Abstract
The history of maize (Zea mays L.) is one of the most debated topics in New World archaeology. Molecular and genetic studies indicate that maize domestication took place in tropical southwest Mexico. Although archaeological evidence for the evolution of maize from its wild ancestor teosinte has yet to be found in that poorly studied region, other research combining paleoecology and archaeology is documenting the nature and timing of maize domestication and dispersals. Here we report a phytolith analysis of sediments from San Andrés, Tabasco, that confirms the spread of maize cultivation to the tropical Mexican Gulf Coast >7,000 years ago (≈7,300 calendar years before present). We review the different methods used in sampling, identifying, and dating fossil maize remains and compare their strengths and weaknesses. Finally, we examine how San Andrés amplifies the present evidence for widespread maize dispersals into Central and South America. Multiple data sets from many sites indicate that maize was brought under cultivation and domesticated and had spread rapidly out of its domestication cradle in tropical southwest Mexico by the eighth millennium before the present.
Keywords: phytoliths, pollen, paleoecology, radiocarbon
Genetic and molecular evidence indicates that maize is descended from an annual species of Mexican teosinte (Zea mays subsp. parviglumis Iltis and Doebley) native to the tropical Balsas River Valley on the Pacific slopes of the states of Michoacán and Guerrero (1–3). The natural vegetation of much of this region is a deciduous tropical forest receiving between 1,100 and 1,600 mm of precipitation annually, largely as summer monsoonal rain from May to November. Genetic and molecular data also provide estimated chronologies for maize domestication. Analysis of a large number of microsatellites from the genomes of maize and teosinte suggests that domestication occurred by 9,100 calendar years before the present (cal BP) (2). Studies of individual domestication genes in modern Zea that control crucial phenotypic attributes in maize, such as the production of naked grains, similarly suggest an early Holocene time frame for the fixation of the alleles in question under artificial selection (e.g., ref. 4).
Despite advances in molecular research, archaeobotanical and other forms of paleobotanical research are needed to determine the age of maize domestication conclusively. Available macrobotanical data recovered from dry highland caves in Mexico are congruent with molecular data in indicating considerable pre-6,200 cal BP [5,400 radiocarbon years before the present (RYBP)] human selection of various maize alleles (e.g., refs. 5 and 6). Nevertheless, archaeological evidence from the crucial pre-7,000 cal BP time period is limited.
Results
San Andrés Phytolith and Pollen Records.
Our pollen data from San Andrés (7) indicated that Zea was present in coastal Tabasco by 7,100 cal BP (6,200 RYBP) (Fig. 1). Z. mays subsp. parviglumis is absent from the Gulf of Mexico watershed today and is unlikely to have been present during the time period covered by our fossil sequences, given that it is rare anywhere now below an elevation of 400 m. We deduced that the Zea at San Andrés was cultivated because it was outside its natural habitat and appeared abruptly with other indicators of land clearance. Because pollen grains of teosinte and maize can overlap in attributes such as size and axis to pore ratios (8), our original work at San Andrés failed to rule out the possibility that the cultivated Zea was teosinte. The pre-6,000 cal BP (5,200 RYBP) Zea pollen from San Andrés falls mostly in the 45- to 58-μm-diameter size range, with two grains in the 74- to 76-μm-diameter size range (7). Under Nomarski phase, these smaller grains exhibited distinctive columella characteristic of Zea grains, and they have thicker walls than the larger post-6,000 cal BP grains. The presence of distinctive intertectile columella rules out all non-Zea wild grasses. The post-6,000 cal BP Zea pollen is larger, reaching 92-μm diameter, and all possess the distinctive Zea features as with the earlier grains.
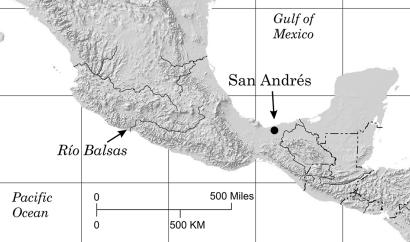
Fig. 1.
Location of San Andrés and the Río Balsas watershed in Mexico.
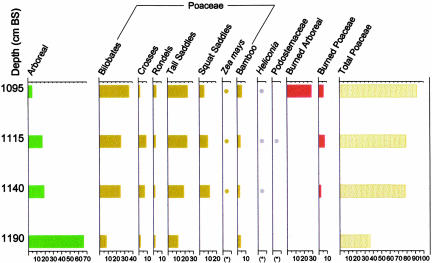
To advance our understanding of the early cultivated Zea at San Andrés and its impact on vegetational history, D.R.P. carried out targeted phytolith analysis on four sediment samples from between 1,190 and 1,095 cm beneath the surface of San Andrés Vibracore (SAV)-4 (Fig. 2). This range of depths encompasses the pre-Zea pollen and predisturbance horizons of the sequence (at 1,190 cm below surface) and includes samples from just below (1,140 cm), within (1,115–1,120 cm), and immediately above (1,095–1,100 cm) the zones that contained the earliest Zea pollen. Phytolith content was high in all of the samples studied.
Fig. 2.
