Abstract
Herpes simplex virus (HSV) entry requires the interaction of glycoprotein D (gD) with a cellular receptor such as herpesvirus entry mediator (HVEM or HveA) or nectin-1 (HveC). However, the fusion mechanism is still not understood. Since cholesterol-enriched cell membrane lipid rafts are involved in the entry of other enveloped viruses such as human immunodeficiency virus and Ebola virus, we tested whether HSV entry proceeds similarly. Vero cells and cells expressing either HVEM or nectin-1 were treated with cholesterol-sequestering drugs such as methyl-β-cyclodextrin or nystatin and then exposed to virus. In all cases, virus entry was inhibited in a dose-dependent manner, and the inhibitory effect was fully reversible by replenishment of cholesterol. To examine the association of HVEM and nectin-1 with lipid rafts, we analyzed whether they partitioned into nonionic detergent-insoluble glycolipid-enriched membranes (DIG). There was no constitutive association of either receptor with DIG. Binding of soluble gD or virus to cells did not result in association of nectin-1 with the raft-containing fractions. However, during infection, a fraction of gB but not gC, gD, or gH associated with DIG. Similarly, when cells were incubated with truncated soluble glycoproteins, soluble gB but not gC was found associated with DIG. Together, these data favor a model in which HSV uses gB to rapidly mobilize lipid rafts that may serve as a platform for entry and cell signaling. It also suggests that gB may interact with a cellular molecule associated with lipid rafts.
Herpes simplex virus (HSV) is typically responsible for mucosal lesions of the mouth and genital organs in humans, from where it spreads and establishes lifelong latent infections in sensory neurons. Periodically, the virus reactivates, multiplies, and is transported through the axon back to a portal of entry (83). Binding to host cell surfaces and entry is a complex process involving the essential viral glycoproteins B (gB), gD, gH, and gL and multiple cellular molecules, each with various levels of affinity and avidity (reviewed in references 11 and 72). In current models, gC and/or gB binds cell surface heparan sulfate proteoglycans, bringing the viral envelope and plasma membrane close enough for fusion to occur (69). As part of this process, gD must bind to a specific receptor, which can be either herpesvirus entry mediator A (HVEM or HveA), a member of the tumor necrosis factor receptor family, nectin-1 (HveC) or nectin-2 (HveB), two members of the Immunoglobulin superfamily, or a particular type of modified heparan sulfate proteoglycans (HSPG) 3-OST-3 (23, 46, 68). These interactions may then recruit the other essential viral glycoproteins into a functional fusion unit. In addition, entry may involve plasma membrane rearrangement, signaling events, and/or recruitment of additional cellular molecules.
Accumulated evidence indicates that plasma membrane microdomains, or lipid rafts, that are highly enriched in cholesterol and sphingolipids play a crucial role in the lateral organization of the plasma membrane (9, 27, 71). It has been proposed that constitutive or transient enrichment of a variety of signaling molecules in these defined microdomains plays a major role in the organization of signal transduction. These domains retain substantial lateral mobility and are viewed as highly ordered moving platforms that carry specific proteins. Several viruses have taken advantage of lipid rafts for one or more aspects of their replication cycle (reviewed in references 12, 48, 67, and 76). Such mechanisms include viral particle assembly (4, 28, 38, 43, 63, 66, 86), budding from the plasma membrane (38, 49, 65), signaling (13, 18, 29), fusion (1), and virus entry (3, 5, 39, 42, 55, 74). It was proposed that human immunodeficiency virus (HIV) entry is inhibited by the presence of drugs that remove cholesterol (42, 59). The inhibitory effect was reversed by addition of exogenous cholesterol, indicating that cholesterol-enriched lipid rafts play an important role in HIV entry. Since HIV and HSV enter cells by direct fusion, an intriguing possibility is that rafts also play a role in HSV entry (reviewed in reference 12).
Receptors for HIV, including CD4 (85) and CCR5 (42), and for murine leukemia virus (39) are found in lipid rafts. Binding of gp120 to cells further recruits a larger number of HIV receptors into lipid rafts (42, 59). One evident question is whether the receptors for HSV associate with lipid rafts. It has recently been shown that some members of the tumor necrosis factor receptor superfamily, including CD120a, CD40, and the p75 neurotrophin receptor, are localized in rafts (7, 16, 30, 33). Cross-linking of CD40 with antibodies results in stable association with lipid rafts, leading to activation of tyrosine kinases and mobilization of tumor necrosis factor receptor-associated factors (TRAFs) (78). Such events are essential for downstream events, such as NF-κB activation and interleukin expression.
Epstein-Barr virus has exploited this signaling route by virtue of the localization of the latent membrane protein-1 (LMP1) in rafts. Association of LMP1 with rafts is responsible for signaling events that mimic those of a constitutively active CD40 receptor (10). An essential role for rafts has been described for the initiation of Fas-mediated cell death signaling (32). As a tumor necrosis factor receptor-like protein, HVEM contains TRAF-binding motifs in its cytosolic tail (46), and several reports demonstrated activation of NF-κB during early events of HSV infection (2, 54). In addition, several proteins are rapidly phosphorylated during HSV entry (60). Hence, it is of interest to know whether HVEM is constitutively distributed in raft-like structures on cell surfaces or redistributes there as a consequence of virus binding. If so, is such an association important for virus entry? Similar questions may be asked about a second HSV receptor, nectin-1 (HveC). Strong evidence for membrane raft-dependent scaffolding of signaling complexes has come from studies on the T- and B-cell immunoreceptors and the Fcɛ receptor, all members of the same protein family as nectin-1 (14, 15, 47, 73).
Here we present data investigating the importance of intact lipid rafts at the cell membrane for efficient virus replication. Second, we analyzed whether two HSV receptors, HVEM and nectin-1, as well as HSV glycoproteins showed an association with lipid rafts during virus entry. We found that HVEM and nectin-1 were not associated with rafts in uninfected cells and that this distribution did not change during infection. Similarly, gD, gC, and gH were not found associated with these microdomains during infection. By contrast, a fraction of gB was associated with rafts after virus attachment and during entry. This last observation points to a unique function for gB during HSV entry that involves lipid rafts and suggests the existence of a gB receptor(s) that is enriched in the cholesterol-rich microdomains.
MATERIALS AND METHODS
Cells and viruses.
African green monkey kidney (Vero) and human embryonic kidney (HEK) 293T cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% and 10% fetal calf serum (FCS), respectively. B78-H1 mouse melanoma cells expressing gD receptor HVEM (A10) or nectin-1 (C10) (45) were grown in DMEM supplemented with 5% FCS and 500 μg of G418 per ml. B78-H1 cells (3E5) expressing a green fluorescent protein (GFP)-tagged form of HVEM (enhanced green fluorescent protein [EGFP] at the C terminus of HVEM) were cultured similarly. These cells were shown to be permissive for infection by HSV (C. Whitbeck, unpublished results). HSV-1 (KOS) and an HSV-1 strain (KOS/tk12) that carries the lacZ gene under the control of the ICP4 promoter (80) were both purified on sucrose gradients as described elsewhere (26). HSV-1 (KOS/tk12) and vesicular stomatitis virus were kindly provided by P. G. Spear and R. N. Harty, respectively.
Antibodies and reagents.
Rabbit R47 and R137 sera were raised against HSV gC-1 and gH-1/gL-1, respectively (20, 56). Monoclonal antibody (MAb) DL6 recognizes a linear epitope on gD (19); MAb SS-10 was generated by immunizing mice with gB purified from an extract of HSV-1-infected BHK cells (unpublished data); MAb CW10 was raised against a recombinant form of HVEM (200t) (82) expressed in a baculovirus expression system (C. Whitbeck, unpublished data); MAbs CK6 and CK41 were raised against a recombinant form of nectin-1 (36). CK41 was also labeled with phycoerythrin at Molecular Probes. MAb to flotillin-2/ESA was obtained from BD Transduction Laboratories. Anti-mouse and anti-rabbit immunoglobulin secondary antibodies coupled to horseradish peroxidase (HRP) were purchased from Kirkegaard and Perry Laboratories. Cholera toxin B subunit peroxidase conjugate (CTB-HRP) was from Sigma and used at 40 ng/ml. 7-Amino-actinomycin D (BD Pharmingen) was used for the exclusion of nonviable cells in flow cytometric assays following the manufacturer's instructions.
Production and purification of HSV-1 glycoproteins.
gC(457t) contains the full ectodomain of gC1 truncated at residue 457 (61). gD(285t), a form of the ectodomain of gD truncated at residue 285 (62), binds the receptor molecules HVEM and nectin-1 with higher affinity than gD(306t) (52, 84). The ectodomain comprising the first 730 amino acids of gB was expressed by recombinant baculovirus-infected insect cells. Based on the nucleotide sequence of the HSV-1 gB open reading frame, we synthesized two PCR primers in order to amplify and modify the gB ectodomain coding region for cloning and expression in a recombinant baculovirus. The first primer, 5′-CGGGATCCGGCGGCTCCGACTTC-3′, hybridized to the noncoding strand of the gB ORF immediately beyond the region coding for the predicted signal sequence and incorporated a BamHI restriction enzyme cleavage site (bold letters). The second primer, 5′-GCGTGATCAGGCGGCGTTGGCGTCGGCGTGGATGAC-3′, hybridized to the coding strand of the cloned gB open reading frame immediately prior to the transmembrane region coding sequence (residue 730) and incorporated a BclI restriction enzyme cleavage site (bold letters).
