Abstract
In this paper, we review evidence from comparative studies of primate cortical organization, highlighting recent findings and hypotheses that may help us to understand the rules governing evolutionary changes of the cortical map and the process of formation of areas during development. We argue that clear unequivocal views of cortical areas and their homologies are more likely to emerge for ‘core’ fields, including the primary sensory areas, which are specified early in development by precise molecular identification steps. In primates, the middle temporal area is probably one of these primordial cortical fields. Areas that form at progressively later stages of development correspond to progressively more recent evolutionary events, their development being less firmly anchored in molecular specification. The certainty with which areal boundaries can be delimited, and likely homologies can be assigned, becomes increasingly blurred in parallel with this evolutionary/developmental sequence. For example, while current concepts for the definition of cortical areas have been vindicated in allowing a clarification of the organization of the New World monkey ‘third tier’ visual cortex (the third and dorsomedial areas, V3 and DM), our analyses suggest that more flexible mapping criteria may be needed to unravel the organization of higher-order visual association and polysensory areas.
Keywords: visual cortex, evolution, development, visuotopic organization, architecture, marmoset
1. Introduction
In 1886 a young Australian, Alfred Walter Campbell, moved to Great Britain to pursue a medical education. Following a degree from The University of Edinburgh, postgraduate experience in London, Vienna and Prague, and a subsequent doctorate in Medicine, Campbell took up a position as resident doctor and neuropathologist at the Rainhill Asylum near Liverpool. There he worked for the next 13 years, during which time he completed several highly influential studies investigating correlations between neurological lesions and histological observations (for review, see Eadie 2003). Campbell's principal contribution, Histological studies on the localisation of cerebral function was published in 1905, the same year in which he returned to Australia to become the country's first full-time neurology specialist. This study, the centenary of which is celebrated by the present volume, was ‘…prepared for publication in the Philosophical Transactions, but the manuscript, when laid before the Council, was adjudged of inordinate length…’ (Campbell 1905). Having gone through recent similar experiences with certain of our own journal submissions, we find a measure of solace in this fact. Fortunately for Campbell and for following generations of neuroscientists, The Royal Society had the insight of offering a special grant to enable the publication of his study as a book, in recognition of its perceived importance and quality. Campbell's monography was published approximately at the same time as Brodmann's most famous papers in the Journal für Psychologie und Neurologie (Brodmann 1903a,b, 1905a,b, 1906, 1908a,b), and only slightly predated Brodmann's own magnum opus (Brodmann 1909). While Brodmann's scheme of nomenclature, which recognized a larger number of cortical areas, became the more commonly used over the subsequent 100 years, Campbell's more conservative approach (proposing fewer areas, while recognizing that some of those changed gradually in character across the surface of the brain) resulted in what remains an essentially accurate description of the main cortical subdivisions and their topographic distribution, even by today's standards (see also Von Bonin 1970).
The present paper has been built around three principal concepts, all of which were already reflected in both Campbell's and Brodmann's works. First, we argue that one is unlikely to attain a complete understanding of the human cortex without a detailed knowledge of the organization of animal brains. Both investigators invested substantial time and effort in studying mammals of different orders, with the objective of determining shared and derived features of cortical organization, and hence understanding the rules governing brain evolution.
Second, we propose that a full understanding of the organization and function of the adult cerebral cortex can be achieved only through a knowledge of brain development. In this respect, Campbell was particularly influenced by the developmental studies of cortical myelogenesis conducted by Flechsig (for review, see Flechsig 1920), which in his opinion provided important clues for the parsing of primary from higher-order sensory areas. Brodmann went further in recognizing this principle, by conducting his own investigations on the genesis of the histological differences between cortical areas; these provided a sound scientific rationale for his proposal of six principal layers in the neocortex, which are modified at later stages of development to produce the variety of laminar organizations seen in adults (Brodmann 1909).
Third, we suggest that cortical areas show genuine variations in their degree of definition, and that these variations are linked to their evolutionary and developmental histories. A degree of uncertainty was explicitly acknowledged by both of the authors we now celebrate, in particular with regards to their definition of areas forming the large association fields of the primate temporal, parietal and frontal lobes. Unlike the primary sensorimotor fields, these were reported as having somewhat indistinct borders, such that they changed gradually in character or blended with their neighbours without sharp limits. Whereas this uncertainty has traditionally been seen as the result of technical limitations, we argue that this is not necessarily the case.
Here, we will examine the results of our own investigations, primarily those based on the visual cortex of New World monkeys, in the context of these ideas. Simian primates belong to two superfamilies, Platyrrhini (or New World monkeys, which include the squirrel, owl, marmoset and capuchin monkeys) and Catarrhini (including Old World ‘monkeys’, such as macaques, apes and humans). Studies of New World monkeys offer unique views of aspects of brain organization, which help to illuminate the general rules governing the development and evolution of the primate cerebral cortex. For example, as noted by Brodmann, the lissencephalic nature of the brain in many of the smaller species of New World monkeys results in particular advantages for the cortical investigator, facilitating experiments that would otherwise be difficult or impossible, and simplifying the analysis of histological material.1 Knowledge about the cortical organization of New World monkeys is therefore important in allowing informed interpretation of the manner in which scientific data obtained in different animal models impact on our understanding of the human brain. This requires information about the relative importance of factors such as brain size, habits and phylogenetic history in determining systematic differences in cortical organization, particularly among species within the same taxon (e.g. Huffman et al. 1999).