Phytolith diagram showing the percentages through time of major taxa identified at San Andrés. Dots indicate that phytoliths are present as 1% of the sum. Percentages of burned phytoliths were computed independently of the other classes in the phytolith sum.
Data for distinguishing phytoliths of maize from teosinte and non-Zea wild grasses, produced by ourselves and other phytolith analysts working throughout the Americas, indicate the following: (i) the fruitcases of teosinte and the cupules and glumes of maize cobs form recognizable phytolith assemblages that can be distinguished from each other, (ii) phytoliths from the fruitcases of Tripsacum spp. are diagnostic to the genus and separable from those of teosinte and maize, (iii) teosinte fruitcase and maize cob phytoliths can be distinguished from those produced by the vegetative organs of Zea and reproductive and vegetative structures of non-Zea grasses, and (iv) certain types of phytoliths originating in glumes and cupules of Zea ears are diagnostic of either teosinte or maize (e.g., refs. 9–17). Distinguishing phytoliths from teosinte and maize is based on a number of distinctive morphological attributes, not simply size as is the case with pollen [see supporting information (SI) Figs. 4–6]. Benz' assertion (18) that it is easier to distinguish teosinte and maize by using pollen rather than phytoliths is incorrect.
Another important factor is that phytolith formation and morphology in Zea fruits are largely under the control of the crucial locus teosinte glume architecture 1 (tga1), a genetic locus that regulates the development of the hard cupulate fruitcase that surrounds teosinte grains and the degree of glume induration (lignification) in wild and domesticated Zea (10, 19) (for more information on the diagnostic characteristics of phytoliths from maize and teosinte and how they are underwritten by the tga1 genetic locus, see SI Text). Studies of size and morphological attributes of other types of phytoliths, called “cross-shaped,” that are produced in grass leaves indicate they can be used effectively to distinguish maize from non-Zea wild grasses and many types of teosinte (10, 13, 20).
The San Andrés phytolith analysis confirms maize presence and appears to rule out contribution from teosinte. In the SAV-4 core, wavy top rondels and ruffle top rondels from maize cobs occurred in three of the four samples analyzed: 1,095–1,100, 1,115–1,120, and 1,140 cm (Fig. 3 a–c). Maize phytoliths were lacking in the deepest sample (1,190 cm), a finding consistent with our original pollen study (7). Rondels as a whole constituted significant proportions of the phytolith assemblages from the samples where maize was identified, and many of the rondel types are those that commonly occur in maize but not teosinte (e.g., “spooled” kinds and rondels with undecorated tops and bottoms). These other types of rondels (those that are not wavy tops or ruffle tops) cannot be unequivocally assigned to maize, because they occur in a few non-Zea grasses, but they are not typical of teosinte, which makes different kinds of rondel phytolith assemblages (see SI Text). Also significant is the fact that the kinds of long epidermal cell phytoliths that are always present in significant numbers in teosinte but are largely absent from modern maize varieties were absent in the SAV-4 samples (some of these phytoliths are called “IRP, short rectangular, elongate WCE, and oblong and one-half decorated;” ref. 10, p. 62). Furthermore, phytoliths from Tripsacum are absent. We cannot suggest which or how many varieties of maize were responsible for creating the phytolith record, but the rondel phytolith assemblages are compatible with those found in modern races, indicating that a substantial degree of divergence from teosinte had taken place by the time maize arrived at San Andrés. The absence of Zea phytoliths that would indicate influence from teosinte alleles at the tga1 locus confirms this picture. The data are congruent with recent molecular and genetic work on the timing of divergence of various maize domestication genes including tga1 (2, 4).
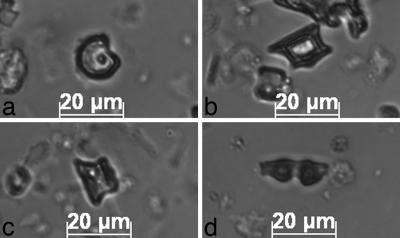
Fig. 3.
Phytoliths from San Andrés. (a–c) Maize cob phytoliths. (a) A ruffle top rondel from 1,115 cm. (b) A wavy top rondel from 1,115 cm. (c) A ruffle top rondel from 1,140 cm. (d) A burned Heliconia phytolith from 1,115 cm.
Cross-shaped phytoliths derived from grass vegetative decay occurred frequently in the SAV-4 samples, and in the samples containing maize cob phytoliths, their 3D morphologies were like those of maize, not teosinte (the fewer cross-shaped phytoliths occurring at 1,190 cm had 3D morphologies like those of bamboos). Their average sizes (12 μm and less in width) were too small to be classified as maize, however. The high input of phytoliths from field weeds belonging to wild genera of the Panicoid subfamily of grasses (below) likely masked the presence of maize vegetative decay.
