Abstract
Cryptococcus neoformans is the etiologic agent of cryptococcosis. Two mating types exist in this fungus, MATα and MATa. The CPRa gene of C. neoformans is a MATa strain-specific gene and encodes a putative seven-transmembrane domain pheromone receptor. Unlike the other reported fungal pheromone receptors, CPRa shows functional diversity. Deletion of CPRa drastically affects mating efficiency but does not abolish mating. CPRa expression is developmentally regulated and is not affected by deletion of the transcriptional regulator STE12a. The expression of CPRa is markedly increased by shifting cultures from liquid to solid media. CPRa also plays a significant role in virulence. Δcpra cells produce smaller capsules in the brains of mice than the wild-type cells, and the mice infected with Δcpra survive significantly longer than those receiving the wild-type strain. Our results suggest that the MATa pheromone receptor of C. neoformans is not only required for mating but also important for survival and growth of the fungus in host tissue.
Cryptococcus neoformans is the etiologic agent of cryptococcosis, one of the most serious fungal diseases encountered by immunocompromised patients worldwide (16). This fungus is a bipolar heterothallic species in which mating is controlled by two alleles, MATα and MATa (15). The sexual reproduction cycle begins when two strains of the opposite mating type are crossed under nutrient deprivation conditions in the laboratory. Hyphal cells can be detected within a few hours after crossing. Typical basidiomycetous clamp connections, basidia, and basidiospores are formed within 24 h in crosses of strains with a high mating efficiency. Basidiospores germinate to produce haploid yeast cells which multiply by polar budding.
The mating pathway of Saccharomyces cerevisiae has been well studied (14, 18, 26). It is known that haploid cells mate with cells of the opposite mating type through a complex process that involves the production of mating-type-specific peptide pheromones and pheromone receptors. These receptors belong to a large class of seven-transmembrane domain receptors and are mostly expressed at the tip of shmoos where cell fusions subsequently occur. These receptors are coupled to a set of heterotrimeric G proteins and a mitogen-activated protein (MAP) kinase cascade. Transduction of the signal by the MAP kinase cascade leads to activation of the transcription factor Ste12p, which in turn promotes the expression of genes involved in mating-specific functions.
In C. neoformans, MATα-specific homologs of several S. cerevisiae genes involved in mating, such as STE20α, STE11α, and STE12α, three copies of the MATα pheromone gene (MFα), as well as the pheromone receptor gene (CPRα), were found to be imbedded in the MATα locus (10, 13, 19, 20, 30). In addition, a non-mating-type-specific G-protein β subunit was also identified and it was thought to control the pheromone response MAP kinase cascade in C. neoformans (29). The initial interaction between cells of the two compatible mating strains is believed to involve pheromone-receptor pairs of both mating types (23). As with other known pheromone receptors in basidiomycetes, C. neoformans CPRα contains seven potential membrane-spanning domains characteristic of receptors that couple to heterotrimeric G proteins (9). In homobasidiomycete species such as Coprinus cinereus and Schizophyllum commune, pheromones and receptors play no role in mate attraction and fusion but promote the formation and maintenance of a dikaryon after cell fusion (3). In Ustilago maydis, a heterobasidiomycete species, pheromones and receptors are essential for mate attraction and haploid cell fusion, which lead to the formation of infectious dikaryons (2). For this reason, pheromone and receptor genes of U. maydis are considered pathogenicity genes.
Deletion of CPRα from C. neoformans resulted in a marked decrease in mating efficiency, demonstrating its importance in mating (9). In addition, the ability to sense the presence of synthetic MATa pheromone was dramatically reduced in Δcprα cells. The role of the pheromone receptor gene of C. neoformans in virulence, however, was not considered (9). It has been shown that components of the pheromone response MAP kinase cascade are important for mating but not required for virulence (J. Heitman, personal communication). Recent studies on MATα- and MATa-specific genes showed that both STE12α and STE12a are important for virulence in serotype D strains of C. neoformans (7, 8). These studies suggest that members of mating-type-specific gene families are not only important for saprobic reproduction but also play an important role in the survival of the organism in host tissue. To further our understanding of the functions of MATa-specific genes, we isolated and characterized a MATa-specific pheromone receptor gene, CPRa. The CPRa gene showed unusual expression patterns at different developmental stages as well as under different environmental conditions. Our results suggested that mechanisms such as physical or nutritional sensing may have evolved in C. neoformans to regulate pheromone receptor signaling. As a result, the pheromone receptor of C. neoformans plays an important role in mating as well as in virulence.
MATERIALS AND METHODS
Strains, media, synthetic pheromones, and general methods.
Strains used for this study are as follows: B-4476 (also called JEC20, MATa, congenic strain of B-4500), B-4476FO5 (MATa ura5), C468 (MATa ura5 ade2 Δcpra::CPRa), C481 (MATa Δcpra::ADE2), C486 (MATa Δcpra::CPRa), C525 (MATa lys1 Δcpra::ADE2), C542 (MATα ura5 ade2 Δcprα::ADE2), JE31 (MATα lys1), JEC33 (MATα lys2) (7), LP8 (MATa ura5 ade2), TYCC384 (MATα ura5 ade2 Δste12α::ADE2) (7), TYCC419 (MATa ura5 ade2 Δcpra::ADE2). Crosses were performed on V-8 juice agar. To prepare V-8 liquid medium, agar was omitted (17) and the solution was autoclaved and filtered to remove particulate debris. Yeast extract-peptone-dextrose (YEPD) and RPMI agar were described previously (4). Culture conditions for growth on RPMI agar were 30 or 37°C with or without 5% CO2. Minimal media (YNB) contained 6.7 g of yeast nitrogen base without amino acids (Difco) with 20 g of glucose (pH 7.0). The presumptive farnesylated MFa pheromone (EEAYGSGQGPTYSC) and the MFα (QEAHPGGMTLC) pheromone were chemically synthesized based on the method described previously (9, 23). The quantitative determination of mating frequency was described previously (8). Briefly, two auxotrophic MATα strains (JEC31 and JEC33) were used as tester strains to determine the mating frequency of any given MATa strain carrying different auxotrophic markers. The relative mating frequency was expressed as a percentage of the mating frequency of the reference strain (LP8). The data represent the average of results derived from mating of MATa strains with JEC31 as well as with JEC33.
