Abstract
Alveolar macrophages from Pneumocystis carinii-infected hosts are defective in phagocytosis (W. Chen, J. W. Mills, and A. G. Harmsen, Int. J. Exp. Pathol. 73:709-720, 1992; H. Koziel et al., J. Clin. Investig. 102:1332-1344, 1998). Experiments were performed to determine whether this defect is specific for P. carinii organisms. The results showed that these macrophages were unable to phagocytose both P. carinii organisms and fluorescein isothiocyanate (FITC)-conjugated latex beads, indicating that alveolar macrophages from P. carinii-infected hosts have a general defect in phagocytosis. To determine whether this defect correlates with the recently discovered down-regulation of the GATA-2 transcription factor gene during P. carinii infection, alveolar macrophages from dexamethasone-suppressed or healthy rats were treated with anti-GATA-2 oligonucleotides and then assayed for phagocytosis. Aliquots of the alveolar macrophages were also treated with the sense oligonucleotides as the control. Cells treated with the antisense oligonucleotides were found to have a 46% reduction in phagocytosis of P. carinii organisms and a 65% reduction in phagocytosis of FITC-latex beads compared to those treated with the sense oligonucleotides. To determine whether the defect in phagocytosis in alveolar macrophages from P. carinii-infected hosts can be corrected by overexpression of GATA-2, a plasmid containing the rat GATA-2 gene in the sense orientation driven by the cytomegalovirus (CMV) promoter was introduced into alveolar macrophages from P. carinii-infected rats. Aliquots of the same cells transfected with a plasmid containing GATA-2 in the antisense orientation relative to the CMV promoter served as the control. Alveolar macrophages treated with the sense GATA-2 expression construct were found to increase their phagocytic activity by 66% in phagocytosis of P. carinii organisms and by 280% in phagocytosis of FITC-latex beads compared to those that received the antisense GATA-2 construct. The results of this study indicate that GATA-2 plays an important role in the regulation of phagocytosis in alveolar macrophages during P. carinii infection.
Pneumocystis carinii, recently renamed Pneumocystis jiroveci (38) for those organisms that infect humans, causes pneumonia in immunocompromised individuals. In AIDS patients, Pneumocystis pneumonia (PcP) accounts for much morbidity and mortality and is usually fatal if untreated (5). Although the incidence of PcP has declined with the advent of highly active antiretroviral therapy (25), PcP is still the most common opportunistic disease in AIDS patients (5). Host responses to the organism have been studied but are still not completely understood. A defect in cellular immunity is important for pathogenesis, since most patients with PcP have an underlying disease linked to suppression of cellular immunity, i.e., AIDS, cancer, or malnutrition (26, 34, 36). Also, immunosuppression with steroids, which suppresses the cellular immune system, predisposes humans and animals to PcP (34, 36).
The importance of alveolar macrophages is shown by the demonstration that Pneumocystis organisms are not cleared in alveolar macrophage-depleted rats (21). In addition, administration of granulocyte-macrophage colony-stimulating factor, which has been shown to activate alveolar macrophages during Pneumocystis infection (30), decreases the severity of PcP (22). Alveolar macrophages from normal animals can be activated by the whole organism or the major surface glycoprotein of Pneumocystis organisms. Activated alveolar macrophages release inflammatory mediators such as tumor necrosis factor alpha and the eicosanoid metabolites prostaglandin E2 and leukotriene B4 (4, 13, 14, 32). This activation is enhanced by vitronectin or fibronectin, which accumulates in the lung during Pneumocystis infection (29). Alveolar macrophages are thought to interact with Pneumocystis organisms through the macrophage mannose receptor or the β-glucan receptor (20, 37).
Although alveolar macrophages from normal hosts are able to bind, phagocytose, and degrade Pneumocystis organisms (11, 21, 23), alveolar macrophages from Pneumocystis-infected hosts appear to be defective in phagocytosis. Using a SCID mouse model, Chen et al. (6) demonstrated that phagocytosis of Pneumocystis organisms is not common. Phagocytosis of Pneumocystis organisms by macrophages is reduced in human immunodeficiency virus-positive patients with PcP, and the production of mannose receptors in alveolar macrophages from human immunodeficiency virus-positive patients with PcP is also found to be decreased (18).
Recently, the expression of the transcription factor GATA-2 gene was found to be down-regulated during infection with P. carinii (rat-derived Pneumocystis organisms) in alveolar macrophages (39). GATA-2 has been shown to play a crucial role in the development of hematopoietic cells (33, 40, 41) and in the regulation of a variety of genes (3, 15, 16). In this study, experiments were performed to determine whether down-regulation of GATA-2 correlates with the defect in phagocytosis of alveolar macrophages from P. carinii-infected hosts.
MATERIALS AND METHODS
Animal model.
Female Sprague-Dawley rats, 130 to 140 g in weight, were divided into three groups. The first group of rats were immunosuppressed with dexamethasone (Dex) at 1.8 mg/liter (0.36 mg/kg/day) in their drinking water; this group is referred to as Dex-suppressed rats. The second group of rats were P. carinii-infected rats; they were Dex suppressed and then infected with P. carinii. P. carinii infection in rats was achieved by transtracheal inoculation with 7.8 × 106 P. carinii trophozoites as previously described (1). The third group of rats were healthy rats, nonimmunosuppressed and noninfected.
Isolation of alveolar macrophages by BAL.
Bronchoalveolar lavage (BAL) and quantitation of lavaged cells were performed as described previously (19). To confirm that the lavaged cells were alveolar macrophages, the cells were cytospun onto a Superfrost+ slide (Fisher, Pittsburgh, Pa.) and then reacted with a mouse monoclonal antibody against the rat macrophage activator antigen (RMA) (BD PharMingen, San Diego, Calif.). This antibody reacts with a 120-kDa cell surface antigen found on alveolar macrophages and a small subset of pulmonary dendritic cells in rats (43). A 1:500 dilution of peroxidase-conjugated rabbit anti-mouse immunoglobulin G (Sigma Chemical Co., St. Louis, Mo.) was used as the secondary antibody. The bound antibody was visualized by reacting the cells with diaminobenzidine (DAB Liquid Substrate Dropper System; Sigma) for 2 min. The reaction signal was enhanced by addition of 0.03% (wt/vol) cobalt chloride to the diaminobenzidine solution according to the manufacturer's instructions.
Phagocytosis assay.
Two different substrates, radiolabeled P. carinii trophozoites and fluorescent latex beads, were used for the phagocytosis assay. Alveolar macrophages were incubated in suspension in complete medium at 37°C in a 5% CO2 atmosphere for 18 h before the phagocytosis assay. Viability of the cells was assessed by trypan blue exclusion at the time of harvest and after the phagocytosis assay. The cells were suspended in fresh medium to a concentration of one million macrophages per ml based on the percentage of cells that are alveolar macrophages in various BAL fluids (Table 1). Lymphoblasts were ignored, since they are not phagocytic and do not react with the macrophage-specific anti-RMA antibody.
TABLE 1.
