Abstract
Basic fibroblast growth factor (bFGF) is overexpressed in most high-grade human gliomas, implying that it is involved in the pathogenesis of these tumors. To assess the biological effect of inappropriate production of bFGF in normal astrocytes, we developed a system for glia-specific gene transfer in transgenic mice. A transgene encoding the receptor for subgroup A avian leukosis virus and controlled by the astrocyte-specific glial fibrillary acidic protein promoter permits efficient glia-specific transfer of genes carried by subgroup A avian leukosis virus vectors. With this system, we have demonstrated that bFGF induces proliferation and migration of glial cells in vivo, without the induction of tumors.
Gliomas form the most common category of primary brain tumors in humans. The four grades of gliomas exhibit increasing degrees of cell growth, angiogenesis, and necrosis (1). The most aggressive form of the disease, glioblastoma multiforme, is typified by diffuse infiltration of tumor cells into surrounding brain structure, resistance to conventional therapies, and death within a year from the time of diagnosis (2, 3).
The molecular abnormalities that account for the pathogenesis of gliomas are not completely described. However, several genetic alterations are frequently encountered in such tumors, including loss of the tumor suppressor genes p53, p16, RB, or PTEN; increased expression of the protooncogenes bFGF, VEGF, or cdk4; and amplification or mutation of the EGFR gene (4–9).
In hopes of defining the roles played by these alterations during gliomagenesis and progression, we have devised a strategy to mimic the genetic changes observed in human gliomas by rendering glial cells in transgenic mice specifically susceptible to infection by viral vectors. Glial cells have been targeted for infection with high-titer subgroup A avian leukosis viruses (ALV-A) by programming animals to produce TVA, the avian cell surface receptor for ALV-A, under the control of an astrocyte-specific promoter from the gene encoding glial fibrillary acidic protein (GFAP).
TVA is the product of the tv-a gene and is essential for infection by ALV-A in chicken cells (10, 11). The introduction of an avian tv-a gene into cultured mammalian cells permits infection by ALV-A and expression of genes carried by ALV-A vectors (12, 13). Because ALV vectors are replication-competent in avian cells, high-titer viral stocks can be produced without a helper component. ALV does not, however, make infectious progeny in mammalian cells, preventing cell-to-cell spread of infection in a population of TVA+ mammalian cells exposed to ALV-A. Furthermore, because the ALV-A env gene is poorly expressed in mammalian cells, the TVA receptor is not occupied or down-regulated by viral envelope protein, so that infected TVA+ mammalian cells remain susceptible to repeated rounds of infection with ALV-A vectors (14, 15). In this manner multiple genes, including those providing histologic identification, can be delivered to a single TVA+ cell.
In this report we document high-efficiency transfer of multiple genes to primary astrocytes in culture. By using the product of the alkaline phosphatase gene (AP) as a histologic marker, we then describe astrocyte-specific gene transfer in vivo and show that simultaneous transfer of the bFGF gene results in migration and proliferation of astrocytes without tumor formation.
MATERIALS AND METHODS
Constructs.
The plasmid pGfa 2lac-1, expressing the lacZ gene from a 2.2-kb fragment of the human GFAP promoter and containing part of the mouse protamine gene (MP-1) to supply an intron and polyadenylylation site was obtained from Mike Brenner (National Institutes of Neurologic Disorders and Stroke). The lacZ gene was removed by digestion with BamHI and replaced with a BglII–BamHI fragment containing the tv-a cDNA from pSP73(0.8) (11). RCAS-puro was obtained from Steve Hughes (National Cancer Institute) (16, 17) (where RCAS is replication-competent ALV). RCAS-AP and RCAS-bFGF were constructed by ClaI digestion of RCAS-puro to remove the puromycin-resistance gene and its replacement with ClaI fragments of cDNAs encoding human placental AP (Steve Hughes) and mouse basic fibroblast growth factor (bFGF; Gail Martin, University of California, San Francisco).
Cell Culture.