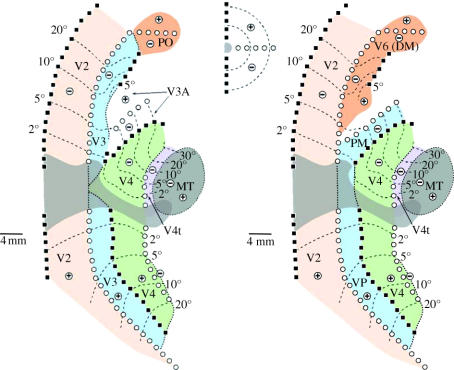
Over the last decade, we have systematically investigated the visuotopic maps, connections and architecture of the striate and extrastriate areas in the marmoset monkey (Callithrix jacchus), resulting in a progressively refined map of the organization of the posterior neocortex (figures 1 and 2). Given the increasing use of marmosets for the study of cortical development and neurophysiology, as well as their role as models of neurological disease, understanding the visual cortical organization in this species is justified as a potential reference atlas for investigators working in different fields. More interesting, however, for the purpose of the present review, is the fact that marmosets are among the smallest living monkeys, and in this way provide an important means of comparison for understanding the evolutionary effects of massive changes in brain size among primates. Put simply, comparing the marmoset's 8 g brain with the 80 g brain of the macaque may help to illuminate the types of differences to be expected between the brains of monkeys and humans (more than 1000 g), as well as the likely developmental mechanisms and evolutionary events responsible for these differences across primates in general. We have recently discussed the evidence for the various areas and the types of visuotopic maps therein (Rosa 1997, 2002; Rosa & Tweedale 2004); the reader is directed to these references for additional detail. The present review will concentrate on a few cortical areas, which illuminate concepts of the organization of extrastriate cortex emerging from our comparative studies.
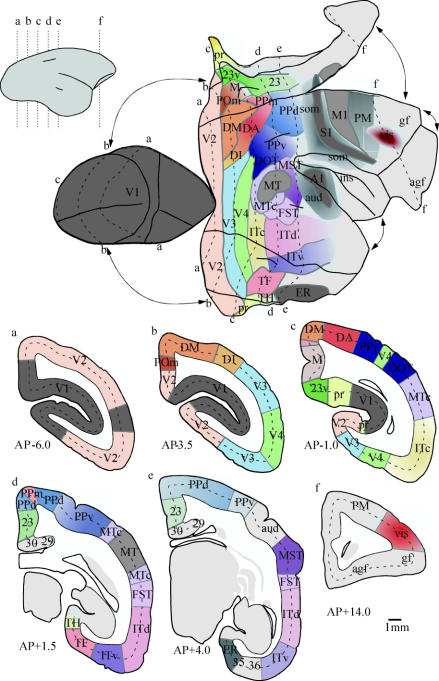
Figure 1.
(Opposite.) Organization of the cerebral cortex in the marmoset, one of the smallest simian primates. Top: graphical reconstruction of the cerebral cortex of the right hemisphere, created from coronal sections stained for myelin. Thick lines indicate major folds (e.g. the boundary between the cortex exposed on the lateral surface of the brain and that buried along the midline fissure, and the lips of the calcarine and lateral sulci). To minimize distortions, discontinuities were introduced at several points along the perimeter (as indicated by the arrows). The presently recognized visual areas, and territories associated with other modalities, are shown in different colours. The contours corresponding to six anteroposterior levels are indicated by dashed lines. Bottom: the corresponding coronal sections, with areal borders indicated in colour. The names of the areas and their sources are listed below, from posterior to anterior in the brain. Other abbreviations: agf, agranular frontal cortex; gf, granular frontal cortex; ins, insular cortex. V1—primary visual area (Fritsches & Rosa 1996). V2—second visual area (Rosa et al. 1997b). DM—dorsomedial area (Rosa et al. 2004). V3—third visual area: corresponds to the ‘ventrolateral posterior area’ defined in marmosets (Rosa & Tweedale 2000). DA (dorsoanterior), DI (dorsointermediate) and PPm (posterior parietal medial) areas—putative functional subdivisions of a densely myelinated architectural field located rostral to DM (Rosa & Schmid 1995). M (medial) and POm (parietooccipital medial) areas—putative functional subdivisions of a more lightly myelinated architectural field located medial to DM (Rosa & Schmid 1995). 23 and 23v—subdivisions of area 23, defined by cytoarchitectural criteria (Kobayashi & Amaral 2000); subdivision 23v is more directly related to visual function (Kobayashi & Amaral 2003). Pr—prostriate area; a visually responsive subdivision of retrosplenial cortex (Rosa et al. 1997a,b). V4—fourth visual area: corresponds to the ‘ventrolateral anterior area’ defined in marmosets (Rosa & Tweedale 2000). TF—visually responsive cytoarchitectural field of the parahippocampal cortex, which may include more than one functional subdivision; bordered medially by field TH (Suzuki & Amaral 2003). ITc—caudal subdivision of the inferior temporal cortex (Rosa & Tweedale 2000). MT—middle temporal area. Surrounded by the middle temporal crescent (MTc; Rosa & Elston 1998). DOT—dorsal occipitotemporal area (Rosa & Tweedale 2000). PPd and PPv—architecturally defined dorsal and ventral subdivisions of the posterior parietal cortex. FST—fundus of superior temporal area (Rosa & Elston 1998). ITd and ITv—major architectural subdivisions of the inferior temporal area; correspond roughly to Brodmann's areas 20 and 21. ER—entorhinal cortex (Brodmann's area 28). MST—medial superior temporal area; likely to include at least two functional subdivisions (Rosa & Elston 1998). A1—densely myelinated, auditory ‘core’ field; surrounded by other auditory areas (‘aud’). S1—densely myelinated, somatosensory ‘core’ field; surrounded by other somatosensory areas (‘som’; Krubitzer & Kaas 1990a). M1 and PM—motor and premotor areas, defined on the basis of cytoarchitectural criteria. vis—visuomotor sector of the frontal lobe, defined on the basis of connections with extrastriate cortex; may include various subdivisions (Krubitzer & Kaas 1990b).