Evidence for a significant degree of burning and forest clearing indicative of agriculture was present in the three samples that contain maize (Fig. 2). In the premaize sample from 1,190 cm, phytolith data indicate that an undisturbed to minimally disturbed tropical forest grew near the coring locale. Phytoliths from a variety of arboreal taxa and understory herbs characteristic of older forest (e.g., Chrysobalanaceae, Fabaceae, and Marantaceae) dominate the profile. Many of the grass phytoliths are from bamboos (e.g., tall saddles, cross-shaped phytoliths diagnostic of bamboos), which probably derived from the forest understory or small natural tree fall gaps. A single burned phytolith was observed of the total of thousands scanned on the slides; this finding correlates with the charcoal curve generated for SAV-4, which showed presence of few charcoal particles at that time (7).
A significant change in the phytolith record at 1,140 cm indicates the appearance of maize agriculturalists. The frequency of arboreal taxa declines greatly, and grasses begin to dominate the record. “Squat” saddle-shaped phytoliths are present for the first time. They are predominantly from the Chloridoid subfamily of grasses, which are typically weeds of agricultural fields in the lowland Neotropics. Bilobate and cross-shaped phytoliths from the common weed subfamily Panicoideae occur in high numbers; a few are burned. The assemblage from 1,140 cm shows close similarities with modern examples constructed from slash-and-burn fields containing maize and manioc. This 1,140-cm assemblage is unlike any constructed from modern tropical forest experiencing little or no human interference (10, 21, 22). In the samples from 1,115–1,120 and 1,095–1,100 cm, signs of agricultural activity involving forest clearing and burning continue and intensify. The archetypal Neotropical weed Heliconia (Fig. 3d) is present for the first time, and every phytolith of this taxon that was observed was burned. Both the frequencies of grass phytoliths and the proportions that are burned increase, and at 1,095–1,100 cm, there is a very high number of burned arboreal phytoliths.
We assigned ages to the phytolith samples based on the four lowest-most radiocarbon dates from the SAV-4 core (4,513 ± 45 RYBP at 837 cm; 5,805 ± 49 RYBP at 993 cm; 5,517 ± 51 RYBP at 1,020 cm; and 6,208 ± 47 RYBP at 1,125 cm) (7). These dates were calibrated to calendar ages by using the Calib (rev 5.0.1) program of Stuiver and Reimer (23), and the weighted averages of the 2-σ ranges (averages = 5,230, 6,611, 6,328, and 7,123 cal BP) were used in a linear regression with depth (r = 0.97) to assign ages. These ages differ only slightly (<100 yr) from our earlier analysis of the San Andrés cores (7), largely because of the use of weighted averages instead of intercept dates in the regression analysis (The former is now known to be the preferred method; ref. 24). The results indicate that the premaize sample at 1,190 cm dates to 7,700 cal BP (6,800 RYBP), the earliest maize phytoliths at 1,140 cm to 7,300 cal BP (6,300 RYBP), and the other maize phytolith samples at 1,115–1,120 and 1,095–1,100 cm date to 7,200 and 7,100 cal BP (6,200 RYBP) respectively (all ages rounded to nearest 100 years). Given the errors inherent in radiocarbon dating and the uncertainties in our regression model, a conservative estimate of the age uncertainty for the earliest maize in SAV-4 is ±100–200 years.
These new San Andrés phytolith data confirm and expand on our previous research at San Andrés. They demonstrate that maize cultivators were active in the area by ≈7,300 cal BP, before the pollen and charcoal data mark a dramatic expansion of maize cultivation at 7,100 cal BP (6,200 RYBP) (7). The phytolith data demonstrate that the introduced cultigen was maize rather than teosinte. Differences in pollen size (early pollen 74–66 μm, post-6,000 cal BP pollen up to 92 μm) may suggest changes in cob size or the introduction of different maize varieties over time (25), an issue that needs further investigation through studies of pollen size and cob characteristics in modern plants. Analysis of core sediments indicates that the early cultivators were on a barrier island between a coastal lagoon and the sea, where they could take advantage of both aquatic resources and cultivated plots.
Discussion
Methodological Issues in Studying the Prehistory of Maize.
Sluyter and Dominguez (26) have questioned the antiquity of maize pollen discovered in our previous Tabasco study based on a ≈5,000 cal BP maize date on pollen from a core they analyzed from the coastal plain in the neighboring state of Veracruz. They concluded that the earliest firm evidence for maize is the macrofossil data from Guilá Naquitz dated to 6,200 cal BP (5,400 RYBP) (6). In light of Sluyter and Dominguez's comments (26) and those of some others attempting to interpret the paleoecological record, we discuss issues relating to microfossil data in general and to the Gulf Coast Mexican studies in particular, focusing on taphonomy, preservation, bioturbation, and dating.
Considerable research in Central and South America shows that pollen and phytoliths recovered from paleoecological sites provide strong evidence for the spread of maize (below). Moderate- to large-sized swamps and lakes receive input from plants representing a wide area, organic preservation is often good, sedimentary sequences are continuous or nearly so across thousands of years of time, and the stratigraphic integrity of the sediments is frequently robust. Multiproxy analysis of terrestrial plant remains from these contexts is usually productive, because these environments provide good conditions for the preservation of pollen, phytoliths, and charcoal. Thus, the construction of chronologies for these records often involves dating a suite of traits that indicate an event horizon: spikes of charcoal, forest decline, and the appearance of weedy vegetation, all often in association with evidence for the introduction of Zea itself (e.g., refs. 7, 27–34). The synchronicity of these phenomena is often strongly supported. Such studies sometimes register signals of maize agriculture (or human activity in general) in their watersheds earlier than existing archaeological records from the region do, especially if the archaeological data are derived from macrofossil studies (attempted recovery of cobs and kernels), and microfossil research (e.g., phytolith, starch grain, and pollen analysis) is lacking (e.g., refs. 27, 29–37).