Primary brain cell cultures from newborn transgenic mice were obtained by mechanical dissociation of the whole brain, followed by digestion with 0.25% trypsin for 15 min at 37°C. Large debris was allowed to settle, and single cells were plated and grown in DMEM with 10% fetal calf serum (GIBCO/BRL). Two types of chicken cells were used to produce vectors: Chicken embryo fibroblasts (CEFs) were used in short-term experiments (up to 7 days) and DF-1 cells were used in long-term experiments. CEFs were obtained by mechanical dissociation of day 10 chicken embryos followed by digestion with 0.25% trypsin for 15 min at 37°C. Large debris was allowed to settle, and single cells were plated and grown in DMEM with 5% fetal calf serum, 5% calf serum, 1% chicken serum, and 10% tryptose phosphate broth (GIBCO/BRL). DF-1 cells (a gift from Doug Foster, University of Minnesota) are propogated as a continuous line in DMEM with 5% fetal calf serum, 5% calf serum, 1% chicken serum, and 10% tryptose phosphate broth (GIBCO/BRL).
Cell Culture Infection.
The supernatant from DF-1 or CEF cells infected with RCAS vectors was filtered through a 0.45-μm (pore size) filter and plated directly onto astrocytes for infection. The murine leukemia virus (MLV)-based lacZ retroviral vector (pHIT 222), the MLV gag-pol expression plasmid (pHIT 60), and the ALV-A env expression plasmid (pHIT envA) were gifts from Paul Bates (University of Pennsylvania). 293T cells were transfected with a mixture containing 5 μg of each plasmid by calcium phosphate precipitation. Medium from these cells was collected 48 h after transfection, filtered, and used as viral stock for infection. Transgenic primary brain cells in culture were infected with filtered medium from RCAS-puro-producing cells and then selected in puromycin (4 μg/ml). After two passages, the astrocytes were infected with a mixture of RCAS-AP and MLV-lacZ (ALV-A) viral stocks and maintained in puromycin selection conditions.
In Vivo Infection.
CEFs or DF-1 cells infected with RCAS vectors were pelleted by centrifugation, and the cell pellets were resuspended in approximately 100 μl of medium and placed on ice. A single intracranial injection of 1 μl containing 104 cells was made in the right frontal region, just anterior to the striatum, with the tip of the needle just touching the skull base.
Brain Sectioning and Staining.
The animals were sacrificed, and the brains were fixed in 4% formaldehyde/0.4% glutaraldehyde/1× PBS for 36 h and then dehydrated in 20% sucrose/2% glycerol/1× PBS. Frozen sections (40 μm) were obtained with a sledge microtome. The sections were then stained for AP with 5-bromo-4-chloro-3-indolyl phosphate and 4 nitroblue tetrazolium chloride (Boehringer) after treatment at 65°C, pH 9.5, for 30 min to remove endogenous AP activity. Fixed frozen sections (20 μm) were stained in solution for AP as described and then incubated in solution with a monoclonal antibody to GFAP (Boehringer) and detected with immunoperoxidase (Vectastain).
RESULTS
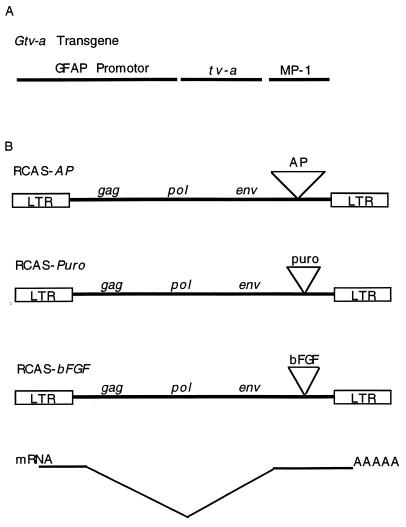
Tissue-specific production of TVA in transgenic mice has been shown to allow myoblast-specific transfer of a histological marker gene encoding AP (AP) carried by the ALV-A-derived vector RCAS-AP (18). For glia-specific gene transfer, we constructed a transgene (Gtv-a) that expresses tv-a from the GFAP promoter (ref. 19 and Fig. 1A) and established two mouse lines that transmit the Gtv-a transgene to progeny in a simple Mendelian pattern.