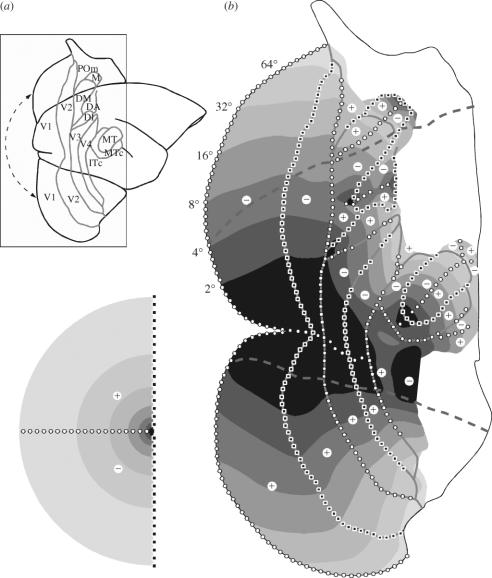
Figure 2.
Visuotopic organization of the marmoset visual cortex. (a) Lateral view of the right hemisphere, showing the anatomical relationships within the cortical regions illustrated in part (b). Parts of the cerebral cortex normally hidden from view (those located along the midline and ventral surface) were ‘unfolded’, and a discontinuity was introduced along the representation of the horizontal meridian in V1 (dashed arrows). Grey lines indicate borders of cortical areas, labelled as in figure 1. (b) Unfolded map of the posterior neocortex. The thick dashed lines indicate the dorsal and ventral limits of the cortex that is normally exposed on the surface of the brain. The numbers to the left of V1 indicate the range of receptive field centre eccentricities observed within the regions coded by different shades of grey (0–2, 2–4, 4–8°, etc.). Representations of the horizontal and vertical meridians are labelled by black squares and white circles, respectively, and the representations of upper and lower quadrants by the ‘+’ and ‘−’ signs, as indicated in the visual field diagram (bottom left).
2. Studying the evolution of ‘primate’ brains
Neuroscience is firmly based on the study of a few ‘model species’, which have been largely determined by the choices made by pioneer investigators. While good optics and frontalized eyes have certainly been important elements in studies of the primate visual system, these early choices were often determined by more practical factors, including a prior tradition of use in other types of physiological experiments (meaning well-tested preparations and drug regimes), the existence of good neuroanatomical information, size, and last, but certainly not least, availability. It is in no small measure owing to such historical contingencies that present-day references to the ‘monkey’ or ‘primate’ cortex are normally understood to refer to Old World monkeys, typically the rhesus (Macaca mulatta) or long-tailed (Macaca fascicularis) macaque species. In contrast with other species, macaques were readily available to investigators on both sides of the Atlantic at the time when the foundations of our current understanding of visual cortical processing were established. Moreover, they quickly proved suitable for studies of the anatomy (Kuypers et al. 1965; Zeki 1969) and physiology (Daniel & Whitteridge 1961; Hubel & Wiesel 1968; Dubner & Zeki 1971) of both striate and extrastriate areas.2
Good choices by pioneers in a given field tend to perpetuate, as similar practical constraints apply to most laboratories exploring the same or related questions. With time, the sheer mass of prior literature on a given species tends to become the key issue, as researchers will naturally prefer to build upon a solid foundation of knowledge, rather than retrace the basic steps and controls that are needed when investigating a new animal model. Today, most of our knowledge regarding the primate brain has been realized in studies using macaques, and this genus remains a touchstone of modern systems neuroscience. Yet it is important to recognize that the study of a single species or genus cannot alone unravel basic rules governing the anatomy, physiology and development of the primate brain in general, or the human brain in particular. Heuristic mistakes commonly found in the scientific literature include the uncritical extrapolation of observations made only in macaques as directly applicable to the human brain, and the justification of the choice of the macaque for a given study owing to its being ‘closer to human’ in terms of brain organization. Such statements need to be carefully qualified. While it is true that the macaque brain offers an excellent guide to the anatomy and physiology of the human brain, clear differences in the size, location, visuotopic organization and even cellular structure of visual areas have also been documented (Preuss et al. 1999; Van Essen et al. 2001; Tootell et al. 2003; Preuss 2004). More importantly, and also more interestingly in terms of illuminating aspects of cortical development and evolution, these differences appear to be localized to specific cortical regions, rather than reflecting a uniform expansion of the same underlying cortical organization (see below). At the same time, the correct assertion that Old World monkeys such as the macaque are more closely related to humans than New World monkeys needs to be tempered by an appreciation of the evolutionary distances involved. Current minimum estimates place the divergence between the lineages leading to present-day New World and Old World simians (the latter including humans) at around 35 Myr, while the divergence between Old World monkeys and hominoids is placed at 25 Myr (Glazko & Nei 2003; Schrago & Russo 2003). Thus, present-day New World and Old World monkeys have both developed independently from the human lineage for much of their evolutionary history. If one proposes that the organization of the New World monkey brain is less representative of the type of information needed to understand the human brain because of an additional 10 Myr of independent evolution, one must also be prepared to accept that much larger differences could have arisen in the 25 Myr separating humans and macaques.