Taphonomic characteristics of these different types of plant fossils are responsible. For example, in one growing season, a single maize plant produces millions of pollen grains and phytoliths, which are dispersed within a few hundred meters and accumulate in the sediment near the maize fields. Thus, sediment samples from lakes and swamps, where pollen and phytoliths tend to accumulate, have a high probability of containing maize microfossils if maize was cultivated in the vicinity. Conversely, maize cobs and kernels originate in several orders of magnitude fewer numbers than pollen and phytolith grains, and in humid environments they have a high probability of being destroyed before their deposition in the soil. Therefore, in the tropical forest, the recovery of maize pollen or phytolith grains at a given level in a sediment layer is a high-probability event if maize was being grown near the site, whereas the recovery of maize cobs from an archaeological excavation is a low-probability event even if maize was a significant food source. Given these differences in the taphonomy of micro vs. macro remains, it is statistically more probable that the earliest evidence for maize will come from microfossils. This fact is true both of paleoecological and archaeological situations.
Some archaeologists and botanists, who are interested in prehistoric plant domestication but who lack experience with retrieving and interpreting paleocological data and who are unfamiliar with the general limnological literature, have raised questions about the stratigraphic integrity of lake sediments with regard to their robustness for dating maize presence (38–40). Claims are made that pollen and phytoliths in lacustrine sediment are routinely subject to bioturbation from burrowing benthic invertebrates and other forms of mixing and displacement (39). Such blanket statements applied to the universe of lacustrine settings are uninformed. An assertion that bioturbation has disturbed stratigraphy is incorrect for any lakes >3 m deep studied so far in the lowland tropics and subtropics. All such lacustrine bodies, including major sites providing pollen and phytolith evidence on maize dispersals and associated forest clearance, are anoxic in the bottom waters and unsuitable for supporting animal life that could cause sediment mixing (e.g., refs. 27–29, 41, 42).
In lake sediments with well developed stratigraphies, including fine laminations and distinct sedimentary zones, it is especially clear that vertical displacement is absent. Analyses of laminae reveal them to be physically and chemically distinct, suggesting little movement even of dissolved chemicals between levels, let alone particles (e.g., refs. 27–29). In short, the sedimentary sequences of many swamps and lakes cannot be compared with those of some archaeological settings where burrowing animals and human activity can move plant remains and other cultural materials up and down through the profile. Similarly archaeologists (e.g., refs. 39 and 40) should resist conflating “indirect” forms of archaeological dating, where identified plant remains are assigned a chronology by reference to other dated organic materials, such as wood charcoal, occurring in the same or nearby stratigraphic level, with how paleoecologists determine chronologies for plant fossils retrieved from lake records. A well stratified swamp or lake sequence is often a completely different kind of sedimentary record, from the initial arrival of pollen, phytoliths, and other particles, to their subsequent deposition and postdepositional history.
In a similar misunderstanding of bioturbation, Blake (40) erroneously asserts that problems noted by von Nagy (43) at San Andrés give reason to question the pollen evidence for early maize from the same site. The bioturbation at San Andrés proposed by von Nagy (43) derived from the recovery of a few Formative-period ceramic fragments from the upper Late Archaic strata in the excavations and had nothing to do with the early evidence for maize, which came from analyses of pollen from sediment cores. Sampling pollen or phytoliths is different from screening for sherds in an excavation, because the recovery of a few grains of maize pollen or phytoliths in a core sample is possible only when such grains are abundant, and high abundances are achieved only in sediments with a large influx of primary grains. The probability of maize pollen or phytoliths being reworked ≥1 m downward into sediments lacking such grains and then recovered in a cm3 sediment sample from a core is extremely low. Such mixing rapidly reduces the concentration of maize grains below detectable limits. Of course, bioturbation and sediment mixing may be a potential problem in some sedimentary records, affecting accuracy of radiocarbon dating through macrobotanical (wood, charcoal, and seeds), macrofaunal (clam and snail shells), and bulk sediment samples recovered from cores. Our field strategy combining multiple cores with archaeological excavation produced abundant organic material for radiocarbon dates resulting in a robust, stratigraphically consistent chronological record.