Identification of the CPRa gene.
The plasmid pYCC365 was a CPRa genomic clone obtained by screening a JEC20 genomic library constructed in the λSHlox vector (gift of J. Edman) with a STE12a probe, pYCC360 (7). The cDNA clones of CPRa were isolated from a B-3502 cDNA library by PCR. The GenBank accession number for CPRa is AF250141.
Deletion and reconstruction of CPRa.
The 3.0-kb EcoRI/XbaI ADE2 fragment of pYCC123 (5) was cloned into the PstI/SpeI site of pYCC365 to yield pYCC412. The 2.0-kb ApaI/NotI URA5 fragment of pCIP3 (11) was cloned into ApaI/NotI site of pYCC412 to yield the final deletion construct, pYCC419. To delete CPRa, we used the biolistic transformation method (27) and positive-negative selection protocol (4) to achieve a high frequency of gene disruption in a MATa strain, LP8. Of the nine transformants analyzed, four clones were cpra deletion mutants. The deleted cpra locus was reconstituted back to the wild type with plasmids pYCC365 and pYCC331 (5) by a biolistic-based cotransformation method (8). The putative reconstituted adenine auxotrophs were transferred three times on YEPD to cure the strains of the cotransformed telomere-based plasmid, pYCC331. Uracil and adenine auxotrophs were isolated, and their genomic DNAs were analyzed by Southern blotting.
Preparation and analysis of nucleic acids.
Genomic DNA isolation and analysis were performed as described previously (7). To obtain RNA from different developmental stages, two opposite mating type strains were grown to early to mid-log phase in YEPD medium. Cells were harvested and washed with 0.9% NaCl. Equal numbers of cells (109) from each mating type strain were mixed and filtered onto 82-mm-diameter nitrocellulose papers. Filters were placed on V-8 agar and incubated at 30°C for the indicated period of time. As controls, sets of filters were also placed on YEPD plates for the same lengths of time. Cells were washed off the filters, and total RNA was isolated by using the FastRNA kit (Bio 101, La Jolla, Calif.). To obtain RNA from V-8 liquid cultures, cells from the mid-log phase grown in YEPD medium were harvested, washed with 0.9% NaCl, transferred to V-8 liquid medium, and incubated for 6 h. To isolate RNA from cells treated with synthetic pheromones, 20 μl of pheromone solution (0.2 mg of synthetic pheromone dissolved in dimethyl sulfoxide) was spread on a SLAD agar plate and allowed to dry overnight. Approximately 108 cells of a 24-h YEPD culture were patched on the area of the SLAD agar where the pheromone had been applied (9). After 2 h of exposure to the synthetic pheromone, the cells were harvested and total RNA was isolated as described previously (9). To isolate C. neoformans RNA from mouse brains, mice were sacrificed by cervical dislocation. Brains were quickly removed, chilled on ice, and ground in ice-cold water. The homogenate was treated with 0.5% KOH on ice for 5 min to digest the host tissue. Yeast cells were collected by centrifugation and washed repeatedly with ice-cold phosphate-buffered saline, and RNA was isolated using the FastRNA kit (Bio 101). Two micrograms of total RNA was treated with RNase-free DNase (Ambion, Austin, Tex.), and cDNA was synthesized with Superscript II reverse transcriptase (Life Technologies, Gaithersburg, Md.). The same amount of cDNA was used in real time reverse transcription-PCR (RT-PCR) with TaqMan universal PCR master mix and the ABI PRISM 7700 sequence detection system (Applied Biosystems, Foster City, Calif.). The CPRa primers used in RT-PCR were CCCTTTTGGAATCTCACTGCTT and ATGTTACGAGCACGCCAATG, and the probe was AGTCCTTGTTCTCCTACCTGCGCCCTG. Data were expressed as the relative amounts of the ACTIN gene.
Northern blot analysis was performed as previously described (6). For quantitative northern analysis, the experiments were repeated at least twice and the blot was exposed to a PhosphorImager screen and quantified with ImageQuant 1.1 (Molecular Dynamics). Each gene-specific signal was normalized to that of the ACTIN gene. The relative expression levels of each gene were compared and expressed as a percentage of the wild-type levels.
Animal model, virulence, and histopathology.
For all the animal experiments, female BALB/c mice (6 to 8 weeks old) were injected via the lateral tail vein with 0.2 ml of a suspension of each yeast strain. Virulence was studied by monitoring mortality. Kaplan-Meier analysis of survival was performed with JMP software for Macintosh (SAS Institute, Cary, N.C.). To measure the growth rate of each strain at 37°C in vitro, overnight YEPD-grown cells were inoculated into 37°C prewarmed YEPD medium and the turbidity (optical density at 600 nm) was monitored during incubation at several time intervals. To measure the growth rate of each strain in the brain, mice were injected with yeast cells (4 × 106 cells) as described above, and then 3 mice per yeast strain were sacrificed at several intervals after injection (3 h as the starting point and 3, 6, 9, 12, and 15 days postinjection). The brains were homogenized with a mortar and pestle, diluted, and then plated onto YEPD agar. Colonies were counted after 2 days of incubation at 30°C. For histopathological studies, brains were removed and fixed in 10% buffered formalin. Paraffin sections of the brains were stained with hematoxylin and eosin or Gomori methenamine silver (performed by American Histolabs, Gaithersburg, Md.).
RESULTS
Isolation and characterization of CPRa gene.
