:3916–3920. doi: 10.1073/pnas.91.9.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitsky H I, Montgomery J, Ahmadzadeh M, Staveley-O’Carroll K, Guarnieri F, Longo D L, Kwak L W. J Immunol. 1996;156:3858–3865. [PubMed] [Google Scholar]
- 14.Glimcher L H, Kim K J, Green I, Paul W E. J Exp Med. 1982;155:445–459. doi: 10.1084/jem.155.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins M J, Pardoll D M, Mizuguchi J, Chused T M, Schwartz R H. Proc Natl Acad Sci USA. 1987;84:5409–5413. doi: 10.1073/pnas.84.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz R H. Cell. 1992;71:1065–1068. doi: 10.1016/s0092-8674(05)80055-8. [DOI] [PubMed] [Google Scholar]
- 18.Townsend S E, Allison J P. Science. 1993;259:368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Ashe S, Brady W A, Hellstrom I, Hellstrom K E, Ledbetter J A, McGowan P, Linsley P S. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 20.Baskar S, Ostrand-Rosenberg S, Nabavi N, Nadler L M, Freeman G J, Glimcher L H. Proc Natl Acad Sci USA. 1993;90:5687–5690. doi: 10.1073/pnas.90.12.5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levitsky H I. Sem Immunol. 1996;8:281–287. doi: 10.1006/smim.1996.0036. [DOI] [PubMed] [Google Scholar]
- 22.Ashwell J D, DeFranco A L, Paul W E, Schwartz R H. J Exp Med. 1984;159:881–905. doi: 10.1084/jem.159.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzavecchia A. Nature (London) 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 24.Schultze J L, Cardoso A A, Freeman G J, Seamon M J, Daley J, Pinkus G S, Gribben J G, Nadler L M. Proc Natl Acad Sci USA. 1995;92:8200–8204. doi: 10.1073/pnas.92.18.8200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison J P, Krummel M F. Science. 1995;270:932–933. doi: 10.1126/science.270.5238.932. [DOI] [PubMed] [Google Scholar]
- 26.June C H, Bluestone J A, Nadler L M, Thompson C B. Immunol Today. 1994;15:321–331. doi: 10.1016/0167-5699(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 27.Perez V L, Van Parijs L, Biuckians A, Zheng X X, Strom T B, Abbas A K. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 28.Karandikar N J, Vanderlugt C L, Walunas T L, Miller S D, Bluestone J A. J Exp Med. 1996;184:783–788. doi: 10.1084/jem.184.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perrin P J, Maldonado J H, Davis T A, June C H, Racke M K. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 30.Leach D R, Krummel M F, Allison J P. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 31.Ronchese F, Hausmann B. J Exp Med. 1993;177:679–690. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lassila O, Vainio O, Matzinger P. Nature (London) 1988;334:253–255. doi: 10.1038/334253a0. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs E J, Matzinger P. Science. 1992;258:1156–1159. doi: 10.1126/science.1439825. [DOI] [PubMed] [Google Scholar]
- 34.Eynon E E, Parker D C. J Exp Med. 1992;175:131–138. doi: 10.1084/jem.175.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanoue A, Bona C, von Boehmer H, Sarukhan A. J Exp Med. 1997;185:405–414. doi: 10.1084/jem.185.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogen B. Eur J Immunol. 1996;26:2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]