Sluyter and Dominguez (26) challenged our 8th millennium cal BP date for maize from Tabasco based on their analysis of pollen from a single core in coastal Veracruz. Even if the dating of the maize in their core is correct (≈5,000 cal BP), their date has little relevance for establishing the chronology of our Tabasco cores or of when maize was first domesticated. Sluyter and Dominguez assume their date records the first appearance of maize cultivators in Veracruz, even though the evidence for maize cultivation appears after a depositional hiatus and an environmental change from a mangrove swamp to estuary bank at 5,000–6,000 cal BP. An alternative and more likely interpretation is that the appearance of maize at their Veracruz coring location reflects farmers moving into the area along the banks of the estuary in response to the appearance of suitable soils rather than their first appearance in the region. Further evidence for Sluyter and Dominguez's (26) lack of understanding of the meaning of basal maize dates is their citation of Goman and Byrne's (34) basal 4,830 cal BP date as supporting a late introduction of maize into Veracruz. The maize pollen in Goman and Byrne's core extends to the bottom of the core and is mute on when maize was first introduced.
A more relevant issue raised by Sluyter and Dominguez (26) is the potential for errors when dating pollen sequences by radiocarbon dates on associated organic remains. Their 2006 paper proposed a correction to earlier work on the same core (44) based on the more recent attempt to date the pollen directly. The radiocarbon date obtained from the pollen (4,150 ± 50 RYBP) was much younger than the date on a Neritina reclivata shell (5,450 ± 35 RYBP) from 26 cm higher in the core. This finding led Sluyter and Dominguez to conclude that bioturbation was a major problem in coastal lagoon and estuary sediments, and they suggested that our 7,000 cal BP dates for maize in Tabasco must be in error.
Their reasoning is faulty, and their dating is problematic on two counts. First, the suspect date from the Veracruz core was obtained on a N. reclivata shell. Dating shells from brackish-water environments can be erroneous, because of unknown reservoir effects and recrystallization (45). We avoided dating shells in our study because of these factors, and because shells are more durable than uncarbonized wood and more susceptible to being redeposited. Neritidae snail shells in particular are known to produce anomalously old radiocarbon dates, because these grazing snails often digest carbonate-rich substrates (46, 47). Second, the new Veracruz Accelerator Mass Spectrometry (AMS) pollen date was acquired long after the core was taken in 1993 (44). Direct AMS dating of nonsediment types of organic materials such as pollen grains, phytoliths, and macrofossils retrieved from paleoecological cores has been shown to be a valuable approach contributing to increased chronological resolution (48). Sluyter and Dominguez (26) fail to describe how the core samples were curated during the years between when the core was taken and when the AMS date was run, however. Vigilance is needed to guard against growth of fungi and bacteria that can cause modern contamination of organic material that results in artificially young ages (49, 50). Pretreatment that would remove contaminants (strong oxidation) would also destroy the pollen. Furthermore, the smaller the quantity of carbon submitted for a date, the greater the error even a small amount of contaminant material will cause. The potential hazards of contamination in long-term storage precluded our submitting new samples from the SAV-4 core for radiocarbon dates on pollen at San Andrés (pollen extracts included abundant mould spores). Not enough sediment remained from the SAV-4 core to attempt phytolith dating, where appropriate pretreatment would be possible.
The relatively condensed nature of the Veracruz core is another cause for concern. Sediments dating to 5,000–7,000 cal BP occur over only 2–3 m and thus are more susceptible to bioturbation. In contrast, sediments dating to 5,000–7,000 cal BP in our Tabasco SAV-4 core occur over ≈7 m and contain well stratified estuary, beach, and lagoon sediments, some with distinct laminations (see SI Fig. 7). These sediments provide better temporal resolution and are less susceptible to the impact of bioturbation than the sediments from the Veracruz core. Furthermore, Sluyter and Dominguez (26) acquired a single core and seven radiocarbon dates. Our Tabasco study was based on cross-correlations of five cores and four deep excavations with 35 radiocarbon dates. Laminations in the San Andrés cores (see SI Fig. 7), together with the characteristic palynological signature of maize cultivators (the combined indicators of maize presence, tree loss, and rise in weedy species, along with spikes of charcoal associated with burning), and now the phytolith data, provide support for the integrity of the San Andrés stratigraphy. The San Andrés research remains the best-documented and earliest evidence for maize in southeastern Mexico.
In sum, the past 20 years of research have made it clear that studying the origins and dispersals of maize requires multiproxy [e.g., both macrobotanical (cobs, kernels, stalks) and microfossil data (pollen, phytoliths, and starch grains)] from paleoecological and archaeological research. Especially striking are the major differences shown to exist with regard to the preservation and visibility of these different types of maize fossils. Numerous data accumulated from tropical archaeological sites demonstrate that macrobotanical remains of maize (and many other crops) often fail to survive for very long in the tropical forest where high humidity and soil characteristics create conditions inimical to their preservation. In contrast, when microfossils such as phytoliths and starch grains that can withstand the forces of degradation through time are studied from archaeological sediments, stone tools, and ceramics, evidence for maize agriculture is consistently earlier (≈7,800–4,500 cal BP or 7,000–4,000 RYBP) than that obtained from macrofossils (15, 51–54).