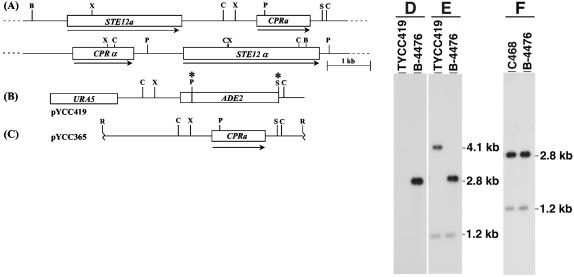
We screened a plasmid genomic library of a MATa strain with an STE12a probe and identified the CPRa gene, a putative pheromone receptor gene, located about 2.0 kb downstream of STE12a (Fig. 1A). The relative genomic arrangement of STE12a and CPRa at the MATa locus is different from that of STE12α and CPRα (Fig. 1A). Like STE12a, CPRa is present only in MATa strains (data not shown). Comparison between genomic and cDNA sequences revealed that CPRa contains three introns and the calculated molecular mass of the encoded protein is 43 kDa. The putative protein encoded by CPRa contains seven transmembrane domains and is more closely related to Bbr1p of S. commune (50% similarity, GenBank accession number P78741) than to the MATα-specific Cprαp of C. neoformans (45% similarity, GenBank accession number AF259519).
FIG. 1.
(A) Genomic arrangement of CPRa. Arrows indicate the direction of transcription, and boxes represent the coding region. B, BamHI; C, ClaI; P, PstI; R, EcoRI; S, SalI; X, XbaI. (B) Map of deletion plasmid pYCC419. The asterisk represents the restriction enzyme site removed during plasmid construction. (C) Map of CPRa reconstitution plasmid, pYCC365. (D, E, and F) Southern blot analysis. Genomic DNA was digested with ClaI and hybridized with a probe of the 1.6-kb PstI/SpeI DNA fragment of pYCC365 (D) or the 3.0-kb EcoRI/XbaI DNA fragment of pYCC365 (E and F).
Phenotype of cpra deletion mutant.
To study the function of the MATa pheromone receptor, we deleted CPRa from a MATa strain. The genomic DNA of putative deletion mutants identified by PCR was isolated, digested, and analyzed by Southern blotting. The blot was hybridized with the pYCC365 PstI/SpeI 1.6-kb DNA fragment that was deleted in pYCC419 (Fig. 1B). While the wild-type MATa strain (B-4476) showed the hybridization signal corresponding to the CPRa gene, no signal was detected in the putative deletion mutant TYCC419 (Fig. 1D). When the pYCC365 EcoRI/XbaI 3.0-kb DNA fragment (Fig. 1C) was used as a probe for the same blot, the signals detected in the putative deletion mutant corresponded to fragments of predicted sizes (Fig. 1E). These results indicated that TYCC419 is a cpra deletion mutant.
To obtain a relevant control strain for further analysis of CPRa function, we also reconstituted the disrupted cpra allele back to the wild type by a cotransformation method. The cpra deletion mutant (TYCC419) was used as a recipient strain and was cotransformed with pYCC365, which contained the entire CPRa gene (Fig. 1C), and pYCC331, which contained the selectable nutritional marker URA5 gene and telomeres. Several putative red-colored adenine prototrophic transformants, which represented the putative gene replacement strains, were isolated and analyzed by Southern blotting. Strain C468 exhibited the same hybridization pattern as the wild-type strain B-4476; therefore, C468 is a CPRa-reconstituted strain (Fig. 1F).
One of the expected phenotypes for the cpra deletion mutant was sterility. Although the mating frequency was dramatically decreased, the cpra deletion mutant, however, still mated with the opposite mating type strains and produced viable spores. Results of quantitative mating experiments showed that crosses between cpra deletion mutants and MATα strains produced very few colonies on YNB plates compared with a wild-type control mating, whereas mating cultures involving CPRa-reconstituted strains produced similar numbers of colonies as the wild-type control. The calculated relative mating frequencies for the cpra deletion mutant (TYCC419) and for the CPRa-reconstituted strain (C468) were 0.065 and 86.7% of the wild-type control strains, respectively. This dramatic reduction in mating frequency was similar to that observed for the cprα deletion mutant (9).
Although the mating frequencies of Δcpra and Δcprα strains were significantly reduced, it was possible that Δcpra and Δcprα strains could still mate with each other and produce viable progeny. To test this possibility, we conducted crosses between Δcpra and Δcprα strains and found no evidence of hyphal formation after prolonged incubation. It was possible that the mating frequencies in these crosses were too low to be detected by visual or microscopic observation. We then introduced different auxotrophic markers separately into Δcprα (MATα ura5) and Δcpra (MATa lys1) strains by genetic crosses. More than 108 cells from both strains were mixed on V-8 agar and incubated for 14 days to allow mating processes to reach maturity. The entire culture was scraped off the plate and plated on YNB medium. If mating had occurred, prototrophic progeny should have been obtained. However, no viable offspring were isolated in these experiments. These data suggested that C. neoformans requires at least one intact pheromone receptor in either mating type strain to undergo the sexual life cycle.
Expression of CPRa.
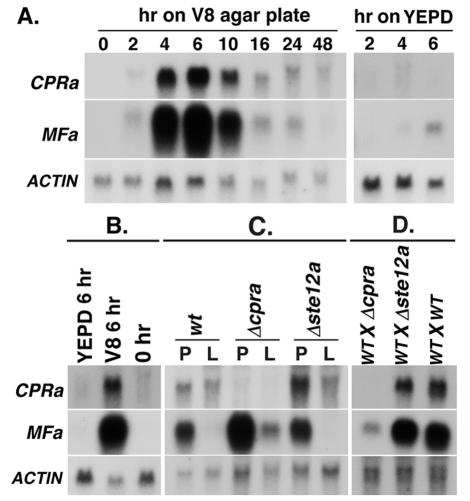
To study the patterns of CPRa expression in response to mating, RNA was isolated at different time points from mating cultures and analyzed by Northern blotting. Expression of CPRa was barely detectable during yeast-phase growth in YEPD broth (Fig. 2A, 0 h V-8 agar). However, 2 h after crossing MATa and MATα cells on V-8 agar, expression of the CPRa gene increased and peaked at 6 h (198-fold over the 0-h culture). CPRa transcript levels declined by 10 h and decreased drastically by 16 h after crossing. When MATa and MATα strains were crossed on YEPD agar, a medium not suitable for mating, CPRa transcript was barely detectable (Fig. 2A, right panel).