It is in this context that comparative information about the cortical organization in different species becomes crucial. One needs to determine robust patterns of common organization, which can give strong indications of how the human brain is likely to be organized, even where no direct evidence exists. Finding features of cortical organization that are presently shared by species of New World and Old World monkeys strongly implies that the developmental mechanisms responsible for their generation were already present in a common ancestor group that existed long before the first apes; hence all present-day simians, including humans, would in all likelihood have inherited the corresponding genetic machinery. However, it is also important to recognize that the morphologies of adult animals are the result of a complex interaction between genetic and epigenetic factors. Relatively small variations of a similar set of developmental ‘instructions’, or even different environmental influences, have the potential to give rise to different adult brain phenotypes (Striedter 1998; Kornack 2000). One clear example of this fact comes from the observation that New World and Old World monkeys of similar brain size (Cebus apella and M. fascicularis) have similar patterns of ocular dominance columns in the primary visual area (V1; Rosa et al. 1988, 1992), while smaller New World monkeys (e.g. Saimiri and Callithrix) have ocular dominance columns that are less sharply segregated, more fragmented, and may even be absent in a substantial fraction of the population (Adams & Horton 2003). This strongly suggests that, while all simian primates have the potential to express ocular dominance columns as adults, at least in terms of their genetic code (the only trait that can be directly inherited by offspring), certain other conditions have to be fulfilled at the time of brain development for these columns to persist into adulthood (Markstahler et al. 1998). In this particular example, the conditions may include certain types of visual experience involving a minimum degree of decorrelation between the images seen by the two eyes (hence, species with smaller interocular separations may tend to have weak or absent columns). Thus, without additional information one cannot readily assume that every monkey, or even the hypothetical common ancestors, would necessarily have ocular dominance columns as adults. We will return to the concept of adult brain characters as ‘attractors’ in a potentially multistable developmental landscape (Striedter 1998) at various points in this review, particularly when we discuss the different configurations of cortical areas found in primates. For the moment, it is important to emphasize that noting the existence of shared morphological characteristics is only part of what is required to understand cortical evolution. Equally important is to pinpoint characters that vary in predictable ways, according to factors such as brain size and habits (de Winter & Oxnard 2001), and to understand the rules governing this variability. Finally, whenever possible, one needs to identify autapomorphic characters, which are shared only by members of a related group of species to the exclusion of other branches of the primate phylogenetic tree.
3. The concept of homology as applied to the cerebral cortex
Central to any analysis of evolutionary patterns are the concepts of analogy and homology. Two structures with completely distinct evolutionary origins may perform the same functions, in which case they are deemed analogous. On the other hand, homologous structures are those which reflect the common inheritance of a given biological character. Although homologous structures do tend to share a given morphology and to perform similar functions in related species, this is not necessarily the case; herein lies a major challenge to present-day neurobiologists (Striedter 1998). Establishing homologies is a particularly important step in tracing evolutionary patterns specific to a given structure. The most objective way of deciding whether or not two neural structures are homologous (be they cortical areas, columnar systems, or classes of neurons) is by consideration of their embryological origin. This point of view has been particularly well articulated by Romer (1955)3 and was also championed by Brodmann (1909), who made extensive use of embryological material in defending the concept of homogenetic isocortex, and hence establishing the view of most neocortices as evolutionary and developmental variations of a common, six-layered plan. Here we defend the point of view that a one-to-one correspondence between cortical areas in different species of primates is unlikely to exist, particularly among higher-order sensory and association fields. Rather, in many cases it makes more sense to apply the concept of homology to larger fields, which comprehend variable, developmentally multistable configurations of areas in different species.