This situation is also true of extratropical environments, for example in subtropical South America and the High Andes (e.g., refs. 13 and 55). It is becoming apparent even in relatively recent human occupations from the same regions dating to 3,300–2,000 cal BP (3,000–2,000 RYBP), where microfossil records of maize are substantially earlier than demonstrated by macrofossils, often by 1,000 years (17, 56). At San Andrés itself, the maize pollen and phytoliths recovered from sediment cores are 5,800 years older than the earliest maize macrofossil and 4,600 years older than the earliest cultigen macrofossil (sunflower) from the same site (7). This fact may in part be due to the fact that the early Archaic levels at the site lie at depths beyond the reach of excavation, but it also emphasizes the value of paleoecological studies using geological coring.
The San Andrés Data in Light of Other Evidence for Maize Dispersals.
The San Andrés data are significant both because they affirm the power of using multiproxy paleoecological methods, and because they reveal that maize had spread from its postulated Balsas River Valley homeland across the Isthmus of Tehuantepec to tropical Tabasco, Mexico, by the eighth millennium cal BP. The San Andrés data expand on the evidence for an early and rapid dispersal of maize through Neotropical lowland regions documented in Central and South America. We summarize this information, which is more comprehensive (including starch grain data) and accurate than that reported in Blake (40) (see SI Fig. 8 a and b).
The actual or potential vegetation of much of the Central Balsas region is deciduous tropical forest with between 1,100 and 1,600 mm of rain annually. The data demonstrate an initial spread of maize into ecologically compatible tropical areas to the south beginning before 7,800 cal BP. Evidence derives from multiproxy lines of investigation, including phytolith and starch grain studies both of stone tools used to process plants and of sediments from the same well studied human occupations, as well as combined pollen and phytolith analysis of major lakes and swamps with long, well stratified and well dated sedimentary sequences (see the legends to SI Fig. 8 a and b). Several sites located in western and central Panama, for example, have produced archaeological and paleoecological phytolith, pollen, and starch grain studies, all indicating that maize arrived in Panama by ≈7,800 cal BP (7,000 RYBP) (51, 53, 54, 57). In southwest Ecuador, preceramic societies already practicing food production incorporated maize into their subsistence systems by 7,500 cal BP (6,600 RYBP), and maize was well established as a food plant in the earliest ceramic (Valdivia) cultural tradition of the region by 6,100 cal BP (5,300 RYBP) (10, 15, 52, 53, 58).
There is paleoecological (pollen) and archaeological (starch grain and phytolith) evidence for maize by 7,500 cal BP (6,600 RYBP) in midaltitude Colombia (Cauca and Porce Valleys) (59–61) and 5,800 cal BP (pre-5,000 RYBP) in Amazonian Colombia (e.g., ref. 62) and Ecuador (27), with maize appearing in the eastern Amazon as late as the fourth millennium on present evidence (53). Starch-grain and phytolith evidence from preceramic sites in the southern Peruvian Andes and southeastern Uruguay indicates that maize had moved into these regions by 4,000 cal BP (3,700 RYBP) (55) and 4,700 cal BP (4,000 RYBP) (13), respectively.**
In conclusion, through applications of techniques capable of providing robust data, combining archaeological, paleoecological, and molecular research, we now understand that in all likelihood maize was brought under cultivation and domesticated in southwestern Mexico during the early Holocene period (≈10,000–7,000 RYBP or 11,000–8,000 cal BP), a chronology in accordance with one predicted from a molecular clock (2). The San Andrés data document the dispersal of maize across the Isthmus of Tehuantepec to southeastern Mexico by ≈7,300 cal BP. Additional research incorporating both paleoecological and archaeological strategies will further elucidate the domestication and early dispersals of maize, including the story of its origin in the Río Balsas drainage.
Materials and Methods
Phytoliths were analyzed by standard techniques (for details, see SI Text). Phytoliths were identified by comparison to a large modern reference collection of >2,000 Neotropical species, including vegetative and reproductive structures from 25 races of Latin American maize, all known races of teosinte and most species of Tripsacum, and hundreds of species of wild grasses native to Mexico and the Neotropics (see refs. 9 and 10 for a review of the data).
Supplementary Material
Acknowledgments
Douglas Kennett and José Iriarte provided valuable comments on the manuscript. Brenda Green prepared the illustrations, and Enrique Moreno (Smithsonian Tropical Research Institute) helped prepare Fig. 2. Tabasco research was funded by National Science Foundation Grant SBR-9617389 and by a grant from the Foundation for the Advancement of Mesoamerican Studies, Inc. D.R.P.'s phytolith work was funded by the Archaeobiology Program of the National Museum of Natural History and the Smithsonian Tropical Research Institute. We thank the Instituto de Antropología e Historia of Mexico for permission and the New World Archaeological Foundation of Brigham Young University for material field support.
Abbreviations
- cal BP
calendar years before the present
- RYBP
radiocarbon years before the present
- SAV-4
San Andrés Vibracore 4.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701425104/DC1.
The suggestion that maize moved into northern South America shortly before 4,000 cal BP, based on phytoliths recovered from a small sample of early fourth millennium BP pottery from Ecuador (63), is unsupportable in light of the accumulated data.