Figure 1.
DNA constructs. (A) GFAP tv-a (Gtv-a) transgene. A 2.2-kb fragment of the GFAP promoter drives expression of the tv-a cDNA. The fragment from the mouse protamine gene (MP-1) supplies an intron and signal for polyadenylylation. (B) RCAS vectors. These vectors carry an exogenous gene 3′ of env. RCAS-AP, RCAS-puro, and RCAS-bFGF carry the human placental AP cDNA, the bacterial gene for puromycin resistance, and the mouse bFGF cDNA, respectively. These exogenous genes are expressed in both avian and mammalian cells from a spliced message as illustrated, although viruses are produced only by avian cells.
Transfer of Multiple Genes to Astrocytes in Culture.
To demonstrate that astrocytes from these mice could be repeatedly infected with ALV-A vectors, we exposed primary cultures of astrocytes to RCAS vectors encoding human placental AP (RCAS-AP) and the bacterial puromycin-resistance gene (RCAS-puro) (Fig. 1B). In addition, we infected the cells with an MLV vector carrying the Escherichia coli lacZ gene, pseudotyped with ALV-A envelope protein [MLVlacZ (ALV-A)] (20, 21). Primary cultures were first infected with RCAS-puro, selected for puromycin resistance, and then reinfected with a mixture of RCAS-AP and MLV-lacZ (ALV-A). More than half of the cells that acquired puromycin resistance as a consequence of infection with RCAS-puro also expressed at least one and often both of the other marker genes (Fig. 2A). Cultured astrocytes from nontransgenic mice were not infectable by either RCAS-AP or MLV-lacZ (ALV-A) vectors. These findings indicate that multiple genes can be transferred to astrocytes sequentially or simultaneously with this system.
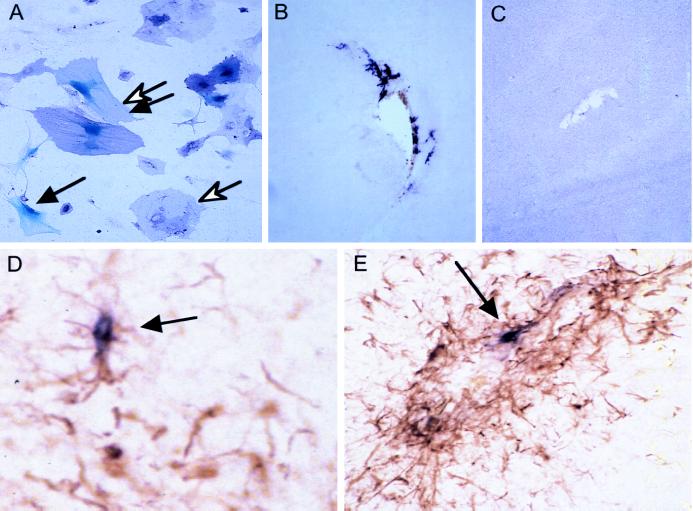
Figure 2.
Gene transfer to transgenic astrocytes. (A) Cultured cells. Primary brain cultures from newborn mice were infected with RCAS-puro, selected for puromycin resistance, then reinfected with a mixture of RCAS-AP and MLV-lacZ (ALV-A) vectors, and stained for AP and β-galactosidase. An open arrow identifies a cell that stains for AP (purple), a solid arrow identifies a cell with nuclear β-galactosidase staining (blue) and the double arrows identifies a cell expressing both markers. (B) Transfer of the AP gene to astrocytes in vivo. Six days after intracranial injection of a newborn Gtv-a transgenic mouse with CEFs producing RCAS-AP, gene transfer can be seen by AP staining in cells with astrocytic morphology around the injection track. (×100) (C) A nontransgenic litter mate injected in the same fashion as in B shows no AP+ cells. (×100) (D and E) GFAP and AP double staining of the injection track 10 days after injection with DF-1 cells producing RCAS-AP demonstrates an increase in number of GFAP+ cells and presence of AP+ cells (arrows). (D, ×100; E, ×200.)