Reaction of cells with anti-RMA antibody
| Source of alveolar macrophages | % of cells (mean ± SD) that reacted with anti-RMA antibody in:
|
|
|---|---|---|
| BAL fluid | Phagocytosis assay | |
| Normal rats | 98 ± 1.3 | 99 ± 0.1 |
| Dex-suppressed rats | 97 ± 1.8 | 99 ± 0.2 |
| P. carinii-infected rats | 43 ± 1.1 | 88 ± 1.0 |
P. carinii trophozoites were isolated from infected lungs as described previously (8) and then labeled by incubating 4 × 107 organisms in 2 ml of Dulbecco's minimum essential medium (DMEM) containing 250 μCi of ProMix reagent (Amersham Biosciences, Piscataway, N.J.) (containing a mixture of [35S]methionine and [35S]cysteine) for 18 h at 37°C with 5% CO2 as described by Limper et al. (21). Unincorporated label was removed from P. carinii by washing the organisms repeatedly in 500 μl of DMEM containing 1 mg of bovine serum albumin (BSA) per ml until an insignificant amount of radioactivity was detected in the wash.
To perform the phagocytosis assay, 5 × 106 radiolabeled P. carinii trophozoites in 1 ml of DMEM containing 1 mg of BSA per ml were incubated with 1 × 106 alveolar macrophages for 2 h at 37°C with 5% CO2. The alveolar macrophages were then pelleted at 400 × g for 5 min. This low-speed centrifugation was employed so that damage to the alveolar macrophages that would release internalized organisms would be minimal. P. carinii organisms that were bound to macrophages but not internalized were detached by incubating the cells with 1 ml of DMEM containing trypsin (2 μg/ml in 0.2 mg of EDTA per ml) for 15 min on ice. The alveolar macrophages in the trypsin reaction mixture were pelleted at 400 × g for 5 min, leaving P. carinii organisms in the supernatant. The cells in the pellet were resuspended and washed repeatedly with DMEM containing 1 mg of BSA per ml until an insignificant amount of radioactivity was detected in the wash. Radioactivity in the cell pellet after the wash represented P. carinii organisms that were phagocytosed by alveolar macrophages. Counts were converted to organism number by dividing the counts from the phagocytosed organisms by the average counts per organism. The average counts per P. carinii organism were the total number of counts in a control reaction divided by the total number (5 × 106) of P. carinii organisms added and were 1.3 ± 0.2 counts per organism. The background counts, averaging 92 ± 14 counts per assay, were subtracted from all results. All phagocytosis assays were performed in triplicate with three different sets of rats. Each set consisted at least three rats.
To perform the phagocytosis assay with fluorescent latex beads, 50 million fluorescein isothiocyanate (FITC)-conjugated, 1-μm-diameter, carboxylated latex beads (Sigma) were added to 1-ml aliquots of macrophages (106/ml) and incubated at 37°C with 5% CO2 for 2 h with gentle agitation every 10 min. The sample was then centrifuged through 3 ml of fetal bovine serum at 300 × g for 5 min at 25°C to remove any beads that were not phagocytosed. Approximately 50,000 cells were placed on a Superfrost+ slide (Fisher) by cytospinning (Cytospin II; Shandon, Pittsburgh, Pa.) at 750 rpm for 5 min at 25°C, stained with Giemsa stain, and examined under a fluorescence microscope (BH-2; Olympus, Tokyo, Japan) at a magnification of ×400. Duplicate slides of each group of macrophage samples were reacted with the anti-RMA antibody to determine the numbers of alveolar macrophages that were retained through the incubations and centrifugations of the assay mixture.
A count was generated by counting at least 300 random macrophages from at least 50 random fields. Macrophages containing beads as well as those not containing beads were used to obtain an average number of FITC-latex beads per macrophage. No FITC-latex beads were found on the slides in areas where there were no cells, indicating that centrifugation through 3 ml of fetal bovine serum adequately removed unphagocytosed latex beads. The cells were also examined with an LSM 510 confocal imaging system (Carl Zeiss Inc., Jena, Germany) and an Axiovert 100 M inverted microscope (Carl Zeiss Inc.) after 45 min of counterstaining with 5 μmol of Cell Tracker Orange (Molecular Probes, Eugene, Oreg.) per liter to determine whether beads were intracellularly located.
Cloning of the coding region of the rat GATA-2 gene.
Total RNA was isolated from the lungs of Sprague-Dawley rats by the method of Chomczynski and Sacchi (9). Reverse transcription was then performed to convert mRNA to cDNA by using an oligo(dT) primer. The cDNA thus generated was used as the template for PCR with GATA-2 primers (forward, 5′-ATGGAGGTGGCGCCTGAGCAG-3′ [nucleotides 155 to 175 of mouse GATA-2 cDNA; GenBank accession no. NM 008090]; reverse, 5′-CTAGCCCATGGCAGTCACCATG-3′ [nucleotides 1576 to 1597 of the same sequence]) to amplify the entire coding region of the GATA-2 gene. The PCR conditions were denaturation at 94°C for 30 s, annealing at 62°C for 30 s, and extension at 72°C for 2 min for 40 cycles. The PCR product was electrophoresed on 1% agarose, purified by elution with QIAEX II resin (Qiagen, Valencia, Calif.), and cloned into the TOPO TA vector (Invitrogen, Carlsbad, Calif.). The sequence and the orientation of the cloned fragment were determined by DNA sequencing.
Treatment of alveolar macrophages with GATA-2 antisense or sense oligonucleotides.
The nucleotide sequence of the GATA-2 gene was analyzed with the RNAdraw software package (24) to identify regions of the transcript where the secondary structure would allow hybridization of the transcript with the antisense oligonucleotide. The sequence located from nucleotide 628 to 645 (473 bp downstream from the initiation codon) of the mouse GATA-2 cDNA (GenBank accession no. NM 008090) was chosen. Both sense (5′-GCTGCAGTGGGGGTGAGG-3′) and antisense (5′-CCTCACCCCCACTGCAGC-3′) oligonucleotides were synthesized (Synthetic Genetics, San Diego, Calif.), using phosphorothioated deoxynucleoside triphosphates. The sense oligonucleotide was used as a control in each transfection experiment. The oligonucleotides were delivered to the cells complexed with the cationic lipid formulation Superfect (Qiagen). Fifteen micrograms of oligonucleotide was complexed with 30 μg of cationic lipid in a tube for 10 min at room temperature and then added to a 1-ml aliquot containing 106 alveolar macrophages. The cells were incubated with sense or antisense oligonucleotides for 6 h at 37°C with 5%CO2 and then treated with gamma interferon for 2 h and with lipopolysaccharide for 30 min prior to phagocytosis assay. An aliquot of alveolar macrophages was incubated with cationic lipid alone to control for any effects that this reagent may have on phagocytic ability.
Detection of GATA-2 protein by Western blotting.