In theory, the direct comparison of morphological or biochemical (e.g. gene expression) characteristics in corresponding developmental stages of two species can provide a direct means of establishing homology between brain structures. Given the impossibility of obtaining direct evidence on anything other than the most general morphological characteristics of the brains of the hypothetical common ancestors, developmental studies have the best potential to provide rigorous tests of phylogenetic hypotheses. Yet in practice, with the exception of primary sensorimotor fields (Donoghue & Rakic 1999; Gitton et al. 1999) and phylogenetically older cortices (Levitt 1984; Pimenta et al. 1996), this remains an unfulfilled promise. There is little evidence of developmental steps that are particular to, or promote the definition of, a specific cortical area. Instead, the molecular specification steps currently described for most six-layered cortices usually take the shape of smooth rostrocaudal or mediolateral gradients of ligands, which are relatively conserved between all mammalian species. As discussed in detail below, we interpret this as indicating a particular status for certain areas, including the classically defined primary sensory cortices. By virtue of their genetically ‘hard-wired’ definition during earlier stages of embryogenesis, including sharply defined molecular borders, these areas could act as constant reference points, or ‘molecular anchors’ (Rosa 2002) that guide the subsequent sequential formation of other sensorimotor maps in the cortex. According to this model, most other areas, including those forming the majority of the primate isocortex, could have their boundaries determined purely or mainly by a combination of weak molecular definition (e.g. smooth gradients of ligands) and activity-dependent processes, provided that a temporal hierarchy of maturation similar to that proposed by Flechsig (1920) exists (e.g. figure 5). Whether or not future experimental work proves this to be the case, in practice, the observed smooth gradients of molecular expression provide little in terms of sharp developmental criteria on which to base decisions regarding the homologies of most cortical areas. This issue becomes particularly significant if one is focusing on regions that have, in all likelihood, experienced marked growth and subdivision during the evolution of primates. For example, the posterior parietal cortex includes more subdivisions in primates than it does in non-primates, and appears to be relatively smaller and less subdivided in marmosets than in macaques (Rosa 1999; Manger et al. 2002). In such a case, one could imagine that the same pool of ventricular zone cells or the same region of the cortical plate that gives rise to an area in one of these species may, as a result of additional cell divisions at later stages of development and subsequent regional specialization, form the origin of several areas in the human brain. Thus, the marmoset, macaque and human configurations could be seen as different endpoints of the same underlying ‘epigenetic landscape’ (Striedter 1998). In this situation, although one could certainly say that the human posterior parietal cortex is, as a whole, homologous to the posterior parietal cortex of New World and Old World monkeys, formulating the question ‘which area of the human brain corresponds to a specific area in the monkey?’ may not always result in a unique answer.
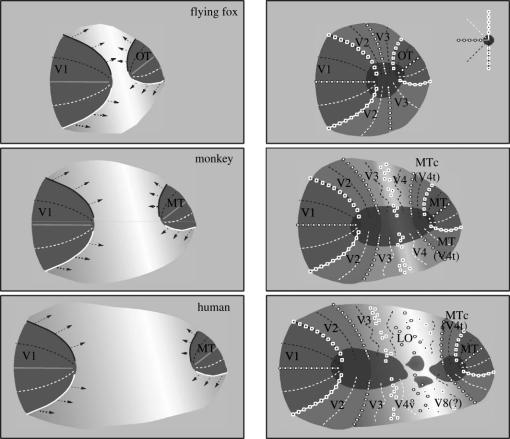
Figure 5.
Formation of visuotopic maps in brains of different sizes, according to the ‘molecular anchors’ hypothesis (for additional details, see Rosa 2002). The visuotopic organization of the cortex is indicated according to the key shown in the top right panel. The left column represents an early stage of development, and the right column a late stage of development. The grey scale represents the sequence of maturation (dark, more mature cortex). Two primary visual maps (corresponding to adult V1 and MT, the only first-order representations in the adult brain) are specified early in development (left), either by gradual distributions of cell surface chemoattractant/chemorepellent molecules (O'Leary et al. 1999) or by spatio-temporal patterning of the afferent projections (Molnár et al. 1998). With the V1 and MT maps defined, the visuotopic maps in adjacent areas (e.g. V2) start to self-organize around these ‘anchors’. Two rules guide this process: (i) the receptive fields of neurons in adjacent columns must overlap; this rule constrains the configuration of maps forming at later stages of development, which must be ‘anchored’ in pre-existing maps; and (ii) the gradient of representation does not revert within a given area (arrows in the left panels); this ensures that the same part of the visual field is not represented more than once in a given area, except along its boundaries. Throughout pre- and postnatal development, activity-dependent mechanisms allow the fine-tuning of the maps. However, for any given area, the degree of plasticity decreases gradually with age (e.g. Waleszczyk et al. 2003). Upper row: the visual cortex of flying foxes is used as an example of small primate-like cortex. In this species, there is little cortex between V1 and the occipitotemporal area (OT; a probable homologue of primate MT). In the adult (right column), this region includes only two visuotopic maps (V2 and V3), which form precise mirror-images (Rosa 1999). Middle row: the visual cortex of a marmoset. Here, four maps (V2, V3, V4 and MTc) exist between V1 and MT. The visual topographies of V3 and V4, which mature last, are the least precise, and most variable between individuals. Bottom row: the human visual cortex, where the cortex between V1 and MT is more expansive, and includes additional areas in which the visuotopic organization is less clear (e.g. Tootell & Hadjikhani 2001). Expansion of the cortex between primary areas results in multiple reversals of visual field representation (and hence ‘areas’). Maps which self-organize at progressively later stages of development are constrained only by areas with progressively larger receptive fields and representational scatter. Thus they can become less and less precise, without violating rules 1 and 2 above.