Astrocyte-Specific Gene Transfer in Vivo.
To achieve persistently high virus titers at injection sites for gene transfer in vivo, we injected CEFs producing ALV-A vectors, rather than cell-free virus stocks, directly into the brain parenchyma of newborn Gtv-a transgenic mice. The producer cells survived for less than 3 days, as shown by tests in nontransgenic animals. It is thought that retroviral particles are too large to diffuse through the brain substance (Eric Oshiro, personal communication). Therefore, we expected gene transfer to be limited to the region around the injection track. Six days after intracerebral injection of CEFs producing RCAS-AP, multiple astrocytic cells surrounding the injection track stained positive for AP (Fig. 2B). After parallel injections into the brains of nontransgenic litter mates, no residual producer cells were evident at the time of sacrifice and no AP staining was detected, implying that AP gene transfer in transgenic mice is receptor-dependent (Fig. 2C). We estimate that a few hundred mouse brain cells were infected after each injection. The RCAS-AP infected cells have continued to stain positively for AP up to 6 months after infection.
Staining for GFAP demonstrates increased numbers of astrocytes in the vicinity of the injection track (Fig. 2 D and E); some of these cells costain for GFAP and AP, identifying these AP+ cells as astrocytes. Injury caused by insertion of the injection needle is expected to induce GFAP expression and division of glial cells and thereby increase infectability of these cells, because receptor availability and cell division are both required for retroviral infection. Increased expression of transgenes expressed from the GFAP promoter has been shown to accompany the induction of GFAP expression in astrocytes (19). Therefore, it is likely that Gtv-a is efficiently expressed in astrocytes adjacent to injection tracks in transgenic mice. Although astrocyte infection in these transgenic mice is receptor-dependent, the amount of TVA on cell surfaces appears low, because TVA was not detected in brain slices by immunohistochemical staining with an anti-TVA antibody† (gift of Andrew Leavitt, University of California, San Francisco; data not shown).
bFGF Expression Induces Astrocyte Migration and Proliferation in Vivo.
To assess the effects of bFGF, a growth factor usually produced abundantly in high-grade gliomas, on glial cell proliferation and localization, we took advantage of our ability to transfer multiple genes simultaneously into astrocytes expressing tv-a. We injected transgenic mice intracerebrally with a mixture of cells, half programmed to produce RCAS-AP (to provide a histologic marker) and the other half producing RCAS-bFGF, and both derived from the chicken cell line DF-1. Two weeks after injection, a few hundred AP+ cells were detected near the injection track, as seen after transfer of the AP gene alone (Fig. 3A). Four weeks after injection, the total number of AP+ cells increased, and cells were found in regions distant from the injection track (Fig. 3B), including the white matter tracts of the striatum, internal capsule, corpus callosum, and hippocampal commissure. We also detected cells on the contralateral side of the brain from the injection site, and the injection track often became difficult to discern because AP+ cells were no longer clustered in that region.
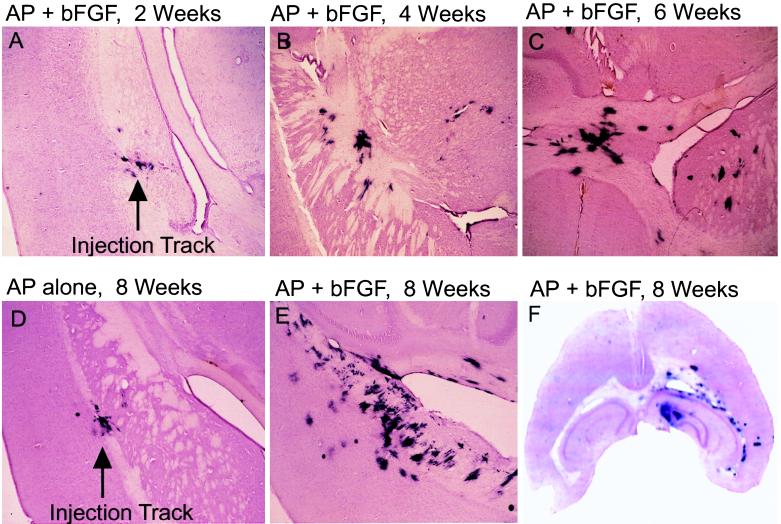
Figure 3.