Alveolar macrophages were lysed in 200 μl of lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 4 mM EDTA, 10 mM dithiothreitol, 1% Triton X-100, 10 μg of chymostatin per ml, 10 μg of leupeptin per ml, 10 μg of aprotinin per ml, 10 μg of pepstatin per ml, and 0.5 mM phenylmethylsulfonyl fluoride). The DNA was sheared by pulling the mixture through an 18-gauge needle twice, and the insoluble materials were pelleted by centrifugation at 14,000 × g for 2 min. The protein concentration in the supernatant was determined with the Coomassie Plus Protein Reagent (Pierce, Rockford, Ill.). Approximately 10 μg of the protein was electrophoresed on a 10% polyacrylamide gel (Nupage system; Invitrogen) and then transferred to a polyvinylidene difluoride membrane (Immobilin-P; Millipore, Bedford, Mass.). The blot was blocked with 5% nonfat milk in TBST (100 mM Tris-HCl [pH 7.5], 0.9% [wt/vol] NaCl, 0.1% [vol/vol] Tween 20) at 25°C for 1 h and then incubated with a 1:15,000 dilution of anti-GATA-2 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) or a 1:15,000 dilution of anti-glyceraldehyde-3-phosphate dehydrogenase (anti-GAPDH) antibody (Research Diagnostics, Inc., Flanders, N.J.) in TBS (TBST without Tween 20) supplemented with 5% nonfat dry milk for 1 h at 25°C. The blot was washed four times for a total of 30 min in TBST and then incubated with a 1:500 dilution (in TBS with 5% nonfat dry milk) of anti-mouse immunoglobulin G conjugated to horseradish peroxidase for 45 min at 25°C. After washing in TBST four times for a total of 30 min, the bands that reacted with the anti-GATA-2 or anti-GAPDH antibody were visualized by incubation for 2 min with equal parts of reagent A and reagent B of the ECL kit (Amersham). The excess luminescence reagent was rinsed off with water, and the blot was exposed to X-ray film for 30 s.
Statistics.
Determinations of significant statistical difference were made by using the one-way analysis of variance method for multiple samples in the SigmaStat software package (Jandel Scientific, San Rafael, Calif.).
Nucleotide sequence accession number.
The nucleotide sequence of the coding region of the rat GATA-2 gene has been deposited in GenBank (accession number AF345897).
RESULTS
Isolation of alveolar macrophages.
Cells were harvested from BAL fluids and reacted with the anti-RMA antibody to identify alveolar macrophages. Cells that reacted with the anti-RMA antibody showed a punctate blue or black precipitate on the cell membrane and were 20 to 25 μm in diameter with a large U-shaped nucleus and a distinct nucleolus, irregular and shaggy cell margins, and visible granules within the cytoplasm. These morphological properties are characteristic of macrophages. Cells that did not react with the anti-RMA antibody were easily identified as erythrocytes, lymphocytes, or polymorphonuclear cells. The results showed that virtually all of the nucleated cells in the BAL fluid from normal rats were alveolar macrophages (Table 1). A similar percentage of alveolar macrophages was seen in the BAL fluid from Dex-suppressed rats, but alveolar macrophages were only 40% of the total cell number in the BAL fluid from P. carinii-infected rats. Anti-RMA antibody reacted with cells cytospun on slides after the phagocytosis assays revealed that the great majority of cells retained through the incubations and centrifugations of the assay were alveolar macrophages (Table 1). Trypan blue exclusion showed that 96% of the lavage cells were viable. Percentages of alveolar macrophages in BAL fluid as determined by anti-RMA reactivity were used to ensure that equal numbers of alveolar macrophages were included in all phagocytosis assays. Nonphagocytic cells were not removed from the incubations, since they did not engulf P. carinii or latex beads and did not affect the results of phagocytosis assays.
Phagocytosis assay with labeled P. carinii and FITC-labeled beads.
A phagocytosis assay was performed to evaluate the phagocytic activity of alveolar macrophages from P. carinii-infected rats. Alveolar macrophages (1 × 106) isolated from BAL fluids of noninfected or P. carinii-infected rats were incubated with radiolabeled P. carinii trophozoites (4 × 106) in 1 ml of DMEM containing 1 mg of BSA per ml for 4 h at 37°C with 5% CO2. Alveolar macrophages were then pelleted, and the radioactivity in the pelleted cells was counted. After deduction of the counts derived from P. carinii that were nonspecifically bound to alveolar macrophages and those that were nonspecifically released from labeled organisms, the alveolar macrophage samples (1 × 106 cells each) from P. carinii-infected rats were found to phagocytose (1.1 ± 0.1) × 106 labeled P. carinii trophozoites (Fig. 1). In contrast, the same number of alveolar macrophages from Dex-suppressed rats phagocytosed (3.0 ± 0.2) × 106 labeled P. carinii trophozoites (Fig. 1). This result indicates that alveolar macrophages from P. carinii-infected rats phagocytosed 65% fewer labeled P. carinii organisms (P < 0.05) than those from Dex-suppressed rats. Alveolar macrophages from normal rats phagocytosed a similar number of labeled P. carinii trophozoites [(2.9 ± 0.3) × 106] as did those from Dex-suppressed rats (Fig. 1), suggesting that Dex treatment did not affect the phagocytic activity of alveolar macrophages. These results indicate that alveolar macrophages from normal rats phagocytosed an average of 2.9 trophozoites per macrophage and those from Dex-suppressed rats phagocytosed an average of 3.0 trophozoites per macrophage but that alveolar macrophages from P. carinii-infected rats phagocytosed only an average of 1.1 trophozoites per macrophage.
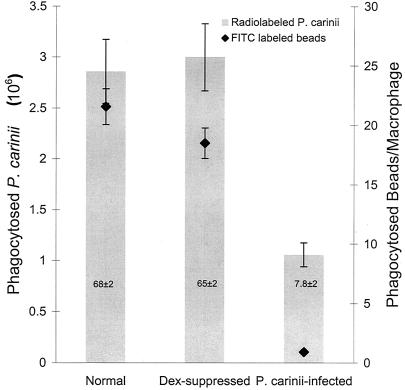
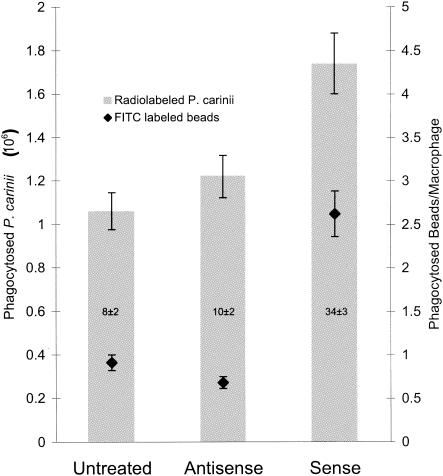
FIG. 1.
Phagocytosis of radiolabeled P. carinii and FITC-labeled latex beads by alveolar macrophages from rats. Alveolar macrophages from normal, Dex-suppressed, and P. carinii-infected rats were isolated and assayed for phagocytic activity with radiolabeled P. carinii trophozoites and FITC-labeled 1-μm-diameter latex beads. Bars represent phagocytosis of radiolabeled P. carinii, and diamonds represent phagocytosis of FITC-labeled latex beads. Numbers in the bars represent the percentage of phagocytically active macrophages from that condition. Results are expressed as the number of P. carinii trophozoites ingested by one million alveolar macrophages or the number of beads ingested per alveolar macrophage and are averages and standard deviations for triplicate reactions from at least three separate experiments.