FIG. 2.
Northern blot analysis. (A) Total RNA was isolated from mating cultures of MATa (B-4476FO5) and MATα (JEC33) strains on V-8 agar or YEPD plates for indicated times. The probes used in each hybridization are as specified. (B) RNA was isolated from cells of a MATa strain (B-4476FO5), which were transferred from YEPD liquid (0 h) onto V-8 agar (V8 6 h) or YEPD plate (YEPD 6 h). (C) RNA was isolated from cells of the wild type (wt, B-4476FO5), ste12a deletion mutant (Δste12a, TYCC384), or cpra deletion mutant (Δcpra, TYCC419), which were transferred from YEPD liquid onto V-8 agar plates (P) or to V-8 liquid medium (L). (D) RNA was isolated from cells from mating cultures containing either the wild type (WT X WT, JEC33 × B-4476FO5), ste12a deletion mutant (WT X Δste12a, JEC33 × TYCC384) or cpra deletion mutant (WT X Δcpra, JEC33 × TYCC419).
Since it was possible that the increase of CPRa transcript levels was due to changes in culture conditions and independent of the mating process, RNA was isolated from cultures of MATa strains grown alone in YEPD broth and shifted onto V-8 agar as well as YEPD agar. CPRa expression was barely detectable in log-phase cells grown in YEPD liquid medium, but it increased noticeably by 6 h on V-8 agar (11-fold higher than that of the 0-h culture) (Fig. 2B, 0 h versus V-8 6 h). This increase was much lower than that of the mating cultures (18-fold differences) (compare Fig. 2B to A). This type of increase did not occur when MATa cells were transferred from YEPD liquid to YEPD agar (Fig. 2B, 0 h versus YEPD 6 h). Furthermore, when cells of the MATa strain were transferred from YEPD broth to V-8 liquid medium, CPRa transcript levels also increased (Fig. 2C) and CPRa transcript levels were twofold higher on V-8 agar than in V-8 liquid. Thus, the CPRa message increased when MATa cells were transferred from YEPD to V-8 medium, and the levels of induction were even higher when transferred to agar plates than to liquid medium.
Because deletion of STE12a greatly reduced the mating efficacy (7), it is believed that STE12a is involved in regulating the mating process. We investigated whether deletion of the STE12a gene affects accumulation of the CPRa transcript during mating. RNA was isolated from cultures of crosses between a MATα strain and either a wild-type, Δste12a, or Δcpra MATa strain. As expected, no CPRa message was detected in RNA isolated from a cross with Δcpra as the MATa parent strain (Fig. 2D). Interestingly, CPRa expression levels were similar in the crosses containing either Δste12a or wild-type STE12a (Fig. 2D). In addition, deletion of STE12a did not affect CPRa expression when the MATa cells alone were transferred from YEPD to liquid or V-8 agar (Fig. 2C, wild type versus Δste12a). These data suggested that the presence or absence of STE12a did not affect CPRa expression under these conditions.
Pheromone and the pheromone receptor belong to the same signaling pathway in fungi. To compare the gene expression patterns between the pheromone and pheromone receptor, we determined the transcriptional profile of MFa under the same conditions as those used for CPRa expression. Expression of MFa was also developmentally regulated during mating, as the transcript level peaked at 6 h after crossing (342-fold over the 0-h culture) and returned to low levels after 24 h (Fig. 2A, left panel). This pattern of expression was much less obvious when crosses were conducted on YEPD medium (Fig. 2A, right panel). As in the case of CPRa, deletion of ste12a did not influence MFa transcript levels during mating (Fig. 2D). However, MFa transcript levels were noticeably reduced, albeit still detectable, in crosses involving Δcpra as one of the parents (Fig. 2D). These data suggested that C. neoformans requires a functional receptor gene CPRa for MFa expression during mating, but the proposed transcriptional regulator of the mating pathway (STE12a) apparently has no significant role in MFa expression.
When MATa cells alone were transferred from YEPD broth onto V-8 agar, MFa transcript levels increased 58-fold, whereas MFa transcript was nearly undetectable in cells transferred onto YEPD agar (Fig. 2B). When cells of a MATa strain were transferred from YEPD broth to V-8 liquid medium, MFa expression was just as low as that of the cells grown in YEPD broth (Fig. 2C). MFa expression under these conditions was unaffected by the presence or absence of STE12a (Fig. 2C). In contrast, MFa transcript levels in cells grown on V-8 agar were 2.7-fold higher in the cpra deletion mutant than in the wild-type strain (Fig. 2C). These data suggested that the presence of a functional CPRa gene slightly deterred the increase of MFa expression when MATa cells were transferred from YEPD broth to V-8 media.