Time course of proliferation and migration of RCAS-bFGF-infected cells in vivo. An equal mixture of DF-1 cells producing RCAS-AP and DF-1 cells producing RCAS-bFGF was injected into the right frontal lobe of Gtv-a transgenic mice. The mice were sacrificed and brains (40-μm sections) were analyzed for AP activity at 2 weeks (A), 4 weeks (B), and 6 weeks (C) after injection. Mice were injected with DF-1 cells producing either RCAS-AP alone (D) or a mixture of RCAS-bFGF and RCAS-AP (E) and analyzed 8 weeks after injection. (×40.) (F) A representative slice of a brain 8 weeks after infection with RCAS-bFGF and RCAS-AP that shows a diffuse spread of infected cells.
Six to 8 weeks after injection, AP-stained regions were frequently bilateral, and there was often 10- to 50-fold more AP+ staining than found in the mice after infection with RCAS-AP alone (Fig. 3 C, E, and F). The amount of AP staining reached a maximum between 8 and 10 weeks after injection; mice sacrificed 16 weeks to 6 months after injection showed no more and often less AP staining than that seen after 8–10 weeks. Transgenic animals injected with the RCAS-AP vector alone showed a relatively small number of AP+ cells near the site of injection 8 weeks after infection, without significant cell migration (Fig. 3D). Again, no AP+ cells were observed in nontransgenic animals. To confirm that the aggregated AP+ cells at sites distant from the needle track were, in fact, infected with RCAS-bFGF, we used PCR with primers designed for RCAS-bFGF proviral DNA to amplify DNA from microdissected AP+ lesions; in both of the analyses, the expected fragments of amplified RCAS-bFGF DNA were detected after gel electrophoresis (data not shown).
We prepared thinner sections of brains 3 months after infection with RCAS-AP and RCAS-bFGF for a more detailed examination of the morphology of the abundant AP+ cells (Fig. 4 A and B). In many regions, the AP+ cells have small nuclei and fine stellate projections, typical of astrocytes (Fig. 4A). In nerve fiber tracts however, the cells exhibit a bipolar morphology and aggregate in parallel rows, aligning themselves in the direction of the axons (Fig. 4B). The projections appear to be long relative to the nuclei; in some sections, the majority of the AP stain is associated with projections from cell bodies found in adjacent sections. Cells in diffuse low-grade fibrillary astrocytomas show similar elongated features (1), implying a possible astrocytic origin for the AP+ cells. Alternatively, the AP+ cells in the white matter may be oligodendrocytes. This would suggest that some subset of the infected cell population has the potential to differentiate into either astrocytes or oligodendrocytes, depending on local environmental signals.
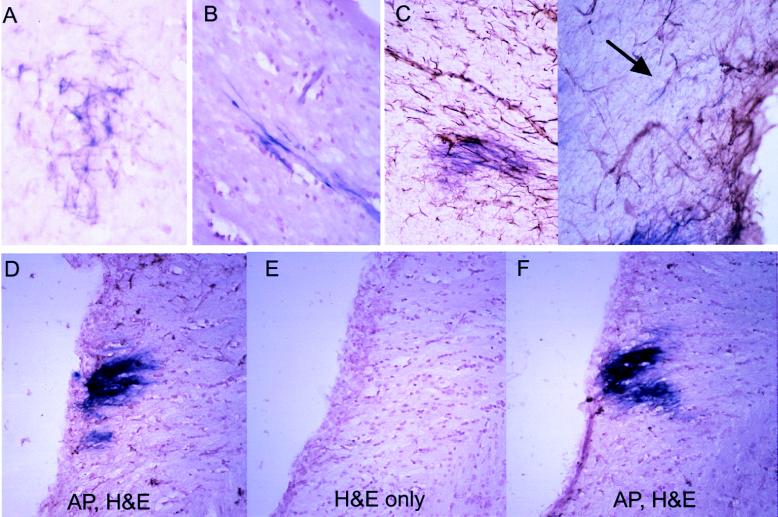
Figure 4.