The results of the experiments described above demonstrated that alveolar macrophages from P. carinii-infected hosts have a reduced ability in phagocytosis of P. carinii, consistent with that reported by Chen et al. (6). Since it was unknown whether this defect is specific for P. carinii or is a general defect in phagocytosis, experiments were performed to determine whether alveolar macrophages from P. carinii-infected rats could phagocytose FITC-latex beads. Alveolar macrophages were obtained from normal, Dex-suppressed, and P. carinii-infected rats. An average of 22 ± 3 beads per macrophage were counted in the macrophage sample from the normal rats (Fig. 1). The macrophage sample from the Dex-suppressed rats showed an average of 18 ± 1 beads per macrophage, and the macrophage sample from the P. carinii-infected rats had an average of only 1.0 ± 0.2 beads per macrophage (a 95% reduction compared to the sample from Dex-suppressed rats; P < 0.05) (Fig. 1).
In addition to changes in the phagocytic activity of cells that were active in phagocytosis, there were similar changes in the percentage of cells that showed any phagocytic activity. Greater than 65% of alveolar macrophages from normal or Dex-suppressed rats phagocytosed beads, but only 8% of alveolar macrophages from P. carinii-infected rats phagocytosed beads (Fig. 1). The results of this study indicate that alveolar macrophages from P. carinii-infected rats have a reduced ability to phagocytose and that the previously discovered inability of alveolar macrophages from P. carinii-infected animals to phagocytose is not specific for P. carinii but is a general defect in phagocytosis, since these alveolar macrophages are also defective in phagocytosis of FITC-latex beads.
Effect of GATA-2 antisense oligonucleotides on phagocytosis.
To determine whether down-regulation of the GATA-2 gene correlates with decreased phagocytic activity of alveolar macrophages, alveolar macrophages from noninfected rats were treated with GATA-2-specific antisense oligonucleotides and then assayed for phagocytic activity by using radiolabeled P. carinii organisms and FITC-latex beads. The cells were also treated with sense GATA-2 oligonucleotides to serve as controls. As described above, normal alveolar macrophages phagocytosed an average of (2.9 ± 0.3) × 106 labeled P. carinii trophozoites (Fig. 2). Treatment of these cells with the sense oligonucleotides did not affect their phagocytic activity; these alveolar macrophages phagocytosed an average of (3.0 ± 0.4) × 106 labeled P. carinii trophozoites (Fig. 2). In contrast, the phagocytic activity was dramatically reduced when these cells were treated with GATA-2 antisense oligonucleotides. An average of only (1.7 ± 0.1) × 106 labeled P. carinii trophozoites were phagocytosed (Fig. 2); this is a 44% decrease (P < 0.05) in phagocytic activity. A decrease in phagocytic activity was also observed on alveolar macrophages from Dex-suppressed rats when they were treated with antisense oligonucleotides. Alveolar macrophages from Dex-suppressed rats phagocytosed (2.8 ± 0.2) × 106 labeled P. carinii trophozoites when they were treated with sense oligonucleotides but phagocytosed only (1.5 ± 0.1) × 106 labeled P. carinii trophozoites when they were treated with antisense oligonucleotides (Fig. 2), a 46% decrease in phagocytic activity (P < 0.0001). Treatment of alveolar macrophages with either the sense or antisense oligonucleotides did not affect their viability, since the trypan blue exclusion assay showed no difference between cells incubated with phosphate-buffered saline (PBS) and those incubated with oligonucleotides.
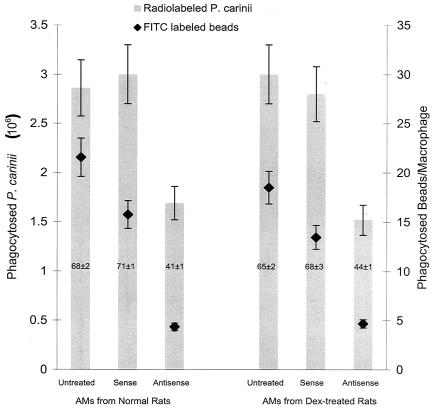
FIG. 2.
Phagocytosis of radiolabeled P. carinii organisms and FITC-labeled latex beads in alveolar macrophages treated with GATA-2 oligonucleotides. Alveolar macrophages (AMs) from normal and Dex-suppressed rats were treated with sense or antisense GATA-2 oligonucleotides. Bars represent phagocytosis of radiolabeled P. carinii, and diamonds represent phagocytosis of FITC-labeled latex beads. Numbers in the bars represent the percentage of phagocytically active macrophages from that condition. Results are expressed as the number of P. carinii trophozoites ingested by one million alveolar macrophages or the number of beads ingested per alveolar macrophage and are averages and standard deviations for triplicate reactions from at least three separate experiments.
The decrease in phagocytic activity by the GATA-2 antisense oligonucleotide was also demonstrated by using FITC-latex beads as the substrate for the phagocytosis assay. The experiment was again performed in triplicate. Alveolar macrophages from normal rats phagocytosed an average of 16 ± 1 beads per macrophage when they were treated with the sense GATA-2 oligonucleotide (Fig. 2). This level of phagocytosis is not statistically different from that by untreated alveolar macrophages, which phagocytosed 22 ± 3 beads per macrophage (Fig. 2). Alveolar macrophages from normal rats phagocytosed an average of 4.3 ± 0.3 beads when they were treated with GATA-2 antisense oligonucleotides, a 72% decrease in phagocytic activity compared to the same cells treated with sense GATA-2 oligonucleotides. Similar results were observed in samples from Dex-suppressed rats. Alveolar macrophages from Dex-suppressed rats phagocytosed 13 ± 1 beads per macrophage when they were treated with sense oligonucleotides but phagocytosed only 4.7 ± 1 beads per macrophage when they were treated with the antisense oligonucleotide. This is a 65% decrease in phagocytic activity caused by the antisense oligonucleotides (P < 0.0001).
In addition, cells treated with the antisense GATA-2 oligonucleotide had a 31% decrease in the number of cells that phagocytosed beads compared to those treated with the sense oligonucleotide (41 versus 72%) (Fig. 2). The percentage of sense oligonucleotide-treated cells that phagocytosed beads was 68%. Only 44% of antisense oligonucleotide-treated cells phagocytosed beads (a 24% decrease) (Fig. 2). Therefore, the decrease in GATA-2 transcription affected not only the activity of the alveolar macrophages but also the number of cells that showed phagocytic activity.
GATA-2 production in alveolar macrophages treated with antisense oligonucleotides.