Given these complications with the application of a developmental definition of homology to cortical areas (at least if one thinks of each area as a separate trait), it is a fact of life that homologies tend to be proposed primarily on the basis of less satisfactory criteria. This in turn has led to endless debates regarding whether or not given cortical areas observed in different species are truly homologous, or whether they represent cases of parallel evolution (having originated from distinct evolutionary events). A particularly illustrative example in this regard is the discussion of whether or not the middle temporal area (MT, also known as V5) is specific to primates. Several authors have demonstrated the existence of visual areas that are probable homologues of MT in various non-primate species, including cats (Creutzfeldt 1988; Payne 1993; Burke et al. 1998), flying foxes (Rosa 1999) and rodents (Paolini & Sereno 1998). In these cases, homology was proposed on the basis of various combinations of criteria, including location relative to V1 and the second visual areas (V2), visuotopic organization, selectivity for direction of motion, and pattern of afferent connections. However, in none of these species has an area that is identical to MT in all respects been described. Key features, such as the particularly dense myelination that characterizes primate MT, are lacking in the putative homologues of cats and squirrels. While the proposed MT homologue in flying foxes is densely myelinated (Rosa et al. 1993a), it is still unknown whether this area has a concentration of direction-selective neurons. At present, we favour the hypothesis that the emergence of a MT homologue was a relatively early event in eutherian evolution (see Rosa 1999 for details); as pointed out above, homologous structures are not necessarily identical. However, it must also be conceded that there is simply not enough information to completely rule out the possibility that this area represents a new evolutionary event, particular to primates (Kaas 2002). As argued below, new developmental evidence pointing to a unique spatial, temporal and chemical delimitation of the neurons forming the MT cortex may provide a way to resolve this issue. From the practical point of view, it is important to realize that, even 100 years after Campbell and Brodmann, cortical maps remain works in progress, particularly when it comes to defining homologous patterns of organization.
4. Comparing primates of different sizes
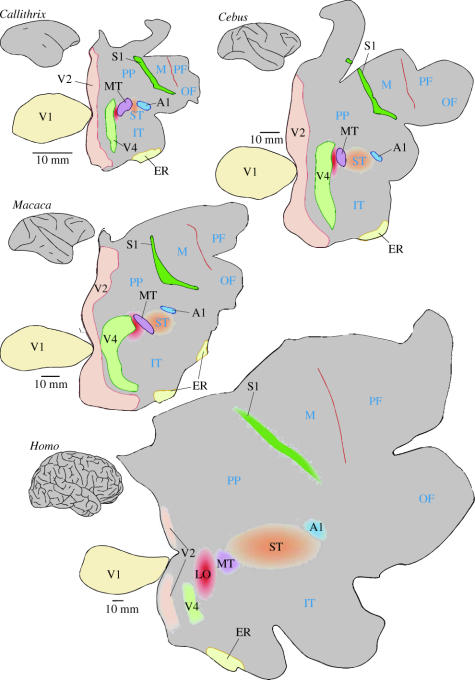
Figure 3 illustrates an unfolded view of the cerebral cortices of diurnal New World (a,b) and Old World (c,d) simians, encompassing nearly the entire range of primate brain sizes (note the different scale bars). As recognized by classical neuroanatomists (e.g. Le Gros Clark 1959), there are fascinating similarities in size and sulcal pattern between the cerebral cortices of capuchins (C. apella, a New World monkey species) and macaques. This provides an interesting counterpoint to the marmoset, allowing objective comparisons between New World and Old World monkeys, which are not likely to be dominated by allometric effects. Because not all cortical areas are equally well defined, for the purpose of illustration we have highlighted only a few subdivisions that can be mapped either on the basis of sharp histological borders or visuotopic organization. The aim here is to demonstrate a simple but important point: the cerebral cortices of larger primates are not merely scaled-up versions of those of smaller primates. While some cortical areas change approximately in proportion to one another, other regions of the cortex become relatively more developed in the larger species. However, New World and Old World monkeys of similar brain mass and sulcal patterns have comparable configurations of cortical areas (figure 3b,c). We interpret this as indicating that there is nothing fundamentally different between New World and Old World monkeys; rather, this variety of configurations stems primarily from the application of the same developmental programme to different masses of neurons, created by different periods of neurogenesis. This concept is explained in more detail in the following section, where we discuss what causes some brain areas to expand in proportion, while others experience marked growth and subdivision in evolution.
Figure 3.
Bidimensional maps of the entire cerebral cortex of four species of simian primates, with selected cortical areas indicated. To minimize distortions, the cortex corresponding to V1 was detached from the map, following the style suggested by Van Essen & Maunsell (1980). Top left: marmoset (Callithrix jacchus); map based on Rosa & Elston (1998); Rosa & Tweedale (2000) and ongoing cytoarchitectural analyses. Top right: capuchin (Cebus apella); map based on Rosa et al. (1993b; 2000a,b;) and unpublished cytoarchitectural data. Middle left: macaque (Macaca fascicularis); based on Felleman & Van Essen (1991). Bottom: human (Homo sapiens); based on Van Essen (2004) and a computer reconstruction of Brodmann's cytoarchitectural areas (available at http://brainmap.wustl.edu/caret/slides/human.03-06/). Note, however, that in generating this illustration the contours of V1 (area 17) were detached from the map, then combined so as to follow the same style as the monkey maps. The contours of human V2 and V4 are incomplete, reflecting the difficulty in stimulating the visual field periphery in fMRI experiments. Measurements of cortical magnification factor (Sereno et al. 1995) indicate that, as in macaques (Sincich et al. 2003), this area is nearly as large as V1.