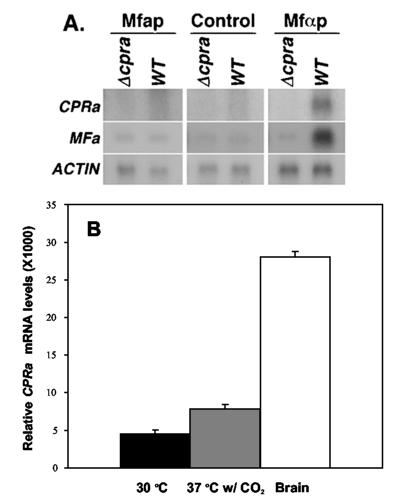
The expected function of CPRa is to sense the MFα pheromone (Mfαp) and to transmit signals downstream to the mating pathway. Although CPRa was required for high expression of MFa during mating (Fig. 2D), this does not directly demonstrate the role of CPRa in sensing the Mfαp and transmitting signals to induce MFa expression. We chemically synthesized Mfαp, treated MATa strains with Mfαp, and monitored MFa expression by Northern blot analysis. Treatment with Mfαp markedly increased the expression levels of CPRa and MFa in the wild-type MATa strain (Fig. 3A right panel), whereas the same treatment in Δcpra showed no increase in MFa expression (Fig. 3A right panel). Furthermore, only the wild-type cells and not the Δcpra produced small sparse hyphal protrusions when treated with Mfαp (data not shown). These data suggested that CPRa is required to sense Mfαp and to transmit the signal to induce expression of MFa. In contrast, when we exposed MATa strains to synthetic Mfap, CPRa and MFa expression were unaffected (Fig. 3A, left panel) and no morphological changes were observed (data not shown).
FIG. 3.
(A) Effect of treatment with synthetic pheromones. MATa strains were treated with synthetic pheromone Mfap or Mfαp or not treated (control) on SLAD agar for 2 h. RNAs were isolated from the wild type (WT, B-4476) or Δcpra (C481) and hybridized as indicated. (B) Quantitation of CPRa expression by real-time PCR. RNAs were isolated from cultures grown on RPMI plates at 30°C or 37°C with 5% CO2 or from the brains of mice 30 days after injection with a MATa strain (B-4476). The results are the means ± standard deviations of three measurements.
Importance of CPRa in virulence.
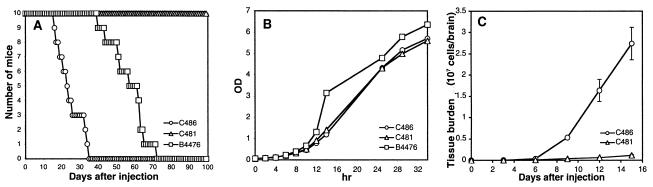
Previous reports showed that mating-type-specific genes, such as STE12a and STE12α, play important roles in the pathobiology of C. neoformans. Furthermore, as described above, CPRa responded to different environmental cues unrelated to the pheromone. These observations prompted us to identify the role of CPRa in virulence with mice as an animal model. All mice that received the wild-type strain (B-4476) or reconstituted F1 prototroph (C486) died within 100 days (Fig. 4A). Although mice showed illness, no mortality was observed in mice that received the F1 prototroph of the Δcpra strain (C481) during the same period (log rank, P < 0.0001). The in vitro growth rate of these strains at 37°C was similar (Fig. 4B). It was likely that the differences in morbidity of the mice were due to the differences in growth rate of the strains in vivo. To address this possibility, we determined the fungal burden from brains of mice infected with different yeast strains at different time points. Both the Δcpra strain and the reconstituted strain reached the brain tissue at similar rates immediately after injection (9.0 × 104 ± 0.1 × 104 versus 8.0 × 104 ± 0.2 × 104 per brain). No significant difference in tissue burden was observed between these two strains during the first 3 days after injection (2.4 × 104 ± 0.2 × 104 versus 3.0 × 104 ± 0.6 × 104 per brain). The number of CFU, however, differed significantly 6 days after injection (1.0 × 105 ± 0.1 × 105 versus 3.9 × 105 ± 0.3 × 105 per brain) and increased exponentially in the mice that received the reconstituted strain while only slight increases were observed in mice that received the Δcpra strain (Fig. 4C).
FIG. 4.
(A) Virulence studies. Mice were injected with yeast strains via the tail vein, and mortality was monitored. The inocula for B-4476, C481, and C486 were 3.4 × 106, 6.6 × 106, and 5.5 × 106 yeast cells/mouse, respectively. (B) In vitro growth curves of yeast. Cells were grown in YEPD liquid at 37°C. The growth rate was determined by measuring turbidity (optical density at 600 nm [OD600]). (C) Growth curves of yeast in mouse brains. Yeast cells were harvested from the brains at the indicated times, and the tissue burden was determined as the number of CFU. B-4476, wild-type; C481, Δcpra; C486, CPRa reconstituted strain.
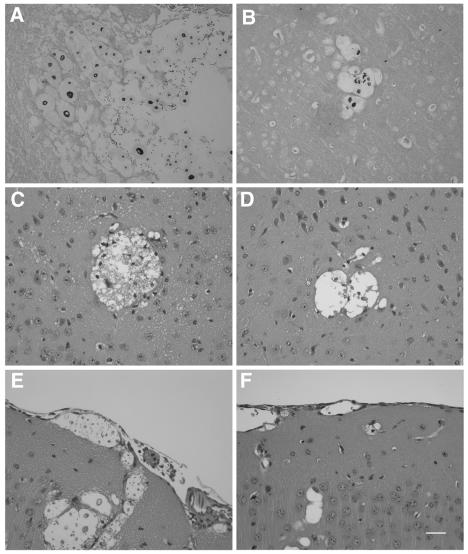
Histopathological analysis of meningoencephalitic cryptococcosis was performed on mouse brains at five different time points for comparative studies. Yeast cells were found in both groups of mice challenged with either the wild-type or the Δcpra strain 3 days postinjection, and the number of yeast cells increased when the infection progressed. However, cystic lesions were more numerous and larger and contained a greater number of yeast cells in mice infected with the reconstituted strain than in those infected with the Δcpra strain (Fig. 5A and B). Inflammation, consisting of a few perivascular and meningeal lymphocytes and granulocytes, was found in mice infected with the reconstituted strain on day 9, and this became more severe as infection progressed (Fig. 5C and E). The number of inflammatory cells on day 9 was considerably smaller in mice that received Δcpra than in mice that received the reconstituted strain (Fig. 5D and F), and a similar degree of inflammation did not develop until much later.
FIG. 5.