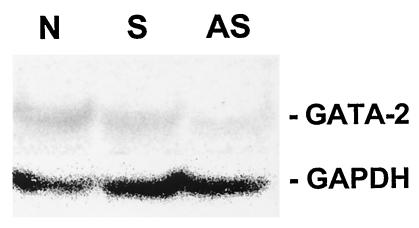
To ensure that GATA-2 antisense oligonucleotides decreased the production of the GATA-2 protein, alveolar macrophages treated with GATA-2 antisense oligonucleotides were examined by Western blotting with an anti-GATA-2 antibody. As an internal control, the same blot was reacted with an anti-GAPDH antibody. Alveolar macrophages were isolated from normal rats. An aliquot of 1 × 106 alveolar macrophages was treated with 15 μg of GATA-2 antisense oligonucleotides. In a separate reaction, a second aliquot of the same number of alveolar macrophages was treated with 15 μg of sense oligonucleotides as a control. Alveolar macrophages treated with only PBS were also used as another control. After 6 h of incubation with sense or antisense oligonucleotides, Western blotting was performed. A 45-kDa protein band was observed in samples from cells treated with PBS or sense oligonucleotides, whereas this band was barely visible in the sample from cells treated with antisense oligonucleotides. In contrast, the GAPDH band was present at approximately equal intensities in all three samples (Fig. 3). These results demonstrated that anti-GATA-2 antisense oligonucleotides did enter the cells and inhibited the production of the GATA-2 protein.
FIG. 3.
Production of GATA-2 protein in rat alveolar macrophages incubated with GATA-2 antisense oligonucleotide. Alveolar macrophages from normal rats were treated with PBS (lane N) or with sense (lane S) or antisense (lane AS) GATA-2 oligonucleotides and then examined for GATA-2 production by Western blotting with an antibody against the rat GATA-2 protein. The same blot was also reacted with an antibody against the GAPDH protein.
Complementation of GATA-2 in alveolar macrophages from P. carinii-infected rats.
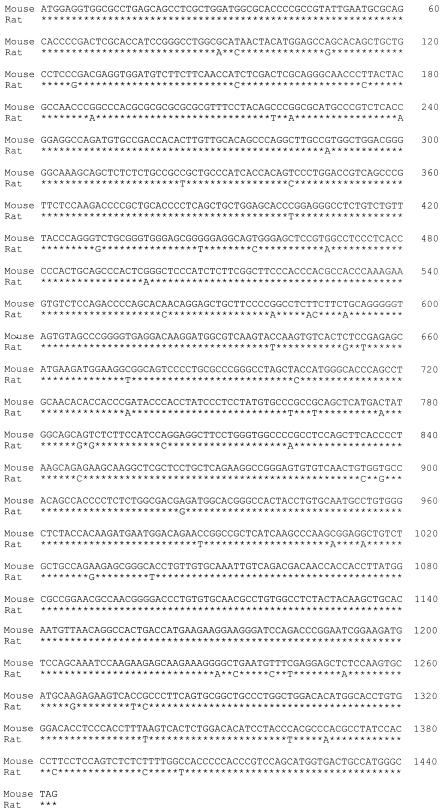
To determine whether the defect in phagocytosis can be corrected, a GATA-2 expression vector was introduced into alveolar macrophages from P. carinii-infected rats. In order to construct the GATA-2 expression vector, the complete coding region of the rat GATA-2 gene was first obtained by reverse transcription-PCR with GATA-2-specific primers. The PCR products were cloned into the TOPO TA cloning vector (Invitrogen). The sequence and the orientation of the cloned fragment were determined by DNA sequencing. The rat GATA-2-coding region was found to be 1,440 bp, encoding 480 amino acids. This sequence differed from that of the mouse GATA-2 gene by 60 nucleotides (Fig. 4) and 7 amino acids (Fig. 5).
FIG. 4.
Nucleotide sequence comparison of the coding regions of the rat and mouse GATA-2 genes. The coding regions of these two genes have exactly the same number of nucleotides. The nucleic acid sequence of the mouse GATA-2 gene is shown in the top lines, and differences in the rat GATA-2 sequence are shown in the bottom lines. Position numbers of nucleotides are shown on the right.
FIG. 5.
Comparison of deduced amino acid sequences of mouse and rat GATA-2. One-letter amino acid codes are used. The amino acid sequence of the mouse GATA-2 gene is shown in the top lines, and differences in the rat GATA-2 sequence are shown in the bottom lines. Amino acid sequences in boldface are the two zinc finger DNA-binding domains of GATA-2 (positions 295 to 319 and 349 to 373). Position numbers of amino acids are shown on the right.
Both sense- and antisense-orientated TOPO TA GATA-2 clones were selected. The XhoI and HindIII DNA fragments containing the GATA-2 gene in the sense or antisense orientation were subcloned into the corresponding sites of pCEP4 (Invitrogen) so that the expression of the GATA-2 gene was driven by the cytomegalovirus (CMV) promoter. pCEP4 recombinant clones, pGATA2sense and pGATA2antisense, with the GATA-2 gene in the same or opposite orientation as the CMV promoter were further confirmed by DNA sequencing. These two GATA-2 constructs were then introduced into alveolar macrophages from P. carinii-infected rats by transfection using liposomes. Cells transfected with pGATA2antisense served as the negative control. The GATA-2 expression constructs pGATA2sense (15 μg) were incubated with 25 μg of Superfect cationic liposome (Qiagen) for 10 min at room temperature. Alveolar macrophages were incubated with this complex for 24 h and then assayed for phagocytosis.
Nontransfected alveolar macrophages from P. carinii-infected rats phagocytosed (1.1 ± 0.1) × 106 labeled P. carinii trophozoites. When these cells were transfected with the sense construct pGATA2sense, they phagocytosed (1.7 ± 0.2) × 106 labeled P. carinii trophozoites, a 68% increase (P < 0.05) in phagocytic activity (Fig. 6). No significant increase in phagocytic activity was observed when the cells were transfected with the control plasmid pGATA2antisense. These cells phagocytosed (1.2 ± 0.2) × 106 labeled P. carinii trophozoites (Fig. 6).
FIG. 6.
Phagocytosis of radiolabeled P. carinii organisms and FITC-labeled latex beads in alveolar macrophages treated with GATA-2 expression vectors. Alveolar macrophages from P. carinii-infected rats were treated with sense or antisense GATA-2 expression vectors. Bars represent phagocytosis of radiolabeled P. carinii, and diamonds represent phagocytosis of FITC-labeled latex beads. Numbers in the bars represent the percentage of phagocytically active macrophages from that condition. Results are expressed as the number of P. carinii trophozoites ingested by one million alveolar macrophages or the number of beads ingested per alveolar macrophage and are averages and standard deviations for triplicate reactions from at least three separate experiments.
Similar results were obtained in phagocytosis experiments performed with FITC-latex beads. Alveolar macrophages from P. carinii-infected rats transfected with antisense construct pGATA2antisense or Superfect alone phagocytosed similar numbers of FITC-latex beads (0.68 ± 0.1 and 0.91 ± 0.1 beads/cell, respectively) (Fig. 6). The same cells transfected with the sense GATA-2 expression construct pGATA2sense regained some phagocytic function, as noted by their increased uptake of FITC-latex beads (2.6 ± 0.3 beads/cell) (Fig. 6). This 280% increase (P < 0.05 versus the antisense construct) indicates that GATA-2 plays an important role in the regulation of phagocytosis.