Morphology and histology of AP+ cells 3 months after infection with RCAS-AP and RCAS-bFGF. (A and B) Astrocytic morphology of infected cells seen in 20-μm sections in gray matter (A) and bipolar morphology in white matter (B) (stained for AP and counterstained with hematoxylin). (C) Staining for AP and GFAP demonstrate a lack of GFAP in most AP+ cells (arrow indicates a rare double-positive cell). (×200.) (D–F) Contiguous hematoxylin/eosin-stained sections with alternate sections also stained for AP. (D and F) AP+ region expected to be found in all three sections. (E) No histologic alteration in the region likely to be AP+. (×100.)
Although the majority of cells infected with RCAS-AP alone appeared to be GFAP+, as seen in Fig. 2D, animals infected with both AP and bFGF vectors had few double-stained cells when analyzed for GFAP and AP 3 months after infection (Fig. 4 C and D). We cannot explain this discrepancy at present. It is possible that bFGF expression reduces GFAP expression in vivo or, alternatively, that a subset of infected cells, initially with low levels of GFAP, preferentially proliferate and give rise to a predominant cell type that no longer expresses GFAP. It is worth noting that astrocytes grown in culture progressively lose expression of GFAP (22).
Regions of AP+ Staining Are Histologically Normal.
To determine whether the AP+ regions were associated with abnormal histologic features as assessed with conventional hematoxylin/eosin staining, we counterstained alternate sections for AP. Fig. 4 D–F illustrates three sequential sections; only sections in D and F were stained for AP. Although the center section (Fig. 4E) almost certainly contains AP activity, it shows no obvious histologic alteration. These findings suggest that FGF signaling promotes proliferation and migration of glial cells that are capable of integrating into the normal brain structure. Even in areas with extensive infiltration by AP+ cells, however, there appear to be no structural distortions indicative of frank gliomagenesis.
DISCUSSION
In this report, we have used glia-specific expression of a receptor for a retrovirus vector to permit the efficient transfer of multiple genes to primary astrocytes in culture and to glial cells in newborn animals. The system described herein is likely to be useful for studying the interaction of multiple exogenous genes in cultured astrocytes, a cell type relatively resistant to multiple gene transfer. However, the system has even greater utility in intact animals as a method for studying the effect of specific genes on restricted cell types in vivo.
After viral transfer of the bFGF gene and AP marker gene to TVA-positive cells in vivo, we observed extensive migration and proliferation of glia over the next several weeks by following the fate of AP+ cells. At this point, we cannot distinguish between direct induction of these behaviors by FGF signaling and the maintenance of glia in a less-differentiated state that inherently displays such behaviors. Furthermore, proliferation and migration appear to be self-limited because the amount of AP staining does not increase beyond 8–12 weeks. We have not assessed the production of bFGF RNA or protein by these cells, and it is possible that levels of bFGF decreases over time. We have not observed frank gliomas arising from doubly infected cells, and the regions staining for AP appear essentially normal by conventional staining in animals up to 13 months after transfer of AP and bFGF.
bFGF has been shown to be a mitogen for astrocytes and to cause migration of both astrocytes and glioma cells in culture (23–25). Furthermore, the Drosophila gene breathless, encoding an FGF receptor homolog, has been shown to be essential for migration of midline glial cells (26) and glial progenitor cells expressing a dominant-negative FGF receptor fail to migrate when transplanted into neonatal rat brains (30). Given that bFGF is overexpressed in most high-grade gliomas and, from results presented herein, appears to be capable of causing migration and proliferation of glia in vivo, it is likely that FGF signaling is at least partly responsible for these characteristics of gliomas. Our data further delineate the role of bFGF in gliomagenesis by separating glial proliferation from overt glioma formation. Thus, heightened expression of bFGF may not be an efficient first step in the induction of gliomas. Conversely, in the model system we have developed, there are only a few hundred cells initially infected; other genetic events required for glioma formation may not occur at sufficient frequency to produce tumors from these cells.