Histopathology of mice infected with the wild type (A, C, and E) and the cpra deletion mutant (B, D, and F). Mice were challenged with yeast cells through the tail vein. Brains were removed and fixed in 10% buffered neutral formalin. Tissue sections stained with Gomori methenamine silver (A and B) were from mouse brains 6 days postinjection for panel A and 9 days postinjection for panel B. Tissue sections stained with hematoxylin and eosin (C, D, E, and F) were from mouse brains 9 days postinfection. Neural parenchyma show locular lesions containing yeast cells (A, B, C, and D). The meningeal areas show cystic spaces that contained cryptococci (E and F). Bar, 30 μm.
Interestingly, the brain smears prepared at early stages of infection showed the yeast cells of the Δcpra strain possessing significantly smaller capsules than those of the wild-type or reconstituted strain (Fig. 6C versus A and B). Yeast cells of the Δcpra strain in brains of mice that were moribund on day 150, however, displayed a capsule size similar to those of the CPRa cells (Fig. 6D). There was no difference in capsule size when these strains were cultured on YEPD media at 30 or 37°C (data not shown). To test if different culture conditions affected the capsule size of the strains, cells were grown on RPMI plates and incubated at 30 and 37°C with or without 5% CO2. We detected no difference in capsule size among strains grown on RPMI at 30°C with or without 5% CO2 or at 37°C without 5% CO2. The Δcpra cultures incubated at 37°C in the presence of 5% CO2, however, displayed slightly smaller capsules than the wild-type strain incubated under the same conditions (data not shown). To determine whether CPRa expression varies under different conditions, RNA was isolated and the CPRa transcription levels were measured by real-time RT-PCR (see Materials and Methods). Figure 3B shows that expression levels of CPRa were 1.7-fold higher at 37°C in the presence of 5% CO2 or 6.2-fold higher in the brains of mice than the expression levels in the culture grown at 30°C. Additionally, a 3.7-fold induction of MFa expression was observed when we compared the cells isolated from mouse brains to the cells grown at 30°C in RPMI medium (data not shown). These results suggested that cryptococcal cells require a functional CPRa in order to respond to different environmental conditions and regulate the size of the capsule.
FIG. 6.
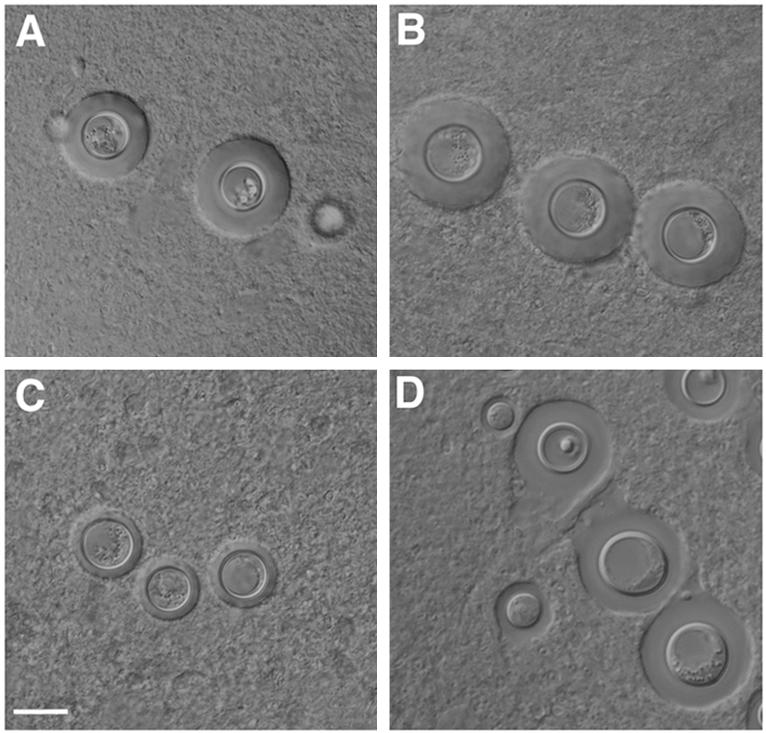
Brain smear showing cells of the wild-type (A) (B-4476), the CPRa reconstituted strain (B) (C486), and the cpra deletion mutant (C and D) (C481). Brain tissue of mice challenged with different yeast strains was smeared on a microscopic slide and examined under a microscope with a Nomarski interference condenser. Brain tissue was harvested 6 days postinjection (A, B, and C) or from the moribund mice challenged with Δcpra (D). Bar, 10 μm.
DISCUSSION
We identified and characterized a pheromone receptor-like gene, CPRa, from a MATa strain of C. neoformans. As is the case in the MATα locus, CPRa is located adjacent to STE12a in the MATa locus, but CPRa lies downstream of STE12a while CPRα is upstream of STE12α (M. Karos, Y. C. Chang, and K. J. Kwon-Chung, Abstr. 100th Gen. Meet. Am. Soc. Microbiol., p. 336, 2000). This observation was confirmed by the recently reported physical map of the mating type loci (19). Gene expression studies showed that genes encoding the pheromone MFa and receptor CPRa of C. neoformans are expressed at low but detectable levels in haploid yeast cells. This low expression may be sufficient for yeast cells to sense the presence of the opposite mating type. Upon mating, the abundance of receptor and pheromone transcripts increased drastically between 2 and 6 h. Our observation is similar to that seen during mating responses in budding yeast and fission yeast where the expression of pheromone and receptor genes is induced during mating (21). It is probable that high levels of pheromone gene expression result in high levels of pheromone secretion in C. neoformans. High levels of secreted pheromone may be advantageous in competition between mating partners, a phenomenon which has been described as courtship for S. cerevisiae (12). Presumably, binding of pheromone to the receptor leads to dissociation of the heterotrimeric G protein, allowing its subunit to interact with downstream effectors in the MAP kinase cascade and cause changes in the expression of downstream genes. The expression of both the pheromone and receptor genes is reduced to a low level 16 h after crossing, but their expression levels are still higher than that of haploid cells unexposed to the opposite mating type. At this point, the products of mating events such as basidia and basidiospores can be observed (data not shown). These data suggest that high levels of expression for both pheromone and receptor genes are not required to continue morphogenesis. Thus, expression of both pheromone and receptor genes is tightly regulated during the process of sexual reproduction, and the proteins encoded by both genes may play crucial roles mainly during the initial stages, such as conjugation and dikaryon formation. Once the genes involved in morphogenesis have been activated and fruiting structures are produced, the receptor and the pheromone appear to be dispensable.