After cloning into the BamHI site of the pVT-Bac transfer vector, the gB coding region was positioned downstream of and in frame with the mellitin signal sequence coding region. The resulting plasmid construct was cotransfected with baculovirus DNA (Baculogold; Pharmingen) into Sf9 cells grown in monolayer culture. After 4 days, the culture supernatant (containing recombinant progeny virus) was plated onto Sf9 cell monolayers under Grace's insect cell medium containing 1% agarose. Recombinant virus plaques were picked and amplified, and infected cell cultures were screened for the expression of gB by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis with R69 antiserum. The truncated protein called gB(730t) was purified from cells with a DL16 immunosorbent column. This MAb recognizes a dimer-specific epitope on gB, and hence all of the purified protein is dimeric. Conditions for elution and concentration were essentially the same as described for gD1(306t) (70).
Cholesterol sequestration and virus entry assay.
Vero, A10, or C10 cells were seeded in 96-well plates and grown overnight to reach about 4 × 104 cells per well. Cells were then incubated for 30 min at room temperature with serial dilutions of methyl-β-cyclodextrin (MβCD) or nystatin (both reagents from Sigma) in cell culture medium containing 30 mM HEPES. After three washes with medium, cells were infected with HSV-1 (KOS/tk12) at a multiplicity of infection of 10 and allowed to attach to the cells at 4°C for 1 h. The temperature was then shifted to 37°C to allow infection to proceed synchronously. Five hours later, cells were lysed by adding an equal volume of DMEM containing 1% NP-40. β-Galactosidase activity was determined by adding substrate (chlorophenol red-β-d-galactopyranoside; Roche) and measuring the absorbance at 570 nm in a microtiter plate reader.
In replenishment experiments, Vero cells were first treated with 7.5 mM MβCD for 30 min. MβCD was washed out as before, and various amounts of water-soluble cholesterol balanced with MβCD (Sigma) in DMEM without serum were added. After 30 min, the cholesterol-containing medium was washed out, cells were infected with HSV-1 (KOS/tk12), and the process of entry was assayed as before. To deplete cholesterol from purified virions, 2 × 106 PFU was incubated for 30 min at room temperature with serial dilutions of MβCD and then diluted 60-fold with cell culture medium containing HEPES. Vero cells seeded into 96-well plates as above were infected with the treated virus at a multiplicity of infection of 10, and entry was assayed as before.
Plaque formation assay.
Vero cell monolayers in 48-well plates were incubated for 30 min at room temperature with serial dilutions of MβCD in cell culture medium containing 30 mM HEPES. After three washes with medium, cells were infected with 100 PFU of HSV-1 (KOS) or vesicular stomatitis virus at 37°C. One hour postinfection, the medium was removed, and the cells were overlaid with DMEM containing 1% carboxymethylcellulose and 5% FCS and incubated for an additional 24 h. The cells were then fixed with methanol-acetone (2:1 ratio) for 20 min at −20°C and air dried. Virus titers were determined by an immunoperoxidase assay (81) with a mixture of anti-gB, -gC, -gD, and -gH/gL polyclonal antisera (HSV) or stained with crystal violet (vesicular stomatitis virus).
Flow cytometry.
C10 or A10 seeded in six-well plates was incubated for 30 min at room temperature with serial dilutions of MβCD in cell culture medium containing 30 mM HEPES. After three washes with medium, cells were detached with 0.02% EDTA in phosphate-buffered saline (PBS) (Versene; Gibco-BRL), pelleted, and resuspended in 100 μl of PBS containing 3% FCS. Cells were stained on ice for 1 h by the addition of 5 μg of CK41 per ml directly labeled with phycoerythrin to detect nectin-1. After a PBS wash, cells were fixed with 3% paraformaldehyde in PBS and analyzed by fluorescence-activated cell sorting (FACS).
Transient transfection of 293T cells.
HEK-293T cells (80% confluent) grown in a T75 flask were transfected with 5 μg of endotoxin-free (Qiagen) purified plasmid pBEC14, encoding HVEM (46), or pBG38, encoding nectin-1 (23), with GenePorter, as recommended by the manufacturer (Gene Therapy Systems). Twenty-four hours later, cells were fractionated on sucrose gradients as described below. Plasmids pBEC14 and pBG38 were kindly provided by P. G. Spear.
Isolation of low-density detergent-insoluble membrane fractions on sucrose gradients.
Low-density detergent-insoluble membrane microdomains were isolated essentially as described by others with some modifications (64). Briefly, confluent cell monolayers in a T75 flask were washed twice with PBS and then scraped into 1 ml of ice-cold MNE buffer (25 mM MES [2-{N-morpholino}ethanesulfonic acid, pH 6.5], 150 mM NaCl, 2 mM EDTA) containing 1% Triton X-100 (Fluka) and a cocktail of protease inhibitors (Roche). Cells were further homogenized with 10 strokes in a Dounce homogenizer, and then 800 μl of the homogenate was adjusted to 45% sucrose (prepared in MNE) and placed at the bottom of an ultracentrifuge tube. A discontinuous gradient was formed by overlaying the homogenate sequentially with 1.6 ml of 35% and of 5% sucrose (both prepared in MNE). These mixtures were centrifuged at 200,000 × g and 4°C for 16 h in an SW50 swinging-bucket rotor. The gradient was fractionated from the top, and 12 fractions of 400 μl each were collected. A 13th fraction was obtained by extracting the pellet at room temperature with 400 μl of 4% SDS in 125 mM Tris, pH 6.8.
Isolation of detergent-resistant membranes by sequential centrifugation.
Nonionic detergent-insoluble glycolipid-enriched membranes (DIG), which are soluble in octylglucoside, were prepared as described by others (17) with minor modifications. Briefly, confluent cells (T25 flask) were scraped into 1 ml of ice-cold MNE buffer containing 1% Brij-96 (Fluka) and a cocktail of protease inhibitors (Roche). After 10 strokes in a Dounce homogenizer, the nuclei were pelleted by centrifugation at 500 × g for 5 min. Supernatants were sequentially centrifuged at 10,000 × g for 10 min and then at 200,000 × g for 1 h with an SW50 rotor. Supernatants containing detergent-soluble membranes as well as cytosolic proteins are referred to as DSM. The insoluble pellet was extracted with 200 μl of octylglucoside solution (50 mM β-octylglucopyranoside in 20 mM Tris [pH 8], 200 mM NaCl, and protease inhibitors). Insoluble membranes still attached to the nuclei and the pellet from the 10,000 × g centrifugation run were also both reextracted with 200 μl of octylglucoside solution. The octylglucoside-soluble fractions were pooled and referred to as DIG.
Measuring binding of virus or soluble glycoprotein to the cell surface.
Confluent C10 cells (T75 flask) were washed once with cold culture medium containing 30 mM HEPES and then incubated for 1 h at 4°C with HSV-1 (KOS) (multiplicity of infection, 10 to 20). Cells were then either left on ice or shifted to 37°C for various times before being washed with cold PBS. Cells were then scraped into 1 ml of ice-cold MNE buffer containing 1% Triton X-100, homogenized, and fractionated as described earlier in the section on isolation of low-density detergent-insoluble membrane fractions on sucrose gradients. Alternatively, cells were grown to confluency (T25 flask), washed, and incubated as before but now in the presence of 0.1 μM purified soluble gB(730t), gC(457t), or gD(285t). Cells were then either left on ice or shifted to 37°C for various times before being washed with cold PBS. The DIG were then isolated as described in the section on isolation of detergent-resistant membranes by sequential centrifugation.
Western and dot blot analyses.
To analyze the distribution of proteins in the fractions obtained from centrifugation experiments, an equal volume of each fraction was mixed with SDS-PAGE sample buffer (Pierce), and the proteins were resolved by SDS-PAGE (Novex) under denaturing conditions, followed by Western blotting. In some experiments, the low-density fractions (3 to 6) from a sucrose gradient were pooled, precipitated with methanol-chloroform, and solubilized in gel sample buffer. After transfer, the membranes were reacted with specific antibodies, washed, and then incubated with secondary antibodies coupled to HRP. Bound antibodies were revealed by enhanced chemiluminescence (ECL; Amersham) and exposure to film. For detection of the ganglioside GM1, 50 μl per fraction was blotted onto a nitrocellulose membrane, blocked with PBS containing 5% nonfat dry milk and 0.2% Tween, and incubated with CTB-HRP diluted in the same buffer. Bound proteins were revealed by ECL as before.
RESULTS
Effect of cholesterol depletion or chelation on HSV entry.