In classical transgenic models for tumors, oncogenes are expressed from tissue-specific promoters in large numbers of cells. These models can be used to detect secondary events that accelerate tumor formation (27). However, it is not possible to follow the fate of individual, genetically modified cells with such models. In contrast, because of the ability to visualize the infected cells with the AP marker, the TVA-based system presented herein allows detection of a gene’s direct effect on specified cell types in vivo.
Weissenberger et al. (28) have reported that transgenic mice expressing the viral oncogene v-src under control of the GFAP promoter occasionally develop astrocytomas. Thus GFAP+ cells are capable of undergoing neoplastic transformation; however, the late and infrequent appearance of tumors implies that even a potent oncogene such as v-src requires other genetic alterations to convert these cells to gliomas. It is also possible that the cells infected in our Gtv-a transgenic animals may be near-terminally differentiated GFAP+ astrocytes with a much lower capacity for forming gliomas and that gliomas may more readily arise from less differentiated precursor cells. These possibilities can be explored by using vectors carrying other dominantly acting genes (such as VEGF, cdk4, or EGFR), by introducing the Gtv-a transgene into genetic backgrounds deficient in various tumor suppressor genes (such as p53, PTEN, p16, or RB), and by driving expression of tv-a transgenes with promoters [such as that derived from the nestin gene (29)] that are active in earlier phases of glial cell development. The ability to combine gain-of-function and loss-of-function genetic alterations in specified cell types in vivo will likely provide insight regarding the effects of the numerous mutations found in human gliomas.
Acknowledgments
We thank Wendy Hively for outstanding technical assistance, Rod Bronson for helpful discussions of pathology, Lisa Garrett and Theresa Hernandez for excellent help with pronuclear injections and animal husbandry, and Tony Wynshaw-Boris and Bill Pavan for critical review of the manuscript. We also thank Doug Foster for the DF-1 cells, Paul Bates for the pHIT vectors, and Steve Hughes for RCAS vectors and invaluable discussion. E.C.H. is a Howard Hughes Medical Institute Physician Postdoctoral Fellow.
ABBREVIATIONS
- RCAS
replication-competent ALV
- AP
alkaline phosphatase
- bFGF
basic fibroblast growth factor
- GFAP
glial fibrillary acidic protein
- ALV
avian leukosis virus
- TVA
receptor for ALV-A
- CEF
chicken embryo fibroblast
- MLV
murine leukemia virus
Footnotes
The rabbit polyclonal antibody was produced by John Young (Harvard University) and Paul Bates (University of Pennsylvania).
References
- 1.Russell D S, Rubinstein L J. Pathology of Tumors of the Nervous System. 5th Ed. Baltimore: Williams & Wilkins; 1989. pp. 95–247. [Google Scholar]
- 2.Nazzaro J M, Neuwelt E A. In: The Practice of Neurosurgery. Tindall G T, Cooper P R, Barrow D L, editors. Baltimore: Williams & Wilkins; 1996. pp. 467–476. [Google Scholar]
- 3.Miller D C. In: The Practice of Neurosurgery. Tindall G T, Cooper P R, Barrow D L, editors. Baltimore: Williams & Wilkins; 1996. pp. 601–635. [Google Scholar]
- 4.James C D, Olson J J. Curr Opin Oncol. 1996;8:188–195. doi: 10.1097/00001622-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Zagzag D, Miller D C, Sato Y, Rifkin D B, Burstein D E. Cancer Res. 1990;50:7393–7398. [PubMed] [Google Scholar]