References
- 1.Doebley J. Annu Rev Genet. 2004;38:37–59. doi: 10.1146/annurev.genet.38.072902.092425. [DOI] [PubMed] [Google Scholar]
- 2.Matsuoka Y, Vigouroux Y, Goodman MM, Sanchez J, Buckler E, Doebley J. Proc Natl Acad Sci USA. 2002;99:6080–6084. doi: 10.1073/pnas.052125199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckler ES, Stevens NM. In: Maize Origins, Domestication, and Selection. Motley TJ, Zerega N, Cross H, editors. New York: Columbia; 2006. pp. 67–90. [Google Scholar]
- 4.Wang H, Nussbaum-Wagler T, Li B, Zhao Q, Vigouroux Y, Faller M, Bomblies K, Lukens L, Doebley J. Nature. 2005;436:714–719. doi: 10.1038/nature03863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz B. Proc Natl Acad Sci USA. 2001;98:2104–2106. doi: 10.1073/pnas.98.4.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piperno DR, Flannery KV. Proc Natl Acad Sci USA. 2001;98:2101–2103. doi: 10.1073/pnas.98.4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope KO, Pohl MED, Jones JG, Lentz DL, von Nagy C, Vega FJ, Quitmyer IR. Science. 2001;292:1370–1373. doi: 10.1126/science.292.5520.1370. [DOI] [PubMed] [Google Scholar]
- 8.Whitehead DR, Langham EJ. Bull Tor Bot Club. 1965;92:7–20. [Google Scholar]
- 9.Piperno DR, Pearsall DM. Smith Cont Bot. Vol. 85. Washington, DC: Smithsonian Institution; 1998a. [Google Scholar]
- 10.Piperno DR. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists. Lanham, MD: AltaMira; 2006. [Google Scholar]
- 11.Boyd M, Surette C, Nicholson BA. J Arch Sci. 2006;33:1129–1140. [Google Scholar]
- 12.Bozarth SR. Plains Anth. 1993;38:279–286. [Google Scholar]
- 13.Iriarte J, Holst I, Marozzi O, Listopad O, Alonso E, Rinderknecht A, Montaña J. Nature. 2004;432:614–617. doi: 10.1038/nature02983. [DOI] [PubMed] [Google Scholar]
- 14.Mulholland SC. Current Research in Phytolith Analysis: Applications in Archaeology and Paleoecology. In: Pearsall DM, Piperno DR, editors. MASCA Res Pap Sci Arch. Vol. 10. Philadelphia: Univ Mus Arch Anth, University of Pennsylvania; 1993. pp. 131–145. [Google Scholar]
- 15.Pearsall DM, Chandler-Ezell K, Chandler-Ezell A. J Arch Sci. 2003;30:611–627. [Google Scholar]
- 16.Piperno DR, Pearsall DM. J Arch Sci. 1993;20:337–362. [Google Scholar]
- 17.Chavez SJ, Thompson R. In: Histories of Maize. Staller J, Benz B, Tykot R, editors. San Diego: Academic; 2006. pp. 415–428. [Google Scholar]
- 18.Benz B. In: Histories of Maize. Staller J, Benz B, Tykot R, editors. San Diego: Academic; 2006. pp. 9–20. [Google Scholar]
- 19.Dorweiler JE, Doebley J. Am J Bot. 1997;84:1313–1322. [PubMed] [Google Scholar]
- 20.Pearsall DM. Paleoethnobotany: A Handbook of Procedures. 2nd Ed. San Diego: Academic; 2000. [Google Scholar]
- 21.Piperno DR. Phytolith Analysis: An Archaeological and Geological Perspective. San Diego: Academic; 1988. [DOI] [PubMed] [Google Scholar]
- 22.Piperno DR, Jones JG. Q Res. 2003;59:79–87. [Google Scholar]
- 23.Stuiver M, Reimer PJ. Radiocarbon. 1993;35:215–230. [Google Scholar]
- 24.Telford RJ, Heegaard E, Birks HJB. Holocene. 2004;14:296–298. [Google Scholar]
- 25.Galinat WC. Econ Bot. 1961;15:320–325. [Google Scholar]
- 26.Sluyter A, Dominguez G. Proc Natl Acad Sci USA. 2006;103:1147–1151. doi: 10.1073/pnas.0510473103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bush MB, Piperno DR, Colinvaux PA. Nature. 1989;340:303–305. [Google Scholar]
- 28.Bush MB, Piperno DR, Colinvaux PA, De Oliveira PE, Krissek LA, Miller MC, Rowe WE. Ecol Mono. 1992;62:251–275. [Google Scholar]
- 29.Bush MB, Miller MC, De Oliveira PE, Colinvaux PA. Holocene. 2000;10:543–554. [Google Scholar]
- 30.Arford MR, Horn SP. J Lat Am Geo. 2004;3:108–115. [Google Scholar]
- 31.Piperno DR. J Arch Sci. 1990;17:665–677. [Google Scholar]