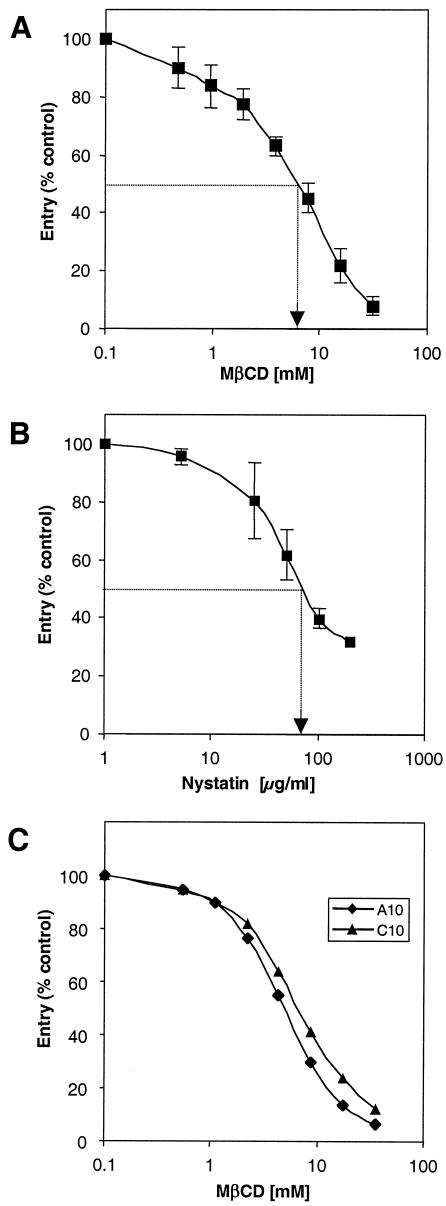
One way to tell if lipid rafts are important for virus entry is to deplete cells of cholesterol with agents that sequester cholesterol, such as cyclodextrins, or agents that chelate cholesterol, such as statins, and then examine the effect of such treatment on virus entry. We treated Vero and B78-H1 cells with methyl-β-cyclodextrin (MβCD) or nystatin for 30 min, washed out the drug, and then infected them with HSV-1 carrying the lacZ (KOS/tk12) gene under the control of the ICP4 promoter (Fig. 1). At 6 h postinfection, the levels of β-galactosidase activity in cell extracts were determined and used as a measure of virus entry (46).
FIG. 1.
Effect of cholesterol depletion or chelation on HSV entry. Vero cells (A and B) and B78 A10 and C10 cells (C) were treated with increasing concentrations of MβCD (A and C) or nystatin (B). The drugs were washed out, and the cells were infected with HSV-1 (KOS/tk12) and assayed for β-galactosidase activity at 6 h postinfection. Results presented in A and B are the mean of three independent experiments done in duplicate, with standard deviations. The results shown in panel C are representative of three experiments. A value of 100% entry represents the β-galactosidase activity at 6 h in the absence of MβCD.
Virus entry into Vero cells was inhibited in a dose-dependent fashion, with 50% inhibition occurring at 7.5 mM MβCD (Fig. 1A) and 80 μg of nystatin per ml (Fig. 1B). To determine whether this effect was dependent on a particular receptor, we repeated the experiment with cell lines bearing a single HSV receptor. A10 cells, which express HVEM, and C10 cells, which express nectin-1, were both derived by transfection of the mouse melanoma cell line B78-H1 (45). MβCD inhibited HSV entry into A10 and C10 cells in a dose-dependent fashion (Fig. 1C). This indicated that cholesterol was important for HSV entry with either the HVEM or nectin-1 receptor. Based on trypan blue dye exclusion or with actinomycin D in a flow cytometric assay, cells remained viable at drug concentrations as high as 30 mM MβCD or 200 μg of nystatin per ml (data not shown).
One possible explanation for the inhibition of HSV entry by cholesterol-sequestering drugs is that residual drug present in or on the cells was toxic for the virus. To address this question, HSV-1 (KOS/tk12) virions were incubated with increasing concentrations of MβCD, then diluted 60-fold, and tested for infectivity on Vero cells (Fig. 2). Treatment of virus with 7.5 mM MβCD inhibited its ability to infect cells by 90%. However, virus treated with 1 mM MβCD entered Vero cells almost as efficiently as control untreated virus. Since cells were washed extensively after MβCD treatment and before infection (Fig. 1A), it is unlikely that residual drug left on the cells was as high as 1 mM. So, although a high concentration of MβCD was toxic for HSV, this toxicity was not sufficient to explain the inhibition of entry into cells treated with MβCD.
FIG. 2.
Effect of cholesterol depletion from virions on HSV entry. HSV-1 (KOS/tk12) particles (2 × 106 PFU) were treated with various amounts of MβCD for 30 min at room temperature. MβCD was then diluted to a final concentration of 0.1 mM or less, a concentration that has no effect on cells, and virus was allowed to infect Vero cells (multiplicity of infection of 10). β-Galactosidase activity at 6 h postinfection was determined as described for Fig. 1. The results presented are representative of two independent experiments done in triplicate, with standard deviations.
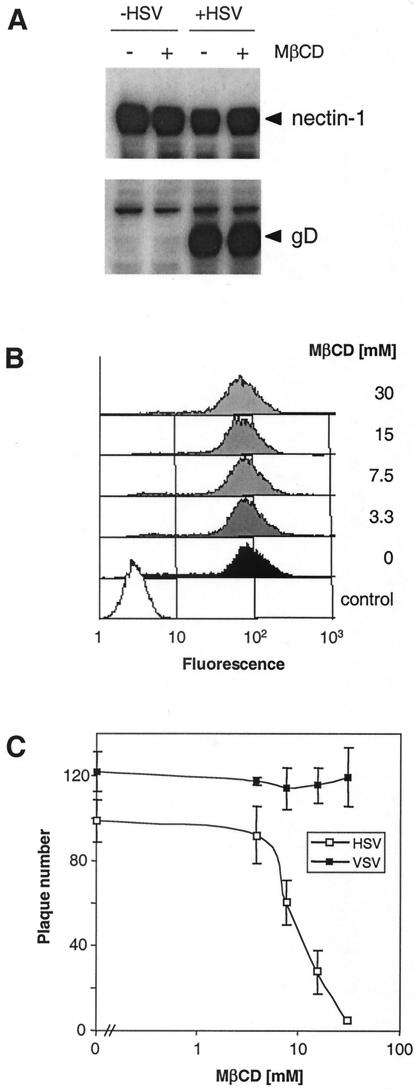
Another possibility is that MβCD inhibited virus attachment to cells. We treated C10 cells with 30 mM MβCD, washed them extensively, and added HSV-1 (KOS) for 1 h at 4°C. Extracts of untreated and MβCD-treated cells contained the same amount of glycoprotein D (gD), as measured by SDS-PAGE and Western blotting (Fig. 3A). In addition, drug treatment did not alter the amount of nectin-1 detected in the same extracts (Fig. 3A). Surface expression of nectin-1 (Fig. 3B) and HVEM (not shown) was not significantly affected by MβCD, as demonstrated by FACS analysis. Together, these observations rule out the possibility that MβCD inhibits HSV entry by either preventing virus binding to cells or changing the receptor levels present in the cells.
FIG. 3.
Effect of cholesterol depletion from cells on expression of nectin-1 and on attachment and replication of HSV. (A) C10 cells were untreated (−) or treated (+) with 30 mM MβCD for 30 min. The drug was washed out, and HSV-1 (KOS) was allowed to bind to the cells for 1 h at 4°C. Total cell proteins were extracted, resolved by SDS-PAGE, and probed with anti-nectin-1 MAb CK6 (upper panel) or anti-gD MAb DL6 (lower panel). (B) C10 cells were treated with increasing concentrations of MβCD (indicated at the right), then stained with anti-nectin-1 MAb CK41 directly labeled with phycoerythrin, and analyzed by FACS. The control (open area) represents the fluorescence of unstained and drug untreated cells. (C) Vero cells were treated with increasing concentrations of MβCD. The drug was washed out, and the cells were infected with HSV1 (KOS) or vesicular stomatitis virus (VSV) for 24 h at 37°C. Then the cells were fixed, and plaques werevisualized by immunoperoxidase staining (HSV) or crystal violet staining (vesicular stomatitis virus).
We also evaluated the effect of cholesterol sequestration on HSV replication in a plaque formation assay. Vero cells were treated with MβCD for 30 min, the drug was washed out, and then cells were infected with HSV-1 (KOS). As a control, MβCD-treated cells were also infected with vesicular stomatitis virus, which is known to be raft independent (42, 59). Plaques were stained 24 h later and counted (Fig. 3C). The number of HSV plaques was reduced in a dose-dependent manner, with 50% inhibition occurring at 10 mM MβCD. In contrast up to 30 μM MβCD did not reduce the number of vesicular stomatitis virus plaques made. These results underscore the importance of plasma membrane cholesterol content for efficient viral replication and support the observations made in the entry assays. In addition, we observed that the few plaques that formed in cells treated with MβCD were markedly smaller (not shown). However, plaque sizes were normal after longer periods of infection, and the reduction in plaque number in drug-treated cells was less severe (not shown). These observations suggest that cholesterol depletion imposes a delay in the onset of infection by HSV.