- 6.Takahashi J A, Mori H, Fukumoto M, Igarashi K, Jaye M, Oda Y, Kikuchi H, Hatanaka M. Proc Natl Acad Sci USA. 1990;87:5710–5714. doi: 10.1073/pnas.87.15.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekstrand A J, Longo N, Hamid M L, Olson J J, Liu L, Collins V P, James C D. Oncogene. 1994;9:2313–2320. [PubMed] [Google Scholar]
- 8.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S, Puc J, Miliaresis Rodgers L, McCombie R, Bigner S H, Giovanella B C, Ittmann M, Tycko B, Hibshoosh H, Wigler M H, Parsons R. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 9.He J J, Olson J J, James C D. Cancer Res. 1995;55:4833–4836. [PubMed] [Google Scholar]
- 10.Vogt P K, Ishizaki R. Virology. 1966;30:368–374. doi: 10.1016/0042-6822(66)90115-2. [DOI] [PubMed] [Google Scholar]
- 11.Bates, P., Rong, L., Varmus, H. E., Young, J. A. T. & Crittenden, L. B. (1998) J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 12.Young J A, Bates P, Varmus H E. J Virol. 1993;67:1811–1816. doi: 10.1128/jvi.67.4.1811-1816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates P, Young J A, Varmus H E. Cell. 1993;74:1043–1051. doi: 10.1016/0092-8674(93)90726-7. [DOI] [PubMed] [Google Scholar]
- 14.Altaner C, Temin H M. Virology. 1970;40:118–134. doi: 10.1016/0042-6822(70)90384-3. [DOI] [PubMed] [Google Scholar]
- 15.Quintrell N, Hughes S H, Varmus H E, Bishop J M. J Mol Biol. 1980;143:363–393. doi: 10.1016/0022-2836(80)90218-1. [DOI] [PubMed] [Google Scholar]
- 16.Hughes S H, Greenhouse J J, Petropoulos C J, Sutrave P. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhouse J J, Petropoulos C J, Crittenden L B, Hughes S H. J Virol. 1988;62:4809–4812. doi: 10.1128/jvi.62.12.4809-4812.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Federspiel M J, Bates P, Young J A, Varmus H E, Hughes S H. Proc Nat Acad Sci USA. 1994;91:11241–11245. doi: 10.1073/pnas.91.23.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brenner M, Kisseberth W C, Su Y, Besnard F, Messing A. J Neurosci. 1994;14:1030–1037. doi: 10.1523/JNEUROSCI.14-03-01030.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau N R, Littman D R. J Virol. 1992;66:5110–5113. doi: 10.1128/jvi.66.8.5110-5113.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frisa P S, Goodman M N, Smith G M, Silver J, Jacobberger J W. J Neurosci Res. 1994;39:47–56. doi: 10.1002/jnr.490390107. [DOI] [PubMed] [Google Scholar]
- 23.Chicoine M R, Madsen C L, Silbergeld D L. Neurosurgery. 1995;36:1165–1170. [PubMed] [Google Scholar]
- 24.Gately S, Soff G A, Brem S. Neurosurgery. 1995;37:723–730. doi: 10.1227/00006123-199510000-00017. [DOI] [PubMed] [Google Scholar]
- 25.Hou Y J, Yu A C, Garcia J M, Aotaki-Keen A, Lee Y L, Eng L F, Hjelmeland L J, Menon V K. J Neurosci Res. 1995;40:359–370. doi: 10.1002/jnr.490400310. [DOI] [PubMed] [Google Scholar]
- 26.Klambt C, Glazer L, Shilo B Z. Genes Dev. 1992;6:1668–1678. doi: 10.1101/gad.6.9.1668. [DOI] [PubMed] [Google Scholar]
- 27.Kwan H, Pecenka V, Tsukamoto A, Parslow T G, Guzman R, Lin T, Muller W J, Lee F S, Leder P, Varmus H E. Mol Cell Biol. 1992;12:147–154. doi: 10.1128/mcb.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weissenberger J, Steinbach J P, Malin G, Spada S, Rulicke T, Aguzzi A. Oncogene. 1997;14:2005–2013. doi: 10.1038/sj.onc.1201168. [DOI] [PubMed] [Google Scholar]
- 29.Lendahl U, Zimmerman L B, McKay R D G. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 30.Osterhout D J, Ebner S, Xu J, Ornitz D M, Zazanis G A, McKinnon R D. J Neurosci Res. 1997;17:9122–9132. doi: 10.1523/JNEUROSCI.17-23-09122.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]