The number of alveolar macrophages that phagocytosed FITC beads was also increased by the introduction of the GATA-2 expression vector. Only 7.8% ± 2.4% of cells from P. carinii-infected rats phagocytosed beads; this value was essentially unchanged in cells transfected with the GATA-2 antisense vector (9.8% ± 1.8%) (Fig. 6). In contrast, introduction of the GATA-2 overexpression vector pGATA2sense increased the number of alveolar macrophages that phagocytosed beads to 34% ± 2.8% (Fig. 6).
Determination of percentage of cells transfected.
To determine the percentage of cells that were transfected and had taken up the GATA-2 expression construct, the uptake of a plasmid carrying a green fluorescent protein (GFP) gene was monitored in alveolar macrophages from Dex-suppressed and P. carinii-infected rats. Alveolar macrophages were lavaged from Dex-suppressed and P. carinii-infected rats. These alveolar macrophages were adjusted to 106 cells per well on a slide chamber in complete medium and then incubated for 24 h with 15 μg of pTracer-EF vector (Invitrogen) complexed to 25 μg of SuperFect cationic liposome (Qiagen). The pTracer-EF vector has a GFP-zeocin recombinant gene that is transcribed under the regulation of the CMV promoter. After washing in PBS, the wells were removed and the slides were dried and examined by fluorescence microscopy. The results of two trials indicate that approximately 29% of alveolar macrophages from Dex-suppressed rats and 23% of those from P. carinii-infected rats took up the GFP construct.
Increase in GATA-2 production in alveolar macrophages transfected with the GATA-2 overexpression vector.
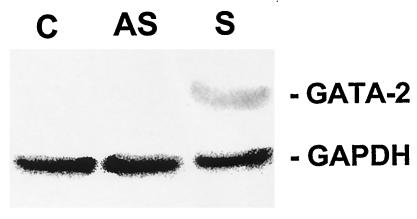
To ascertain that the transfected GATA-2 gene was expressed, pGATA2sense- and pGATA2antisense-transfected alveolar macrophages from P. carinii-infected rats were examined for the production of the GATA-2 protein by Western blotting. The procedures were similar to those used to examine the effect of GATA-2 antisense oligonucleotides on GATA-2 production. An aliquot of 106 alveolar macrophages from P. carinii-infected rats was transfected with pGATA2sense, pGATA2antisense, or the vector pCEP4. Twenty-four hours after transfection, the cells were lysed and examined by Western blotting with an anti-GATA-2 antibody. The same blot was also reacted with anti-GAPDH antibody as described above. As shown in Fig. 7 the 45-kDa GATA-2 protein was detected in the sample derived from cells transfected with pGATA2sense but not in that derived from cells transfected with pGATA2antisense or the vector pCEP4. Since all three samples exhibited GAPDH bands of the same intensity, the results indicate that cells transfected with pGATA2sense did produce the GATA-2 protein. The fact that cells transfected with pGATA2antisense or pCEP4 did not have a detectable 45-kDa GATA-2 protein indicates that the 45-kDa protein found in cells transfected with pGATA2sense was expressed from the GATA-2 expression vector pGATA2sense.
FIG. 7.
Expression of GATA-2 in alveolar macrophages from P. carinii-infected rats incubated with GATA-2 expression vector. Alveolar macrophages from Dex-suppressed rats were transfected with the vector pCEP4 (lane C), pGATA2antisense (lane AS), or pGATA2sense (lane S) and then examined for GATA-2 production by Western blotting with an antibody against the rat GATA-2 protein. The same blot was also reacted with an antibody against the GAPDH protein.
DISCUSSION
In this study, the defect in phagocytosis of P. carinii by alveolar macrophages from P. carinii-infected rats was examined. Alveolar macrophages from P. carinii-infected hosts were found to have a profound decrease in phagocytic activity compared to those from noninfected hosts. This defect is not specific for P. carinii organisms but is a general defect in phagocytosis, because alveolar macrophages from P. carinii-infected hosts were also defective in phagocytosis of FITC-latex beads (Fig. 1). Since FITC-latex beads are commonly used to assay nonopsonized, scavenger receptor-mediated phagocytosis (17, 31), these results suggest that in addition to the mannose receptor-mediated phagocytosis (18), the scavenger receptor-mediated phagocytosis is also defective in alveolar macrophages from P. carinii-infected hosts.
Since the expression of the GATA-2 gene is severely down-regulated during P. carinii infection (39), we hypothesized that the defect in phagocytosis was a result of GATA-2 down-regulation. This hypothesis was proven in this study by showing that anti-GATA-2 oligonucleotides caused a decrease in the phagocytic activity of alveolar macrophages from both normal and Dex-suppressed rats (Fig. 2). The effect of GATA-2 antisense oligonucleotides on GATA-2 production was confirmed by Western blotting with an anti-GATA-2 antibody. A decrease in GATA-2 production was observed in cells transfected with the antisense oligonucleotides but not in those transfected with the sense oligonucleotides (Fig. 3). The hypothesis was further proven by the demonstration that overexpression of the GATA-2 gene in alveolar macrophages from P. carinii-infected hosts significantly restored their phagocytic activity (Fig. 6). In this experiment, overexpression of GATA-2 in alveolar macrophages from P. carinii-infected rats was achieved by introducing a vector (pGATA2sense) containing the rat GATA-2 gene driven by the CMV immediate-early promoter into the cells, and a profound increase (280% increase with FITC-latex beads and 66% increase with labeled P. carinii) in the phagocytic activity of these cells was observed compared to those transfected with the control vector (pGATA2antisense) on which the GATA-2 gene is oriented in the opposite direction to the CMV promoter. GATA-2 appears to have a greater effect on the phagocytosis of FITC-latex beads than P. carinii organisms. This difference may be due to different receptors used to phagocytose these two substrates.
To determine the efficiency of the transfection method used in this study, we transfected pTracer containing the GFP gene into alveolar macrophages from P. carinii-infected cells and found that 23% of these cells took up pTracer. This result agrees well with the phagocytosis assay performed with FITC-latex beads. In this experiment, a 23% increase in the number of alveolar macrophages from P. carinii-infected rats transfected with the GATA-2 expression vector that phagocytosed FITC-latex beads was observed (Fig. 6). The transfection efficiency of alveolar macrophages from Dex-suppressed rats was slightly higher; 29% of these cells took up pTracer. The increase in GATA-2 production in alveolar macrophages from P. carinii-infected rats transfected with the GATA-2 expression vector was also confirmed by Western blotting with an anti-GATA-2 antibody. The GATA-2 protein was not detected in nontransfected cells or in cells transfected with the control vector (pGATA2antisense) but was detected in cells transfected with pGATA2sense.
These results imply that alveolar macrophages from P. carinii-infected hosts would become phagocytically active if the expression of the GATA-2 gene could be reactivated. GATA-2 also appears to affect the number of alveolar macrophages that are phagocytically active, since the percentage of cells that phagocytosed any FITC-latex beads was severely decreased both in alveolar macrophages from P. carinii-infected rats and in normal alveolar macrophages transfected with GATA-2 antisense oligonucleotide (Fig. 1 and 2). These results indicate that the loss of GATA-2 production not only reduces the phagocytic activity in many cells but also completely turns off the phagocytic activity in some cells. We also observed the opposite with GATA-2 overexpression in alveolar macrophages from P. carinii-infected rats. The phagocytic activity of some of these cells was increased, and some cells were transformed from completely phagocytically inactive to active due to GATA-2 overexpression. These results suggest that restoration of GATA-2 will increase the number of phagocytically active alveolar macrophages to clear the offending P. carinii.