Cholesterol replenishment reverses effect of MβCD.
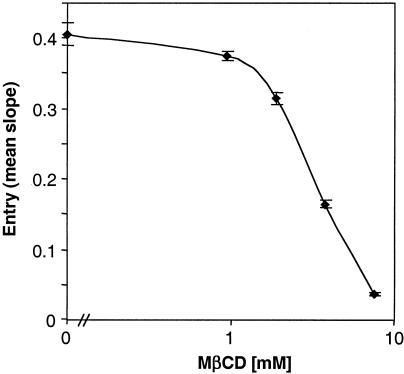
To show that the inhibitory drug effects were specific, we replaced the cholesterol after sequestration and tested whether HSV entry was restored. We treated Vero cells with 7.5 mM MβCD for 30 min, a concentration that inhibited entry by 50% (Fig. 1A, arrow). The drug was washed out, and increasing concentrations of cholesterol were then added to reconstitute plasma membrane cholesterol levels. Excess cholesterol was washed out, and the cells were infected with HSV-1 (KOS/tk12) (Fig. 4). The addition of cholesterol completely reversed the inhibitory effect of MβCD in a dose-dependent fashion. We conclude that plasma membrane cholesterol is very important during HSV entry because sequestration or chelation of cholesterol prior to HSV infection inhibited entry, an effect reversed by the addition of exogenous cholesterol. Since rafts are maintained via cholesterol (reviewed in reference 9), we wondered whether inhibition of HSV entry mediated by cholesterol-sequestering drugs was due to raft dispersion.
FIG. 4.
Effect of cholesterol replenishment on HSV entry. Vero cells were untreated (left, rectangles) or treated (right, rectangles) with 7.5 mM MβCD for 30 min. MβCD was washed out, and various amounts of cholesterol in MβCD were added. After 30 min, the cholesterol was removed, and the cells were infected with HSV-1 (KOS/tk12) and assayed for β-galactosidase activity at 6 h postinfection. The results presented are representative of two independent experiments done in triplicate.
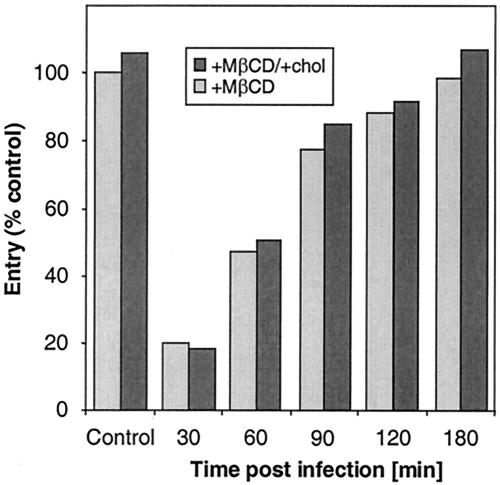
Effect of cholesterol depletion after HSV entry.
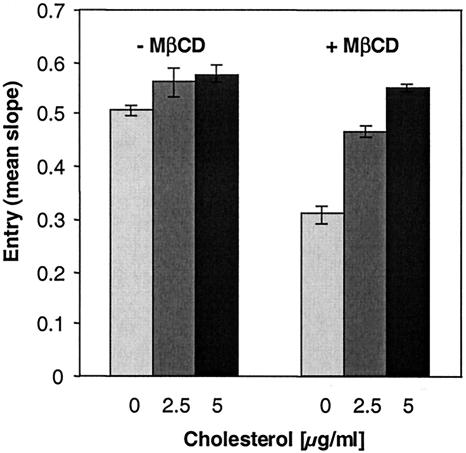
To test whether cholesterol sequestration had an effect after entry but during replication, Vero cells were first infected with HSV-1 (KOS/tk12) and at various times postinfection treated for 30 min with 7.5 mM MβCD (Fig. 5). MβCD markedly inhibited HSV replication when it was added at 30 min postinfection, but the level of inhibition decreased when the drug was added at later times. Normal entry was observed when MβCD was applied to cells between 2 to 3 h after infection. Interestingly, at any time following infection, MβCD-mediated inhibition of entry was not reversible by replenishment of cholesterol. These data suggest that rafts may be important during the early events of HSV replication, like fusion, but play no role later, once virus entry has occurred. They also provide further evidence that MβCD is not toxic for cells.
FIG. 5.
Effect of cholesterol depletion from cells on the course of HSV entry. Vero cells were infected with HSV-1 (KOS/tk12). At various times following infection (indicated on the abscissa), cells were treated for 30 min with MβCD. The drug was washed out, and medium or cholesterol (chol) was added. After 30 min, the cholesterol was removed, and infection was allowed to proceed. β-Galactosidase activity at 6 h postinfection was determined. The results shown are representative of three independent experiments, and 100% entry represents the β-galactosidase activity at 6 h in the absence of MβCD.
Distribution of HSV receptors on the plasma membrane: are the viral receptors HVEM and nectin-1 found in rafts?
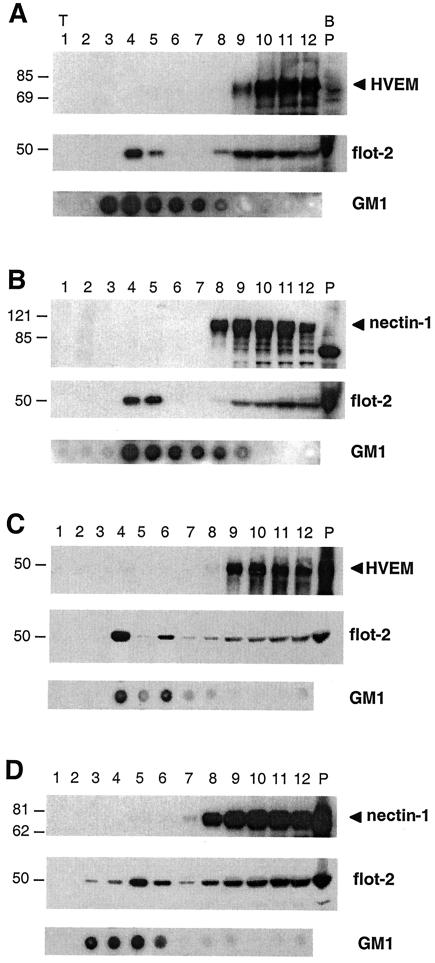
All these experiments suggested that cholesterol-rich rafts might play a role in HSV entry. One possibility is that HSV receptors are associated with rafts or redistribute into rafts as a consequence of binding to virus glycoproteins. To assess these possibilities, we used sucrose gradients to isolate low-density detergent-insoluble fractions from cells. B78 cells stably expressing HVEM-GFP or nectin-1 were extracted in Triton X-100 and analyzed for receptor distribution into low- and high-density fractions (Fig. 6). HVEM-GFP was used because its larger molecular size allowed us to visualize it more easily than native HVEM by Western blotting. The ganglioside GM1 and the protein flotillin-2 are both associated with rafts and were used as markers.
FIG. 6.
Association of HSV receptors with detergent-insoluble low-density sucrose fractions containing rafts. 3E5 cells stably expressing HVEM-GFP (A), C10 cells stably expressing nectin-1 (B), and 293T cells transiently transfected with a plasmid expressing the HVEM (C) or nectin-1 (D) gene were homogenized in Triton X-100 and fractionated on sucrose gradients. Proteins (10 μl of each fraction) were resolved on SDS-PAGE and transferred to nitrocellulose, and the distribution of HSV receptors was analyzed by Western blotting with MAb CW10 to HVEM or MAb CK6 to nectin-1. Fraction numbers are indicated at the top of the figure (P for pellet). T and B refer to the top and bottom of the gradient, respectively. The distribution of the known raft-associated protein flotillin-2 (flot-2) was analyzed with a commercial MAb and served as a positive control for rafts (A, B, C, and D, middle panels). Also shown is the distribution of the raft-specific ganglioside GM1 analyzed by dot blot with CTB-HRP (A, B, C, and D, lower panels). Sizes are shown on the left (in kilodaltons).
GM1 was mostly enriched in the low-density fractions (numbers 3 to 7), and a substantial portion of flotillin-2 was recovered in fractions 4 and 5. This is in agreement with other reports (6, 27). Neither HVEM-GFP (Fig. 6A) nor nectin-1 (Fig. 6B) was found in the low-density sucrose fractions (numbers 1 to 6). These proteins were only recovered in the high-density fractions (numbers 7 to 12), where nonraft proteins are found. Similarly, when 293T cells were transiently transfected with plasmids expressing wild-type HVEM (Fig. 6C) or nectin-1 (Fig. 6D), neither receptor was found in the GM1/flotillin-2-rich fractions.