To facilitate the comparison between species, the maps in figure 3 have been drawn in such a way that the surface area of V1 (a field which can be safely delimited with high precision in all mammalian species) is the same. By doing this, several trends become clear. First, in small-brained primates a much larger proportion of the cerebral cortex is dedicated to primary sensory areas (primary auditory cortex, A1, primary somatosensory cortex, S1, and V1). For example, while V1 occupies about one-fifth of the marmoset cortical map (figure 3a), it corresponds to about one-tenth of the capuchin and macaque maps (figure 3b,c) and less than one-twentieth of the human map. Second, the sizes of the primary sensory and entorhinal (Brodmann's area 28) areas remain more or less constant in relation to each other. Thus, large-scale expansion or compression of the cerebral cortex during primate evolution has mainly involved the rearrangement of the mass of ‘standard’ six-layered neocortex which, being inserted in progressively larger amounts, drives phylogenetically older fields (sensory koniocortices and mesocortical fields) further apart.
This reorganization of the cortex has been achieved in part by changing the relative size of individual fields. When compared with V1, area V2 is small in marmosets (about half of the surface area of V1), but it becomes relatively larger as a function of body (and brain) mass (Rosa 2002). A similar trend applies to area V3, which is much larger in humans than would be predicted from the monkey (Dougherty et al. 2003; Van Essen 2004), and to V4 and its putative homologues, which are similar in size and topographic organization in macaques and capuchins (Gattass et al. 1988; Piñon et al. 1998), but far more compressed in marmosets (Rosa & Tweedale 2000). However, expansion of individual fields can only go so far. New cortical areas, which do not clearly correspond to any area in smaller species, also appear to exist in the human brain. For example, in monkeys there is only a narrow bridge of cortex between the rostral limit of area V4 and area MT (figure 3a–c; see Gattass et al. 1988; Piñon et al. 1998), whereas in humans the topologically equivalent region is expansive (red, in figure 3d), and appears to include functionally unique fields (Grill-Spector et al. 1998; Tootell et al. 1998; Amedi et al. 2002; Ferber et al. 2003; Kourtzi et al. 2003; Stanley & Rubin 2003). The configuration of human areas shown in figure 3 reflects a somewhat conservative interpretation of the evidence in this respect; in a different model, the whole of V4 assumes an even more ventral aspect in humans (Bartels & Zeki 2000). Thus, while the exact extent of this positional shift remains a subject of debate (owing to different points of view regarding which of various areas is the ‘true homologue’ of the monkey V4), it is clear that, rather than a very large V4 and V4t, humans have something without an obvious monkey counterpart in the cortex caudal to MT.
The emergence of this ‘lateral occipital’ complex as one of the best documented cases of an evolutionarily new field is owing to the fact that functional criteria can be used to define, at least to a first approximation, the locations of V4 and MT in both humans and monkeys. However, a similar level of objectivity has been harder to achieve in comparisons involving areas which are non-topographically organized, or whose organization reflects coding of more abstract aspects of behaviour. For these, despite similarities being noted, the detailed nature of the differences between primate species remains largely unknown (e.g. Culham & Kanwisher 2001). Based on comparisons such as that shown in figure 3, it is fair to expect that the relative expansion of the temporal, parietal and frontal association regions, and of non-primary sensory and motor cortices, have resulted from the relative expansion of individual areas, as well as the addition of new areas. That this is the case has, of course, been proposed much earlier, on the basis of cytoarchitectural evidence4 (e.g. Preuss & Goldman-Rakic 1991a,b), but given the relative homogeneity of the isocortex that experiences the most marked changes, a precise view will require direct functional evidence. One region that deserves particular attention in this context is the superior temporal region, highlighted in orange in figure 3. Note that in marmosets and other small primates, visual area MT is located in close proximity to the auditory ‘core’ (Luethke et al. 1989; Rosa & Elston 1998), whereas in macaques several other visual, auditory and polysensory fields exist between these areas (Seltzer & Pandya 1978; Desimone & Ungerleider 1986; Hackett et al. 1998; Lewis & Van Essen 2000; Schroeder & Foxe 2002). This region is even more expansive in the human brain, where it is likely to contain new subdivisions involved, for example, in the processing of speech sounds and integration between visual and auditory language cues (Hackett et al. 2001; Padberg et al. 2003; Puce & Perrett 2003). One interpretation of the comparative evidence is that the addition of this expansive sector of cortex between A1 and MT has allowed better audiovisual integration, a crucial aspect of language. In this light, both the strong activation of area MT by lip movements in humans and the specific deficits MT lesions cause in terms of lip-reading ability (as opposed to all biological motion) could be seen as the result of a recent evolutionary adaptation of a more general-purpose primate motion‐processing system, which was driven by specific needs of hominoid evolution (Campbell et al. 1997; Sekiyama et al. 2003). However, the close proximity of A1 and MT in small primates and the polysensory nature of some of the areas in between may hint at a phylogenetically older role for the MT complex of areas in visual-auditory integration. This possibility needs to be explored by means of a systematic study of this region in smaller primates, including single-cell analyses of visual and auditory responses and more detailed anatomical tracing.