It has been shown that the expression of the GATA-2 gene can be induced by N(G)-monomethyl-l-arginine in Hep3B cells (40), by trichostatin A in lung adenocarcinoma cells (10), and by insulin-like growth factor-1 in skeletal muscle cells (27). Whether these substances will induce GATA-2 expression in alveolar macrophages remains to be investigated. Gene therapy approaches may also be employed to supply GATA-2. A GATA-2 expression construct similar to the one used in this study may be used if means to deliver a construct specifically into alveolar macrophages can be developed. These approaches may become alternative therapeutic methods for Pneumocystis pneumonias.
GATA-2 is a zinc finger transcription factor of the GATA family (27). It plays a crucial role in hematopoiesis (33, 40-42), urogenital development (44), and neurogenesis (2, 45). However, it has been shown not to be involved in the differentiation of macrophages (7, 42). The mechanisms by which GATA-2 regulates phagocytosis are completely unknown and are being investigated. Macrophages are often stimulated by gamma interferon, interleukin-1β, tumor necrosis factor alpha, and interleukin-6 released by CD4+ and/or NK cells (12, 28, 32, 35). Since the number of CD4+ cells in the Pneumocystis-infected host is greatly reduced, the effect of CD4+ cells on phagocytosis of alveolar macrophages may be minimal. This may be a reason why alveolar macrophages from Pneumocystis-infected hosts are not active in phagocytosis.
We conclude from the results of this study that the defects in alveolar macrophages from P. carinii-infected hosts include more than one type of receptor-mediated phagocytosis. We also conclude that the transcription factor GATA-2 regulates phagocytosis via at least one type of receptor in alveolar macrophages. It is conceivable that multiple defects rendering them unable to phagocytose exist in alveolar macrophages from P. carinii-infected hosts. It is quite possible that GATA-2 regulates many other functions. Down-regulation of the GATA-2 gene would therefore disable all of those functions. This could well be a mechanism by which P. carinii survives in the host.
Acknowledgments
This work was supported by NIH grant RO1 HL65170.
Editor: T. R. Kozel
REFERENCES
- 1.Bartlett, M. S., J. A. Fishman, M. M. Durkin, S. F. Queener, and J. W. Smith. 1988. A new rat model of Pneumocystis carinii infection. J. Clin. Microbiol. 26:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell, E., A. Lumsden, and A. Graham. 1999. Expression of GATA-2 in the developing avian rhombencephalon. Mech. Dev. 84:173-176. [DOI] [PubMed] [Google Scholar]
- 3.Bouhassira, E. E., M. F. Kielman, J. Gilman, M. F. Fabry, S. Suzuka, O. Leone, E. Gikas, L. F. Bernini, and R. L. Nagel. 1997. Properties of the mouse alpha-globin HS-26: relationship to HS-40, the major enhancer of human alpha-globin gene expression. Am. J. Hematol. 54:30-39. [DOI] [PubMed] [Google Scholar]
- 4.Castro, M., T. I. Morgenthaler, O. A. Hoffman, J. E. Standing, M. S. Rohrbach, and A. H. Limper. 1993. Pneumocystis carinii induces the release of arachidonic acid and its metabolites from alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 9:73-81. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1997. AIDS-indicator conditions diagnosed in patients reported in 1996, by age group, United States. HIV/AIDS Surveillance Rep. 8:18. [Google Scholar]
- 6.Chen, W., J. W. Mills, and A. G. Harmsen. 1992. Development and resolution of Pneumocystis carinii pneumonia in severe combined immunodeficient mice: a morphological study of host inflammatory responses. Int. J. Exp. Pathol. 73:709-720. [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng, T., H. Shen, D. Giokas, J. Gere, D. G. Tenen, and D. T. Scadden. 1996. Temporal mapping of gene expression levels during the differentiation of individual primary hematopoietic cells. Proc. Natl. Acad. Sci. USA 93:13158-13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chin, K., S. Merali, M. M. Shaw, M. S. Bartlett, and A. B. Clarkson, Jr. 1996. Subpopulations of Pneumocystis carinii separated by a Percoll gradient. J. Eukaryot. Microbiol. 43:53s.. [DOI] [PubMed]
- 9.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 10.Eickhoff, B., S. Ruller, T. Laue, G. Kohler, C. Stahl, M. Schlaak, and J. van der Bosch. 2000. Trichostatin A modulates expression of p21waf/cipl, Bcl-xL, ID1, ID2, ID3, CRAB2, GATA-2, hsp86 and TFIID/TAFII31 mRNA in human lung adenocarcinoma cells. Biol. Chem. 381:107-112. [DOI] [PubMed] [Google Scholar]
- 11.Ezekowitz, R. A. B., D. J. Williams, H. Koziel, M. Y. K. Armstrong, A. Warner, F. F. Richards, and R. M. Rose. 1991. Uptake of Pneumocystis carinii mediated by the macrophage mannose receptor. Nature 351:155-158. [DOI] [PubMed] [Google Scholar]
- 12.Garvy, B. A., R. A. Ezekowitz, and A. G. Harmsen. 1997. Role of gamma interferon in the host immune response and inflammatory responses to Pneumocystis carinii infection. Infect. Immun. 65:373-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hidalgo, H. A., R. J. Helmke, V. F. German, and J. A. Mangos. 1992. Pneumocystis carinii induces an oxidative burst in alveolar macrophages. Infect. Immun. 60:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman, O. A., J. E. Standing, and A. H. Limper. 1993. Pneumocystis carinii stimulates tumor necrosis factor-alpha release from alveolar macrophages through a beta-glucan-mediated mechanism. J. Immunol. 150:3932-3940. [PubMed] [Google Scholar]
- 15.Imagawa, S., M. Yamamoto, and Y. Miura. 1997. Negative regulation of the erythropoietin gene expression by the GATA transcription factors. Blood 89:1430-1439. [PubMed] [Google Scholar]
- 16.Kawana, M., M. E. Lee, E. E. Quertermous, and T. Quertermous. 1995. Cooperative interaction of GATA-2 and AP-1 regulates transcription of the endothelin-1 gene. Mol. Cell. Biol. 15:4225-4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobzik, L. 1995. Lung macrophage uptake of unopsonized environmental particles. Role of scavenger-type receptors. J. Immunol. 155:367-376. [PubMed] [Google Scholar]