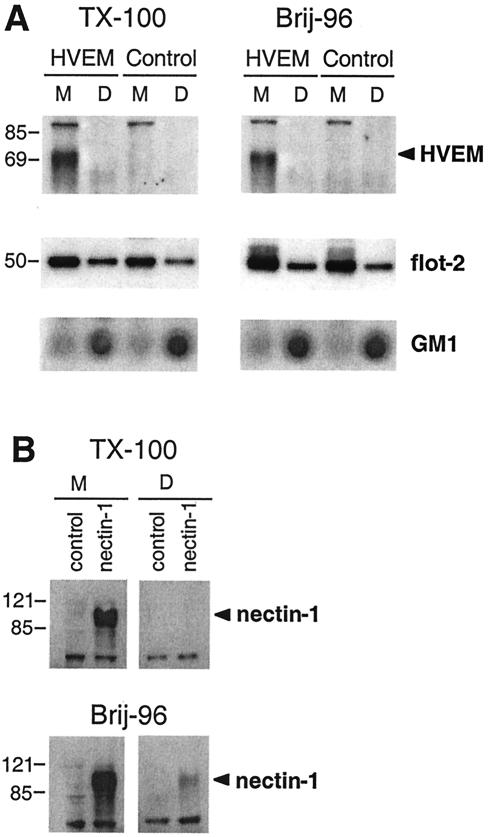
As a second approach, we used a more general procedure for two reasons. First, some proteins, such as prion protein, are enriched in semiordered lipid margins, located at the interface between the highly ordered lipid raft domains and the fluid glycerolipid membrane domain (40). These margin domains are solubilized in Triton X-100 but not in the milder detergent Brij-96. Second, due to strong interactions with the cytoskeleton, some raft-associated molecules, such as TRAF-3, only minimally associate with low-density fractions in sucrose gradients but are mainly recovered in the pellet of the gradient together with the cytoskeleton (29). Nectin-1 associates with afadin, which in turn associates with the cytoskeleton via actin (41, 75). Thus, it was possible that one or both receptors might be minimally associated with rafts.
A less stringent way to examine such interactions is to solubilize the membranes with a milder detergent, such as Brij-96, and then to use centrifugation to separate glycolipid domains (DIG) and the cytoskeleton. Then DIG which also contain rafts are solubilized and separated from the cytoskeleton with octylglycoside (17). Therefore, we lysed HVEM-GFP cells and C10 cells with Triton X-100 or Brij-96 and isolated the DIG fractions (Fig. 7). In no case was HVEM found in fractions containing DIG (Fig. 7A), although a substantial amount of flotillin-2 and all of GM1 were detected in these fractions. These results are consistent with those obtained by sucrose gradient centrifugation. Similarly, nectin-1 was not detected in the DIG recovered from cells lysed with Triton X-100 (Fig. 7B, top panel). However, a small but reproducible fraction of nectin-1 was found associated with DIG recovered from cells solubilized with Brij-96, suggesting that a small amount of the protein may associate with rafts (Fig. 7B, bottom). However, the amount of nectin-1 found in the DIG fraction never exceeded the amount of the transferrin receptor found in this fraction (not shown). The transferrin receptor is a classical marker of a nonraft membrane protein (27). This suggests that nectin-1 detected in DIG did not come from a pool of molecules associated with rafts but rather was a contaminant of the fractionation procedure. We therefore conclude that HVEM and nectin-1 are not associated with lipid raft fractions in HVEM cells or nectin-1 cells regardless of the fractionation technique or the detergent used.
FIG. 7.
Association of HSV receptors with detergent-insoluble glycolipid complexes. 3E5 cells (A), C10 cells (B), and control parental B78-H1 cells were fractionated into detergent-soluble (DSM) and insoluble (DIG) membrane fractions with either Triton X-100 (TX-100) or Brij-96. The distribution of the two HSV receptors was analyzed by Western blotting as described for Fig. 6. At the top are indicated the receptor expressed (control, HVEM, or nectin-1), the detergent used (Triton X-100 or Brij-96), and the fraction, DSM (M) or DIG (D). Sizes are shown on the left (in kilodaltons).
Distribution of HSV glycoproteins on plasma membrane during entry.
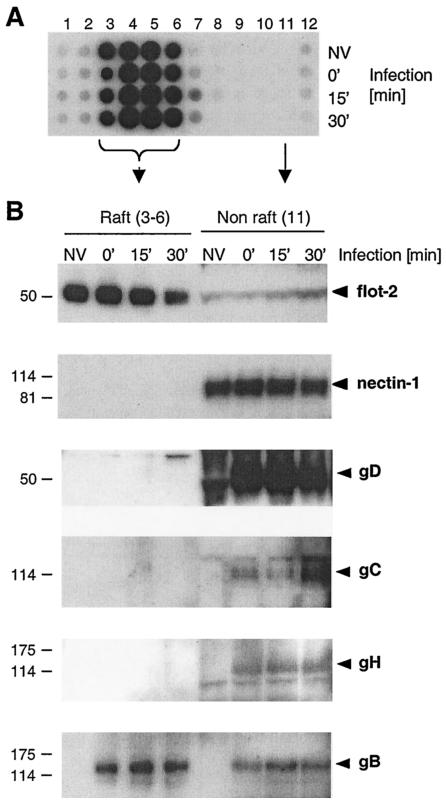
We asked whether HSV receptors redistribute into lipid rafts after initial virus association or during entry. Manes et al. proposed this process for HIV gp120 and its receptors (42). Accordingly, C10 cells were infected with HSV and lysed with Triton X-100 at 0, 15, or 30 min postinfection. Lysates were subjected to sucrose gradient centrifugation (Fig. 8). GM1 was detected with the low-density fractions 3 to 6 at all three times postinfection (Fig. 8A). To facilitate analysis, we chose a representative nonraft fraction (fraction 11) and compared this with pooled fractions 3 to 6 (Fig. 8B and 8C). The raft marker flotillin-2 was found primarily in pooled fractions 3 to 6 (Fig. 8B). However, nectin-1 was excluded from these fractions and instead was located entirely in the nonraft fractions at 0, 15, and 30 min postinfection (Fig. 8B).
FIG. 8.
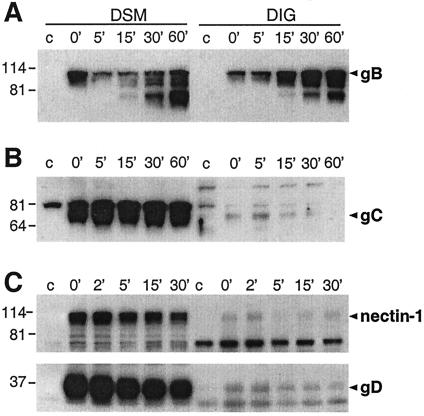
Association of HSV receptors and glycoproteins with detergent-insoluble low-density sucrose fractions during entry. C10 cells were infected with HSV (KOS), and at various times postinfection, cells were extracted in Triton X-100 and fractionated on a sucrose gradient as in Fig. 6. (A) The distribution of GM1 in the different sucrose gradient fractions was analyzed by dot blot in noninfected cells (NV) and after infection for various times, indicated at the right of the panel. (B) Low-density fractions 3 to 6 containing the raft marker GM1 (raft) were pooled, precipitated with methanol-chloroform, resuspended in gel sample buffer, and resolved by SDS-PAGE. The distribution of flotillin-2 (flot-2) and nectin-1 at various times during infection (indicated at the top of the panel) was compared to that found in 10 μl of the GM1-negative fraction 11 (non-raft) by Western blotting on separate membranes. The distribution of the glycoproteins (indicated at the right) in raft and nonraft fractions was analyzed with MAb DL6 to gD, polyclonal antibody R47 to gC, polyclonal antibody R137 to gH, and MAb SS-10 to gB. Sizes are shown on the left (in kilodaltons).
During this time period, gD also was restricted to nonraft fractions (Fig. 8B), suggesting that any association of gD with nectin-1 at the time of or shortly after infection occurred outside lipid rafts. Similarly, gC and gH, two other glycoproteins involved in entry, were restricted to nonraft fractions (Fig. 8B). Interestingly, a substantial fraction of gB associated with rafts at 4°C, and this proportion remained constant through the early steps of entry (Fig. 8B). However, when purified virions were fractionated on a sucrose gradient in the absence of cells, gB could not be detected in raft fractions (not shown). Similarly, gB was not selectively associated with DIG compared to gD and gH/gL when transfected in 293T cells (not shown). Thus, it is unlikely that gB interacts with rafts during the extraction procedure or during sedimentation. The association of gB with rafts requires the initial binding of the virus to a cell surface.
Distribution of soluble HSV glycoproteins after association with plasma membrane.