- 32.Pohl MD, Pope KO, Jones JG, Jacob JS, Piperno DR, deFrance S, Lentz DL, Gifford JA, Valdez F, Jr, Danforth ME, Josserand JK. Lat Am Anti. 1996;7:355–372. [Google Scholar]
- 33.Dull RA. Q Res. 2004;61:159–167. [Google Scholar]
- 34.Goman M, Byrne R. Holocene. 1998;8:83–89. [Google Scholar]
- 35.Blake M, Chisholm JE, Clark JE, Mudar K. In: Transitions to Agriculture in Prehistory. Gebauer AB, Price TD, editors. Madison, WI: Prehistory Press; 1992. pp. 133–151. [Google Scholar]
- 36.Neff H, Pearsall DM, Jones JG, Arroyo B, Collins SK, Freidel DE. Lat Am Anti. 2006;17:287–315. [Google Scholar]
- 37.Kennett DJ, Voorhies B, Martorana D. In: Behavioral Ecology and the Transition to Agriculture. Kennett DJ, Winterhalter B, editors. Berkeley: Univ of California; 2006. pp. 103–136. [Google Scholar]
- 38.Fritz GJ. In: Archaeological Methods. Maschner HDG, Chippindale C, editors. Vol II. Lanham, MD: AltaMira; 2006. pp. 773–834. [Google Scholar]
- 39.Benz B, Staller J. In: Histories of Maize. Staller J, Benz B, Tykot R, editors. San Diego: Academic; 2006. pp. 665–673. [Google Scholar]
- 40.Blake M. In: Histories of Maize. Staller J, Benz B, Tykot R, editors. San Diego: Academic; 2006. pp. 56–68. [Google Scholar]
- 41.Colinvaux PA, Miller MC, Liu K, Steinitz-Kannan M. Nature. 1985;313:42–45. [Google Scholar]
- 42.Steinitz-Kannan M, Colinvaux PA, Kannan R. Arch Hydrobiol/Suppl. 1983;65:61–105. [Google Scholar]
- 43.von Nagy C. New Orleans, LA: Tulane University; 2003. PhD thesis. [Google Scholar]
- 44.Sluyter A. Glob Planet Change. 1997;14:127–146. [Google Scholar]
- 45.Taylor RE. Radiocarbon Dating: An Archaeological Perspective. Orlando, FL: Academic; 1987. [Google Scholar]
- 46.Dye T. Radiocarbon. 1994;36:51–57. [Google Scholar]
- 47.Anderson A, Higham T, Wallace R. Rec Austral Mus. 2001;(Suppl 27):33–42. [Google Scholar]
- 48.Vandergoes MJ, Prior CA. Radiocarbon. 2003;45:479–491. [Google Scholar]
- 49.Wohlfarth B, Skog G, Possnert G, Holmquist B. J Q Sci. 1998;13:137–145. [Google Scholar]
- 50.Geyh MA, Krumbein WE, Kudrass H-R. Mar Geo. 1974;17:M45–M50. [Google Scholar]
- 51.Dickau R, Ranere AJ, Cooke RG. Proc Natl Acad Sci USA. 2007;104:3651–3656. doi: 10.1073/pnas.0611605104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearsall DM, Chandler-Ezell K, Zeidler JA. J Arch Sci. 2004;31:423–442. [Google Scholar]
- 53.Piperno DR, Pearsall DM. The Origins of Agriculture in the Lowland Neotropics. San Diego: Academic; 1998. [Google Scholar]
- 54.Piperno DR, Ranere AJ, Holst I, Hansell P. Nature. 2000;407:894–897. doi: 10.1038/35038055. [DOI] [PubMed] [Google Scholar]
- 55.Perry L, Sandweiss DH, Piperno DR, Rademaker K, Malpass MA, Umire A, de la Vera K. Nature. 2006;440:76–79. doi: 10.1038/nature04294. [DOI] [PubMed] [Google Scholar]
- 56.Perry L. J Arch Sci. 2004;31:1069–1081. [Google Scholar]
- 57.Piperno DR, Clary K, Cooke RG, Ranere AJ, Weiland D. Am Anth. 1985;87:87l–878. [Google Scholar]
- 58.Stothert K, Piperno DR, Andres TC. Q Intern. 2003;109–110:23–43. [Google Scholar]
- 59.Bray WB, Herrera L, Cardale Schrimpff M, Botero P, Monsalve JG. Pre-Hispanic Agricultural Fields in the Andean Region. In: Denevan WM, Mathewson K, Knapp G, editors. Brit Arch Rep Internatl Ser. Vol. 359. Oxford, UK: 1987. pp. 443–481. [Google Scholar]
- 60.Monsalve JG. Pro Calima. 1985;4:40–44. [Google Scholar]
- 61.Bocanegra FJA, Espitia NC. Before Farming. 2005;2:1–17. [Google Scholar]
- 62.Mora SC, Herrera LF, Cavelier I, Rodríguez C. Univ Pittsburgh Lat Am Arch Rep. Vol. 2. Pittsburgh, PA: University of Pittsburgh Department of Anthropology; 1991. Cultivars, Anthropic Soils and Stability. [Google Scholar]
- 63.Staller JE, Thompson RG. J Arch Sci. 2002;29:33–50. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.