- 18.Koziel, H., Q. Eichbaum, B. A. Kruskal, P. Pinkston, R. A. Rogers, M. Y. Armstrong, F. F. Richards, R. M. Rose, and R. A. Ezekowitz. 1998. Reduced binding and phagocytosis of Pneumocystis carinii by alveolar macrophages from persons infected with HIV-1 correlates with mannose receptor down regulation. J. Clin. Investig. 102:1332-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasbury, M. E., P. J. Durant, and C. H. Lee. 2003. Correlation of Pneumocystis carinii burden and alveolar macrophage number during infection and recovery. Clin. Diagn. Lab. Immunol. 10:293-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Limper, A. H. 1998. Alveolar macrophage and glycoprotein responses to Pneumocystis carinii. Semin. Respir. Infect. 13:339-347. [PubMed] [Google Scholar]
- 21.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. The role of alveolar macrophages in Pneumocystis carinii degradation and clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandujano, J. F., N. B. D'Souza, S. Nelson, W. R. Summer, R. C. Beckerman, and J. E. Shellito. 1995. Granulocyte-macrophage colony stimulating factor in Pneumocystis carinii pneumonia in mice. Am. J. Respir. Crit. Care Med. 151:1233-1238. [DOI] [PubMed] [Google Scholar]
- 23.Masur, H., and T. C. Jones. 1978. The interaction in vitro of Pneumocystis carinii with macrophages and L-cells. J. Exp. Med. 147:157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzura, O., and A. Wennborg. 1996. RNAdraw: an integrated program for RNA secondary structure calculation and analysis under 32-bit Microsoft Windows. Comput. Appl. Biosci. 12:247-249. [DOI] [PubMed] [Google Scholar]
- 25.Miller, R. 1998. Clinical aspects of Pneumocystis carinii pneumonia in HIV-infected patients: 1997. FEMS Immunol. Med. Microbiol. 22:103-105. [DOI] [PubMed] [Google Scholar]
- 26.Murray, J. F., C. P. Felton, S. M. Garay, M. S. Gottlieb, P. C. Hopewell, D. E. Stover, and A. S. Teirstein. 1984. Pulmonary complications of the acquired immunodeficiency syndrome. N. Engl. J. Med. 310:1682-1688. [DOI] [PubMed] [Google Scholar]
- 27.Musaro, A., K. J. McCullagh, F. J. Naya, E. N. Olson, and N. Rosenthal. 1999. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581-585. [DOI] [PubMed] [Google Scholar]
- 28.Nakamoto, A., and K. Kitsukawa. 1992. Human lymphocyte proliferative response and gamma-interferon production to Pneumocystis carinii antigen. J. Jpn. Assoc. Infect. Dis. 66:1651-1659. [DOI] [PubMed] [Google Scholar]
- 29.Neese, L. W., J. E. Standing, E. J. Olson, M. Castro, and A. H. Limper. 1994. Vitronectin, fibronectin, and gp120 antibody enhance macrophage release of TNF-alpha in response to Pneumocystis carinii. J. Immunol. 152:4549-4556. [PubMed] [Google Scholar]
- 30.Paine, R., III, A. M. Preston, S. Wilcoxen, H. Jin, B. B. Siu, S. B. Morris, J. A. Reed, G. Ross, J. A. Whitsett, and J. M. Beck. 2000. Granulocyte-macrophage colony-stimulating factor in the innate immune response to Pneumocystis carinii pneumonia in mice. J. Immunol. 164:2602-2609. [DOI] [PubMed] [Google Scholar]
- 31.Palecanda, A., J. Paulauskis, E. Al-Mutairi, A. Imrich, G. Qin, H. Suzuki, T. Kodama, K. Tryggvason, H. Koziel, and L. Kobzik. 1999. Role of the scavenger receptor MARCO in alveolar macrophage binding of unopsonized environmental particles. J. Exp. Med. 189:1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pesanti, E. L. 1991. Interaction of cytokines and alveolar cells with Pneumocystis carinii in vitro. J. Infect. Dis. 163:611-616. [DOI] [PubMed] [Google Scholar]
- 33.Pevny, L., M. C. Simon, E. Robertson, W. H. Klein, S. F. Tsai, V. D'Agati, S. H. Orkin, and F. Costantini. 1991. Erythroid differentiation in chimeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature 349:257-260. [DOI] [PubMed] [Google Scholar]
- 34.Roths, J. B., J. D. Marshall, R. D. Allen, G. A. Carson, and C. L. Sidman. 1990. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant SCID mice. Am. J. Pathol. 136:1173-1186. [PMC free article] [PubMed] [Google Scholar]
- 35.Rudmann, D. G., A. M. Preston, M. W. Moore, and J. M. Beck. 1998. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-γ and type 1 and 2 TNF receptor genes. J. Immunol. 161:360-366. [PubMed] [Google Scholar]
- 36.Shellito, J., V. V. Suzara, W. Blumenfeld, J. M. Beck, H. J. Steger, and T. H. Ermak. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J. Clin. Investig. 85:1686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stehle, S. E., R. A. Rogers, A. G. Harmsen, and R. A. Ezekowitz. 2000. A soluble mannose receptor immunoadhesin enhances phagocytosis of Pneumocystis carinii by human polymorphonuclear leukocytes in vitro. Scand. J. Immunol. 52:131-137. [DOI] [PubMed] [Google Scholar]
- 38.Stringer, J. R., C. B. Beard, R. F. Miller, and A. E. Wakefield. 2002. A new name (Pneumocystis jiroveci) for Pneumocystis from humans. Emerg. Infect. Dis. 8:891-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang, X., M. E. Lasbury, D. D. Davidson, M. S. Bartlett, J. W. Smith, and C. H. Lee. 2000. Down-regulation of GATA-2 transcription factor during Pneumocystis carinii infection. Infect. Immun. 68:4720-4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tarumoto, T., S. Imagawa, K. Ohmine, T. Nagai, M. Higuchi, N. Imai, N. Suzuki, M. Yamamoto, and K. Ozawa. 2000. N(G)-monomethyl-l-arginine inhibits erythropoietin gene expression by stimulating GATA-2. Blood 96:1716-1722. [PubMed] [Google Scholar]
- 41.Tsai, F.-Y., G. Keller, F. C. Kuo, M. Weiss, J. Chen, M. Rosenblatt, F. W. Alt, and S. H. Orkin. 1994. An early hematopoietic defect in mice lacking the transcription factor GATA-2. Nature 371:221-226. [DOI] [PubMed] [Google Scholar]
- 42.Tsai, F.-Y., and S. H. Orkin. 1997. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood 89:3636-3643. [PubMed] [Google Scholar]
- 43.Yamin, M., D. Lazarus, E. E. Schneeburger, K. McCarthy, W. Xia, and R. Kradin. 1990. Anti-RMA: a murine antibody that activates rat macrophages. I. Distribution and characterization of the RMA antigen. Am. J. Respir. Cell. Mol. Biol. 2:207-215. [DOI] [PubMed] [Google Scholar]
- 44.Zhou, Y., K. C. Lim, K. Onodera, S. Takahashi, J. Ohta, N. Minegishi, F. Y. Tsai, S. H. Orkin, M. Yamamoto, and J. D. Engel. 1998. Rescue of the embryonic lethal hematopoietic defect reveals a critical role for GATA-2 in urogenital development. EMBO J. 17:6689-6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou, Y., M. Yamamoto, and J. D. Engel. 2000. GATA-2 is required for the generation of V2 interneurons. Development 127:3829-3838. [DOI] [PubMed] [Google Scholar]