These studies suggested that gB interacts specifically with lipid rafts during virus entry. Because this association was observed at time zero, it was possible that the ectodomain of virion gB might associate with a raft-associated cell molecule. To test this, we incubated cells with purified ectodomains of gB, gC, or gD and evaluated the association of the different glycoproteins with the DIG fraction. Also, to mimic the situation of virus entry, the proteins were added to the cells at 4°C and the temperature was shifted to 37°C for various times (Fig. 9). At 4°C and time zero, soluble gB was already associated with the DIG fraction (Fig. 9A, 0 min). Interestingly, the apparent amount and proportion of gB in the DIG fraction increased during incubation at 37°C for up to 1 h (Fig. 9A, DIG). During the same period, the proportion of the full-size gB ectodomain found in the DSM was constant, although there was an accumulation of degradation products over time (Fig. 9A, DSM).
FIG. 9.
Association of HSV soluble glycoproteins with detergent-insoluble glycolipid complexes. (A) Soluble gB, (B) gC, or (C) gD (0.1 μM each) was bound to C10 cells at 4°C, and the temperature was shifted at 37°C for various times (in minutes), as indicated at the top of the panel. Cells were extracted in 1% Brij-96, and the distribution of each glycoprotein into DSM and DIG was analyzed as described for Fig. 8. For each experiment, the control (c) without glycoproteins (A and B) or on parental nectin-1-negative B78-H1 cells (C) is shown. Panel C also shows the distribution of nectin-1. Sizes are shown on the left (in kilodaltons).
By contrast, gC (Fig. 9B) and gD (Fig. 9C, lower panel) were almost excluded from DIG at all time points. The low levels of gD found in DIG are consistent with the low levels of nectin-1 found in this fraction (Fig. 9C, top panel). The fact that gC was also found exclusively in the DSM is important because it, like gB, associates with heparan sulfate on cells (61). Thus, it is unlikely that the distribution of gB into DIG is related to heparan sulfate binding. If so, gB but not gC would associate with a specific type of HSPG enriched in DIG, or gB and gC could have differential effects when bound to HSPG. The association of soluble gB with DIG corroborates our observations made with virion gB. Together, the data suggest that the interaction of gB with rafts does not depend on its transmembrane domain or carboxy terminus but is mediated by the ectodomain.
DISCUSSION
Requirement of cholesterol for HSV entry.
The entry of HSV into cells is a complex process that is not completely understood. It involves four viral glycoproteins, gB, gD, and gH/gL, and at least one of the gD receptors (reviewed in references 11 and 72). Lipid rafts represent a privileged microdomain that may facilitate virus entry (reviewed in references 9, 12, 48, 67, 71, and 76). We found that sequestration of cholesterol, an essential constituent of rafts, inhibits entry into cells expressing either HVEM or nectin-1. Entry was restored by replenishing the cells with cholesterol, confirming the requirement for cholesterol. Since cholesterol is an essential structural molecule of rafts (9), we hypothesize that rafts may be directly involved during entry. HSV entry may also require the presence of cholesterol in the plasma membrane independently of rafts. In particular, fusion may require cholesterol regardless of the maintenance of rafts.
Fusion of alphaviruses requires the presence of both cholesterol and sphingolipids in target membrane, but the fusion activity is not dependent on the presence of DIG (79). Similarly, depleting plasma membrane cholesterol inhibits HIV-1 entry, but the presence of HIV-1 receptors in rafts is not required for infection (57). These observations suggest that cholesterol modulates the HIV-1 entry process independently of its ability to promote raft formation. Cholesterol is, however, essential for the conformation and function of CXCR4 and CCR5 (50, 51). Alternatively, it was proposed that binding of HIV may be favored by the presence of CD4 in rafts, but rafts may then disperse prior to the membrane fusion reaction (35). Since gB was found in lipid rafts during attachment of HSV, this suggests a function for these structures during entry, in particular for fusion.
HVEM and nectin-1 are not associated with lipid rafts.
HIV infection induces lateral membrane diffusion following interaction of the viral envelope with cell surface receptors, which are necessary for infection (42). We found that HVEM was not associated with rafts in uninfected cells regardless of which detergent was used for extraction. This was a surprise, because other tumor necrosis factor receptors such as CD40, CD120a, and the p75 neurotrophin receptor are associated with rafts (7, 16, 30, 33). The cytosolic tail of CD120a containing the death domain is necessary and sufficient for both localization of the receptor to lipid rafts and signaling apoptosis. This implies that receptor localization to lipid rafts is important for the function of this receptor (16).
The absence of a death domain in HVEM may explain its particular membrane distribution (46). On the other hand, CD40 engagement by MAbs leads to a membrane raft-restricted recruitment of TRAF-3 and TRAF-2 to CD40's cytoplasmic tail. This indicates that the membrane raft structure plays an integral role in CD40 signaling (78). A similar domain is likely important for HVEM function. However, binding of gD did not result in redistribution of HVEM into rafts. Moreover, deletion of part of the cytosolic tail of HVEM comprising the TRAF-2 and TRAF-5 binding sites required for NF-κB activation had no effect on its function as an HSV receptor (31, 46).
Based on the model proposed for the T- and B-cell receptors and the Fcɛ receptor (14, 15, 47, 73), we hypothesized that the immunoglobulin family member nectin-1 would also associate with rafts during entry. Nectin-1 is located at tight junctions (22), and tight junctions are found in membrane microdomains (53). However, we found that nectin-1 was not associated with lipid rafts in uninfected cells and that this distribution did not change during infection. If rafts are involved in HSV entry, it is not because of the localization of the gD receptors in these structures. Localization of nectin-1 in tight junctions and rafts in vivo should be confirmed.
gB associates with lipid rafts during entry.
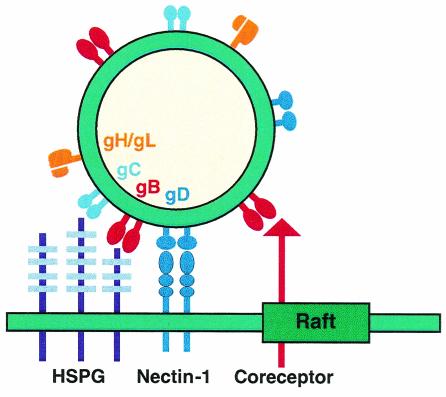
The interesting finding that gB but not gD, gC, or gH associated with rafts after virus attachment and during entry may point to a unique function for gB during HSV entry that involves association with lipid rafts. We hypothesize that a cellular gB receptor(s) is enriched in rafts, a situation that would explain the unique association of gB with microdomains during entry (Fig. 10). Localization of this unknown molecule in rafts would also account for the inhibition of virus entry by MβCD and nystatin. Rafts are likely to be less than 70 nm in diameter (21, 77). Thus, the envelope of HSV (which is 150 to 200 nm in diameter) could potentially interact simultaneously with both raft and nonraft domains during attachment. This would explain how only gB is found associated with rafts (Fig. 10).
FIG. 10.
Model of HSV attachment involving lipid rafts. Initial attachment of HSV is mediated through the interaction of gB and/or gC with HSPG. These interactions are likely to occur in nonraft domains, since gC and a fraction of gB are found in DSM. Then a specific interaction of gD with a cognate receptor (here, nectin-1) is essential for fusion to occur. Both gD and nectin-1 are excluded from rafts during attachment and throughout the entry process. However, a fraction of gB is associated with rafts from the moment of attachment and during entry. Such a distribution can be explained by the interaction of gB with an unknown receptor enriched in rafts. The size of the HSV virion (150 to 200 nm) would allow its envelope to interact with a plasma membrane region that can encompass at least one raft microdomain (70 nm).
Among the herpesviruses, gB is the most highly conserved glycoprotein, suggesting that this molecule possesses a central function which is common for the entry of different herpes viruses (58). It is of interest that cytomegalovirus gB activates the interferon response pathway via an interaction with an unidentified cellular receptor different from HSPG (8). Activation of these genes involves the mitogen-activated protein kinase pathway and protein kinase C. Liquid-ordered domains represent the preferential location for the initiation of the mitogen-activated protein kinase as well as other signaling cascades (reviewed in reference 71). Thus, the potential cytomegalovirus gB receptor may also localize in rafts, where it elicits signaling upon cytomegalovirus attachment. The association of HSV gB with lipid rafts during entry may be necessary to elicit rapid signaling events. Transient tyrosine phosphorylation of several proteins occurs within minutes after infection, followed by activation of NF-κB. Both of these events support the idea that signaling cascades parallel HSV entry (data not shown) (60). The direct involvement of HSV gB in signaling remains to be investigated.
The role of rafts during HSV infection has been investigated by others. Sucrose gradient fractionation suggested that the UL56 gene product is a membrane protein associated with rafts (34). A minor fraction of the tegument protein vhs as well as VP16 and gH localizes to DIG in HSV-infected cells (37). We found that gH as well as gB, gC, and gD was excluded from DIG in the virion envelope (data not shown). Similarly, these glycoproteins were not in DIG from either cells infected with HSV for 8 h or cells transfected with the individual glycoproteins (data not shown). We suspect that the traces detected in rafts are likely a consequence of contamination.