Gene expression of CPRa increases when yeast cultures are shifted from liquid to an agar surface, from rich medium (YEPD) to poor medium (V-8), or from 30 to 37°C with 5% CO2, and, interestingly, in the brains of mice during early periods of infection. Such responses of pheromone receptor expression levels to diverse environmental cues have not been described for other fungal systems. Most interestingly, CPRa plays an important role in virulence in a murine model and deletion of CPRa affects the size of the capsule in vitro and in vivo. Our result of the virulence studies is intriguing because the p21-activated protein kinase homologs, STE20a and STE20α (28), as well as the components of the MAP kinase cascade involved in mating, STE11α, STE7, and CPK1, are important for mating but dispensable for virulence in serotype D strains (J. Heitman, personal communication). It is clear from our studies that CPRa is indeed involved in the mating pathway through sensing the presence of pheromone, but it is not clear how CPRa influences virulence. It is possible that the CPRa protein is charged with sensing different environmental cues. Alternatively, other proteins may actually respond to environmental cues and either directly or indirectly modulate CPRa expression. Although most seven-transmembrane receptors transmit the signal through G-protein-dependent activation of effectors, some receptors are also known to interact with other proteins (24). It would be interesting to know whether the CPRa protein is also coupled to different signal transduction cascades besides the pheromone response MAP kinase cascade or whether it interacts with other molecules besides the G protein.
We showed that synthetic Mfαp, but not Mfap, induces MFa gene expression in MATa strains and causes slight morphological changes in strains incubated on SLAD agar. These results are similar to the results of a previous study which showed that, when MFa was expressed from an episomal plasmid in MATa strains, the resulting cells failed to undergo morphological differentiation on V-8 plates (22). However, expression of the MFa gene from a telomeric plasmid in a MATa strain reportedly induced hyphal protrusions on filament agar (25). Since these experiments were carried out on different media, it is not easy to dissect the differences. We know that the synthetic Mfap used in the present study was functional since the same pheromone induced expression of MFα and caused morphological differentiation in MATα strains (9). It is possible that MATa cells require additional stimuli in addition to the one we used in this study to cause morphological changes. It is also possible that overexpression of MFa from a telomeric plasmid may result in stress that induces hyphal protrusions in MATa strains on filament agar. Another possibility is that the MATa cells could not sense the exogenously supplied Mfap but responded to internal overexpression of MFa.
The expression of MFa was induced by transferring yeast cultures from liquid to an agar surface. The expression of MFa was also induced by switching cultures from rich medium to nitrogen starvation and from neutral pH to acidic pH (unpublished data). However, the levels of induction are not significantly influenced by the presence or absence of CPRa (Fig. 2 and unpublished data). In contrast, expression levels of MFa during mating are dramatically reduced in the Δcpra strain. These data suggested that C. neoformans employs at least two pathways to regulate the expression of MFa. One, which is pheromone receptor dependent, is most likely to function through the MAP kinase signaling pathway during mating. Several other components in the MAP kinase pathway have also been shown to be important in regulating mating and induction of pheromone gene expression in C. neoformans (25). The second pathway appears to be CPRa independent and is manifested when cells are exposed to different environmental conditions. It is not clear whether the later pathway utilizes other components of the mating MAP kinase signaling pathway or components of different signaling cascades. Although the RAS signaling pathway is important for mating (1), a link between MFa expression and the RAS signaling pathway has not been demonstrated. In addition, the reason why the presence of a functional pheromone receptor gene slightly hampered the ability of cells to express MFa under nitrogen starvation conditions or upon switching media from YEPD to V-8 remains unknown.
It has been proposed that C. neoformans STE12a and STE12α may belong to an as yet unidentified pathway and that the effect of both STE12a and STE12α on mating could be a result of cross talk between the unidentified pathway and the pheromone response pathway (7). Recent studies showed that pheromone response MAP kinase components are required for induction of MFα in response to MATa cells, whereas STE12α is not (J. Heitman, personal communication). Our observations that deletion of ste12a does not affect the expression of CPRa and MFa during mating appear to further support this hypothesis. We speculate that C. neoformans contains a different transcription factor that functions in the mating pathway. A homolog of the U. maydis transcription factor Prf1, important for mating, was identified in the cryptococcal genome database at the Stanford Genome Technology Center (http://www-sequence.stanford.edu). It would be interesting to know if this homolog is the target transcription factor for the pheromone response MAP kinase pathway.