Another interesting observation was that treatment of herpes simplex virions with a cholesterol-sequestering drug inhibited entry. Replenishment with cholesterol partially restored entry. Thus, cholesterol may play an important role in determining the structure of the viral envelope. Whether cholesterol is important for the organization of rafts on the virion is not known. However, rafts are found in the Golgi complex (24), a cellular compartment important for the final envelopment of virus (reviewed in reference 44). Thus, it is possible that some rafts may be incorporated in the viral envelope. Similar experiments with HIV showed that MβCD-treated virus was less infectious (25).
The association of gB with rafts during entry could represent a step in our understanding of fusion. Semliki Forest virus fusion protein E1 inserts preferentially in membrane domains enriched in cholesterol and sphingolipid (1). By analogy, HSV gB may preferentially bind to a raft-associated molecule and play a direct role in fusion through these microdomains. The other glycoproteins would contribute through strong and specific interactions with cognate receptors, bringing the viral envelope and plasma membrane into close apposition. Alternatively, conformational changes in the entry glycoproteins induced by binding to receptors could result in the formation of a fusion complex which includes the association of gB with rafts. Identification of the gB binding molecules as well as characterization of the individual involvement of each glycoprotein during this process are essential to answer these questions.
Acknowledgments
This work was supported by Public Health Service grants NS-30606 and NS-36731 from the National Institute of Neurological Diseases and Stroke (R.J.E. and G.H.C.) and grant AI-18289 from the National Institute of Allergy and Infectious Diseases (R.J.E. and G.H.C.).
We thank N. W. Fraser, P. G. Spear, and R. N. Harty at the University of Pennsylvania for reagents. We are grateful to F. Baribaud and B. J. Shenker for help with FACS. We also thank all the members of the Cohen and Eisenberg laboratories for critical advice and helpful discussions.
REFERENCES
- 1.Ahn, A., D. L. Gibbons, and M. Kielian. 2002. The fusion peptide of Semliki Forest virus associates with sterol-rich membrane domains. J. Virol. 76:3267-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of IκB kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, H. A., Y. Chen, and L. C. Norkin. 1996. Bound simian virus 40 translocates to caveolin-enriched membrane domains, and its entry is inhibited by drugs that selectively disrupt caveolae. Mol. Biol. Cell 7:1825-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barman, S., and D. P. Nayak. 2000. Analysis of the transmembrane domain of influenza virus neuraminidase, a type II transmembrane glycoprotein, for apical sorting and raft association. J. Virol. 74:6538-6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bickel, P. E., P. E. Scherer, J. E. Schnitzer, P. Oh, M. P. Lisanti, and H. F. Lodish. 1997. Flotillin and epidermal surface antigen define a new family of caveola-associated integral membrane proteins. J. Biol. Chem. 272:13793-13802. [DOI] [PubMed] [Google Scholar]
- 7.Bilderback, T. R., R. J. Grigsby, and R. T. Dobrowsky. 1997. Association of p75(NTR) with caveolin and localization of neurotrophin-induced sphingomyelin hydrolysis to caveolae. J. Biol. Chem. 272:10922-10927. [DOI] [PubMed] [Google Scholar]
- 8.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 10.Brown, K. D., B. S. Hostager, and G. A. Bishop. 2001. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1). J. Exp. Med. 193:943-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 12.Campbell, S. M., S. M. Crowe, and J. Mak. 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol. 22:217-227. [DOI] [PubMed] [Google Scholar]
- 13.Chen, Y., and L. C. Norkin. 1999. Extracellular simian virus 40 transmits a signal that promotes virus enclosure within caveolae. Exp. Cell Res. 246:83-90. [DOI] [PubMed] [Google Scholar]
- 14.Cheng, P. C., M. L. Dykstra, R. N. Mitchell, and S. K. Pierce. 1999. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190:1549-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherukuri, A., M. Dykstra, and S. K. Pierce. 2001. Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity 14:657-660. [DOI] [PubMed] [Google Scholar]
- 16.Cottin, V., J. E. Doan, and D. W. Riches. 2002. Restricted localization of the tumor necrosis factor receptor CD120a to lipid rafts: a novel role for the death domain. J. Immunol. 168:4095-4102. [DOI] [PubMed] [Google Scholar]
- 17.Doucey, M. A., D. F. Legler, N. Boucheron, J. C. Cerottini, C. Bron, and I. F. Luescher. 2001. CTL activation is induced by cross-linking of TCR/MHC-peptide-CD8/p56lck adducts in rafts. Eur. J. Immunol. 31:1561-1570. [DOI] [PubMed] [Google Scholar]
- 18.Dykstra, M. L., A. Cherukuri, and S. K. Pierce. 2001. Floating the raft hypothesis for immune receptors: access to rafts controls receptor signaling and trafficking. Traffic 2:160-166. [DOI] [PubMed] [Google Scholar]
- 19.Eisenberg, R. J., D. Long, M. Ponce de Leon, J. T. Matthews, P. G. Spear, M. G. Gibson, L. A. Lasky, P. Berman, E. Golub, and G. H. Cohen. 1985. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J. Virol. 53:634-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenberg, R. J., M. Ponce de Leon, H. M. Friedman, L. F. Fries, M. M. Frank, J. C. Hastings, and G. H. Cohen. 1987. Complement component C3b binds directly to purified glycoprotein C of herpes simplex virus types 1 and 2. Microb. Pathog. 3:423-435. [DOI] [PubMed] [Google Scholar]
- 21.Friedrichson, T., and T. V. Kurzchalia. 1998. Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 394:802-805. [DOI] [PubMed] [Google Scholar]
- 22.Fukuhara, A., K. Irie, A. Yamada, T. Katata, T. Honda, K. Shimizu, H. Nakanishi, and Y. Takai. 2002. Role of nectin in organization of tight junctions in epithelial cells. Genes Cells 7:1059-1072. [DOI] [PubMed] [Google Scholar]
- 23.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 24.Gkantiragas, I., B. Brugger, E. Stuven, D. Kaloyanova, X. Y. Li, K. Lohr, F. Lottspeich, F. T. Wieland, and J. B. Helms. 2001. Sphingomyelin-enriched microdomains at the Golgi complex. Mol. Biol. Cell 12:1819-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guyader, M., E. Kiyokawa, L. Abrami, P. Turelli, and D. Trono. 2002. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol. 76:10356-10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Handler, C. G., R. J. Eisenberg, and G. H. Cohen. 1996. Oligomeric structure of glycoproteins in herpes simplex virus type 1. J. Virol. 70:6067-6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harder, T., P. Scheiffele, P. Verkade, and K. Simons. 1998. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141:929-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henderson, G., J. Murray, and R. P. Yeo. 2002. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology 300:244-254. [DOI] [PubMed] [Google Scholar]
- 29.Higuchi, M., K. M. Izumi, and E. Kieff. 2001. Epstein-Barr virus latent-infection membrane proteins are palmitoylated and raft-associated: protein 1 binds to the cytoskeleton through tumor necrosis factor receptor cytoplasmic factors. Proc. Natl. Acad. Sci. USA 98:4675-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hostager, B. S., I. M. Catlett, and G. A. Bishop. 2000. Recruitment of CD40 and tumor necrosis factor receptor-associated factors 2 and 3 to membrane microdomains during CD40 signaling. J. Biol. Chem. 275:15392-15398. [DOI] [PubMed] [Google Scholar]
- 31.Hsu, H., I. Solovyev, A. Colombero, R. Elliott, M. Kelley, and W. J. Boyle. 1997. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5. J. Biol. Chem. 272:13471-13474. [DOI] [PubMed] [Google Scholar]
- 32.Hueber, A. O., A. M. Bernard, Z. Herincs, A. Couzinet, and H. T. He. 2002. An essential role for membrane rafts in the initiation of Fas/CD95-triggered cell death in mouse thymocytes. EMBO Rep. 3:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko, Y. G., J. S. Lee, Y. S. Kang, J. H. Ahn, and J. S. Seo. 1999. tumor necrosis factor-alpha-mediated apoptosis is initiated in caveolae-like domains. J. Immunol. 162:7217-7223. [PubMed] [Google Scholar]
- 34.Koshizuka, T., F. Goshima, H. Takakuwa, N. Nozawa, T. Daikoku, O. Koiwai, and Y. Nishiyama. 2002. Identification and characterization of the UL56 gene product of herpes simplex virus type 2. J. Virol. 76:6718-6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozak, S. L., J. M. Heard, and D. Kabat. 2002. Segregation of CD4 and CXCR4 into distinct lipid microdomains in T lymphocytes suggests a mechanism for membrane destabilization by human immunodeficiency virus. J. Virol. 76:1802-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krummenacher, C., I. Baribaud, J. F. Sanzo, G. H. Cohen, and R. J. Eisenberg. 2002. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J. Virol. 76:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, G. E., G. A. Church, and D. W. Wilson. 2003. A subpopulation of tegument protein vhs localizes to detergent-insoluble lipid rafts in herpes simplex virus-infected cells. J. Virol. 77:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindwasser, O. W., and M. D. Resh. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu, X., and J. Silver. 2000. Ecotropic murine leukemia virus receptor is physically associated with caveolin and membrane rafts. Virology 276:251-258. [DOI] [PubMed] [Google Scholar]