Acknowledgments
We thank L. Penoyer and T. McPadden for technical assistance and A. Varma and R. Tscharke for critical reviews of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Alspaugh, J. A., L. M. Cavallo, J. R. Perfect, and J. Heitman. 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol. Microbiol. 36:352-365. [DOI] [PubMed] [Google Scholar]
- 2.Bolker, M., M. Urban, and R. Kahmann. 1992. The a mating type locus of U. maydis specifies cell signaling components. Cell 68:441-450. [DOI] [PubMed] [Google Scholar]
- 3.Casselton, L. A., and N. S. Olesnicky. 1998. Molecular genetics of mating recognition in basidiomycete fungi. Microbiol. Mol. Biol. Rev. 62:55-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang, Y. C., and K. J. Kwon-Chung. 1994. Complementation of a capsule-deficient mutation of Cryptococcus neoformans restores its virulence. Mol. Cell. Biol. 14:4912-4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang, Y. C., and K. J. Kwon-Chung. 1999. Isolation, characterization, and localization of a capsule-associated gene, CAP10, of Cryptococcus neoformans. J. Bacteriol. 181:5636-5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, Y. C., L. A. Penoyer, and K. J. Kwon-Chung. 1996. The second capsule gene of Cryptococcus neoformans, CAP64, is essential for virulence. Infect. Immun. 64:1977-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang, Y. C., L. A. Penoyer, and K. J. Kwon-Chung. 2001. The second STE12 homologue of Cryptococcus neoformans is MATa-specific and plays an important role in virulence. Proc. Natl. Acad. Sci. USA 98:3258-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, Y. C., B. L. Wickes, G. F. Miller, L. A. Penoyer, and K. J. Kwon-Chung. 2000. Cryptococcus neoformans STE12α regulates virulence but is not essential for mating. J. Exp. Med. 191:871-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, S., M. Karos, Y. C. Chang, J. Lukszo, B. L. Wickes, and K. J. Kwon-Chung. 2002. Molecular analysis of CPRα, a MATα-specific pheromone receptor gene of Cryptococcus neoformans. Eukaryot. Cell 1:432-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke, D. L., G. L. Woodlee, C. M. McClelland, T. S. Seymour, and B. L. Wickes. 2001. The Cryptococcus neoformans STE11α gene is similar to other fungal mitogen activated protein kinase kinase kinase (MAPKKK) genes but is mating type specific. Mol. Microbiol. 40:200-213. [DOI] [PubMed] [Google Scholar]
- 11.Edman, J. C., and K. J. Kwon-Chung. 1990. Isolation of the URA5 gene from Cryptococcus neoformans var.neoformans and its use as a selective marker for transformation. Mol. Cell. Biol. 10:4538-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson, C. L., J. B. Konopka, and L. H. Hartwell. 1991. S. cerevisiae alpha pheromone receptors activate a novel signal transduction pathway for mating partner discrimination. Cell 67:389-402. [DOI] [PubMed] [Google Scholar]
- 13.Karos, M., Y. C. Chang, C. M. McClelland, D. L. Clarke, J. Fu, B. L. Wickes, and K. J. Kwon-Chung. 2000. Mapping of the Cryptococcus neoformans MATα locus: presence of mating type-specific mitogen-activated protein kinase cascade homolog. J. Bacteriol. 182:6222-6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurjan, J. 1993. The pheromone response pathway in Saccharomyces cerevisiae. Annu. Rev. Genet. 27:147-179. [DOI] [PubMed] [Google Scholar]
- 15.Kwon-Chung, K. J. 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67:1197-1200. [PubMed] [Google Scholar]
- 16.Kwon-Chung, K. J., and J. E. Bennett. 1992. Cryptococcosis, p. 397-446. Lea & Febiger, Philadelphia, Pa.
- 17.Kwon-Chung, K. J., J. E. Bennett, and J. C. Rhodes. 1982. Taxonomic studies on Filobasidiella species and their anamorphs. Antonie Leeuwenhoek 48:25-38. [DOI] [PubMed] [Google Scholar]
- 18.Leberer, E., D. Y. Thomas, and M. Whiteway. 1997. Pheromone signalling and polarized morphogenesis in yeast. Curr. Opin. Genet. Dev. 7:59-66. [DOI] [PubMed] [Google Scholar]
- 19.Lengeler, K. B., D. S. Fox, J. A. Fraser, A. Allen, K. Forrester, F. S. Dietrich, and J. Heitman. 2002. Mating-type locus of Cryptococcus neoformans: a step in the evolution of sex chromosomes. Eukaryot. Cell 1:704-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lengeler, K. B., P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 2000. Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc. Natl. Acad. Sci. USA 97:14455-14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marsh, L., A. M. Neiman, and I. Herskowitz. 1991. Signal transduction during pheromone response in yeast. Ann. Rev. Cell Biol. 7:699-728. [DOI] [PubMed] [Google Scholar]
- 22.McClelland, C. M., J. Fu, G. L. Woodlee, T. S. Seymour, and B. L. Wickes. 2002. Isolation and characterization of the Cryptococcus neoformans MATa pheromone gene. Genetics 160:935-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore, T. D., and J. C. Edman. 1993. The alpha-mating type locus of Cryptococcus neoformans contains a peptide pheromone gene. Mol. Cell. Biol. 13:1962-1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce, K. L., R. T. Premont, and R. J. Lefkowitz. 2002. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 3:639-650. [DOI] [PubMed] [Google Scholar]
- 25.Shen, W.-C., R. C. Davidson, G. M. Cox, and J. Heitman. 2002. Pheromones stimulate mating and differentiation via paracrine and autocrine signaling in Cryptococcus neoformans. Eukaryot. Cell 1:366-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sprague, G. F., and J. W. Thorner. 1992. Pheromone response and signal transduction during the mating process of Saccharomyces cerevisiae. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 27.Toffaletti, D. L., T. H. Rude, S. A. Johnston, D. T. Durack, and J. R. Perfect. 1993. Gene transfer in Cryptococcus neoformans by use of biolistic delivery of DNA. J. Bacteriol. 175:1405-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, P., C. B. Nichols, K. B. Lengeler, M. E. Cardenas, G. M. Cox, J. R. Perfect, and J. Heitman. 2002. Mating-type-specific and nonspecific PAK kinases play shared and divergent roles in Cryptococcus neoformans. Eukaryot. Cell 1:257-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, P., J. R. Perfect, and J. Heitman. 2000. The G-protein beta subunit GPB1 is required for mating and haploid fruiting in Cryptococcus neoformans. Mol. Cell. Biol. 20:352-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickes, B. L., U. Edman, and J. C. Edman. 1997. The Cryptococcus neoformans STE12α gene: a putative Saccharomyces cerevisiae STE12 homologue that is mating type specific. Mol. Microbiol. 26:951-960. [DOI] [PubMed] [Google Scholar]