- 40.Madore, N., K. L. Smith, C. H. Graham, A. Jen, K. Brady, S. Hall, and R. Morris. 1999. Functionally different GPI proteins are organized in different domains on the neuronal surface. EMBO J. 18:6917-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandai, K., H. Nakanishi, A. Satoh, H. Obaishi, M. Wada, H. Nishioka, M. Itoh, A. Mizoguchi, T. Aoki, T. Fujimoto, Y. Matsuda, S. Tsukita, and Y. Takai. 1997. Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 139:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manes, S., G. del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. C. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, C. G., C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and N. W. Fraser. 2001. Development of a syngenic murine B16 cell line-derived melanoma susceptible to destruction by neuroattenuated HSV-1. Mol. Ther. 3:160-168. [DOI] [PubMed] [Google Scholar]
- 46.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the tumor necrosis factor/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 47.Montixi, C., C. Langlet, A. M. Bernard, J. Thimonier, C. Dubois, M. A. Wurbel, J. P. Chauvin, M. Pierres, and H. T. He. 1998. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 17:5334-5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nayak, D. P., and S. Barman. 2002. Role of lipid rafts in virus assembly and budding. Adv. Virus Res. 58:1-28. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen, D. H., and D. Taub. 2002. Cholesterol is essential for macrophage inflammatory protein 1 beta binding and conformational integrity of CC chemokine receptor 5. Blood 99:4298-4306. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen, D. H., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121-4126. [DOI] [PubMed] [Google Scholar]
- 52.Nicola, A. V., S. H. Willis, N. N. Naidoo, R. J. Eisenberg, and G. H. Cohen. 1996. Structure-function analysis of soluble forms of herpes simplex virus glycoprotein D. J. Virol. 70:3815-3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nusrat, A., C. A. Parkos, P. Verkade, C. S. Foley, T. W. Liang, W. Innis-Whitehouse, K. K. Eastburn, and J. L. Madara. 2000. Tight junctions are membrane microdomains. J. Cell Sci. 113:1771-1781. [DOI] [PubMed] [Google Scholar]
- 54.Patel, A., J. Hanson, T. I. McLean, J. Olgiate, M. Hilton, W. E. Miller, and S. L. Bachenheimer. 1998. Herpes simplex type 1 induction of persistent NF-kappa B nuclear translocation increases the efficiency of virus replication. Virology 247:212-222. [DOI] [PubMed] [Google Scholar]
- 55.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 56.Peng, T., M. Ponce-de-Leon, H. Jiang, G. Dubin, J. M. Lubinski, R. J. Eisenberg, and G. H. Cohen. 1998. The gH-gL complex of herpes simplex virus (HSV) stimulates neutralizing antibody and protects mice against HSV type 1 challenge. J. Virol. 72:65-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Percherancier, Y., B. Lagane, T. Planchenault, I. Staropoli, R. Altmeyer, J. L. Virelizier, F. Arenzana-Seisdedos, D. C. Hoessli, and F. Bachelerie. 2003. HIV-1 entry into T-cells is not dependent on CD4 and CCR5 localization to sphingolipid-enriched, detergent-resistant, raft membrane domains. J. Biol. Chem. 278:3153-3161. [DOI] [PubMed] [Google Scholar]
- 58.Pereira, L. 1994. Function of glycoprotein B homologues of the family Herpesviridae. Infect. Agents Dis. 3:9-28. [PubMed] [Google Scholar]
- 59.Popik, W., T. M. Alce, and W. C. Au. 2002. Hum. immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qie, L., D. Marcellino, and B. C. Herold. 1999. Herpes simplex virus entry is associated with tyrosine phosphorylation of cellular proteins. Virology 256:220-227. [DOI] [PubMed] [Google Scholar]
- 61.Rux, A. H., H. Lou, J. D. Lambris, H. M. Friedman, R. J. Eisenberg, and G. H. Cohen. 2002. Kinetic analysis of glycoprotein C of herpes simplex virus types 1 and 2 binding to heparin, heparan sulfate, and complement component C3b. Virology 294:324-332. [DOI] [PubMed] [Google Scholar]
- 62.Rux, A. H., S. H. Willis, A. V. Nicola, W. Hou, C. Peng, H. Lou, G. H. Cohen, and R. J. Eisenberg. 1998. Functional region IV of glycoprotein D from herpes simplex virus modulates glycoprotein binding to the herpesvirus entry mediator. J. Virol. 72:7091-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sapin, C., O. Colard, O. Delmas, C. Tessier, M. Breton, V. Enouf, S. Chwetzoff, J. Ouanich, J. Cohen, C. Wolf, and G. Trugnan. 2002. Rafts promote assembly and atypical targeting of a nonenveloped virus, rotavirus, in Caco-2 cells. J. Virol. 76:4591-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sargiacomo, M., M. Sudol, Z. Tang, and M. P. Lisanti. 1993. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J. Cell Biol. 122:789-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 66.Scheiffele, P., M. G. Roth, and K. Simons. 1997. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 16:5501-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shin, J. S., and S. N. Abraham. 2001. Caveolae-not just craters in the cellular landscape. Science 293:1447-1448. [DOI] [PubMed] [Google Scholar]
- 68.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 69.Shukla, D., and P. G. Spear. 2001. Herpesviruses and heparan sulfate: an intimate relationship in aid of viral entry. J. Clin. Investig. 108:503-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sisk, W. P., J. D. Bradley, R. J. Leipold, A. M. Stoltzfus, M. Ponce de Leon, M. Hilf, C. Peng, G. H. Cohen, and R. J. Eisenberg. 1994. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 68:766-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smart, E. J., G. A. Graf, M. A. McNiven, W. C. Sessa, J. A. Engelman, P. E. Scherer, T. Okamoto, and M. P. Lisanti. 1999. Caveolins, liquid-ordered domains, and signal transduction. Mol. Cell. Biol. 19:7289-7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 73.Stauffer, T. P., and T. Meyer. 1997. Compartmentalized IgE receptor-mediated signal transduction in living cells. J. Cell Biol. 139:1447-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stuart, A. D., H. E. Eustace, T. A. McKee, and T. D. Brown. 2002. A novel cell entry pathway for a DAF-with human enterovirus is dependent on lipid rafts. J. Virol. 76:9307-9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van der Goot, F. G., and T. Harder. 2001. Raft membrane domains: from a liquid-ordered membrane phase to a site of pathogen attack. Semin. Immunol. 13:89-97. [DOI] [PubMed] [Google Scholar]
- 77.Varma, R., and S. Mayor. 1998. GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394:798-801. [DOI] [PubMed] [Google Scholar]
- 78.Vidalain, P. O., O. Azocar, C. Servet-Delprat, C. Rabourdin-Combe, D. Gerlier, and S. Manie. 2000. CD40 signaling in human dendritic cells is initiated within membrane rafts. EMBO J. 19:3304-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Waarts, B. L., R. Bittman, and J. Wilschut. 2002. Sphingolipid and cholesterol dependence of alphavirus membrane fusion. Lack of correlation with lipid raft formation in target liposomes. J. Biol. Chem. 277:38141-38147. [DOI] [PubMed] [Google Scholar]
- 80.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 81.Watson, R. J., J. H. Weis, J. S. Salstrom, and L. W. Enquist. 1984. Bacterial synthesis of herpes simplex virus types 1 and 2 glycoprotein D antigens. J. Investig. Dermatol. 83:102s-111s. [DOI] [PubMed]
- 82.Whitbeck, J. C., C. Peng, H. Lou, R. Xu, S. H. Willis, M. Ponce de Leon, T. Peng, A. V. Nicola, R. I. Montgomery, M. S. Warner, A. M. Soulika, L. A. Spruce, W. T. Moore, J. D. Lambris, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1997. Glycoprotein D of herpes simplex virus (HSV) binds directly to HVEM, a member of the tumor necrosis factor receptor superfamily and a mediator of HSV entry. J. Virol. 71:6083-6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Whitley, R. J., and B. Roizman. 2001. Herpes simplex virus infections. Lancet 357:1513-1518. [DOI] [PubMed] [Google Scholar]
- 84.Willis, S. H., A. H. Rux, C. Peng, J. C. Whitbeck, A. V. Nicola, H. Lou, W. Hou, L. Salvador, R. J. Eisenberg, and G. H. Cohen. 1998. Examination of the kinetics of herpes simplex virus glycoprotein D binding to the herpesvirus entry mediator, with surface plasmon resonance. J. Virol. 72:5937-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xavier, R., T. Brennan, Q. Li, C. McCormack, and B. Seed. 1998. Membrane compartmentation is required for efficient T cell activation. Immunity 8:723-732. [DOI] [PubMed] [Google Scholar]
- 86.Zhang, J., A. Pekosz, and R. A. Lamb. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]