While the exact developmental mechanism underlying the non-uniformity of evolutionary changes across the surface of the cortex remains conjectural, an educated guess can be offered on the basis of what we know about the dynamics of cell proliferation giving rise to different parts of the brain (Kornack 2000; Finlay et al. 2001). During embryonic development of the cortex, the pool of progenitor cells in the ventricular zone undergoes a gradual change in the mode of cell division (Kornack 2000; Caviness et al. 2003). Initially, most cell divisions are symmetric non-terminating (whereby both daughter cells become progenitor cells). This gradually changes to an asymmetric mode (i.e. one of the daughter cells remains as a progenitor cell in the ventricular zone, while the other migrates as a postmitotic neuron to form the developing cortex), and then to a symmetric terminating mode (in which both daughter cells become postmitotic neurons). Even slight changes in the duration of these phases can have dramatic effects on the resultant number of neurons that comprise a particular structure. This has been well documented with respect to the fact that primates tend to have a larger fraction of the cortex formed by supragranular layers, in comparison with rodents. In this case, the inside-out pattern of cortical development means that postmitotic cells generated in ‘late’, additional cell division cycles (enabled by the longer gestation in primates) tend to aggregate in layers two to four. This effect is compounded by the fact that the duration of the cell cycle also accelerates towards the end of the primate gestation period, in such a way that relatively more cell divisions occur during the final third of cortical neurogenesis (Kornack 2000). A localized expansion of certain regions of the cortex could happen if these corresponded to sectors of the proliferative epithelium that underwent slightly later transitions between modes of division. One might conjecture, for example, that cells in the ventricular zone sectors giving rise to V1 (and other ‘core fields’) would tend to enter the symmetric terminating mode earlier than those in sectors giving rise to V2, hence the relative enlargement of the latter area in species that undergo a longer period of corticogenesis. This is essentially an extension of Finlay & Darlington's (1995) evolutionary/developmental argument to subdivisions of the cerebral cortex. These authors proposed the existence of differential effects of order of neurogenesis (and other developmental events) on the size of brain components (‘late-generated structures become disproportionately large in large brains’; Darlington et al. 1999, p. 367). This argument will be expanded in § 5, where we deal with the relative phylogenetic stability of area MT.
5. Primary and non-primary areas in development and evolution: the case of area MT
What makes the relative size of some cortical areas constant across species, whereas other regions show marked variation? A possible clue to the answer to this question comes from the fact that one rostral visual area, MT, defies the trend among extrastriate cortices, in that it corresponds to a nearly constant proportion of the size of primary cortical fields in all primates (Rosa 2002). We propose that this can be explained according to the hypothesis that MT is part of a framework of primordial cortical areas, which are specified through well-defined transitions at relatively early stages of pre- and postnatal development (Rosa 2002), and which were already present from early stages of primate evolution. By contrast, the main focuses of isocortical expansion and differentiation (where, as outlined above, areas show more variability between related species) would correspond to regions that form and mature later in development, with a lesser degree of molecular specification; these also correspond to more recent evolutionary acquisitions.
Of particular relevance for this argument is the fact that MT is noteworthy among extrastriate areas, owing to its accelerated maturation in pre- and postnatal life. The early development of MT in relation to other extrastriate areas was first suggested on the basis of an anatomical comparison between the pattern of myelination in newborn human brains (Flechsig 1920) and functional mapping of motion selectivity in adults; as pointed out by Watson et al. (1993), human MT seems to correspond to one of the few regions of cortex that are already myelinated at birth (Flechsig's Feld 16). That the functional maturation of MT also occurs earlier than that of other visual areas (with the exception of V1) has been suggested by research showing the comparatively early development of pattern motion selectivity (Dobkins et al. 2004) and other presumed MT-related visual functions (Fine et al. 2003). The validity of these inferences was confirmed by recent studies of the postnatal development of cytoskeletal and calcium-binding protein expression in marmosets, which have confirmed a precise correspondence between a sharply defined region of early maturing cortex in the superior part of the temporal lobe and the borders of developing area MT (Bourne et al. 2004). Significantly, this same study demonstrated that the histological maturation of MT is concomitant with the maturation of the primary sensory fields A1, S1 and V1, preceding that of most other cortical areas by a few weeks (Bourne et al. in press).
This developmental observation has added support to the concept of MT as a primary sensory area, which has so far been argued primarily from functional and anatomical points of view (Rosa & Elston 1998; Rosa 2002). In traditional schemes of hierarchical processing, MT is usually seen as a higher-order visual area, occupying the fourth or fifth level of cortical processing. However, MT has a number of characteristics that are shared with primary sensory areas, but are rare among other fields. For example, MT neurons receive direct retinal innervations through a small, but well-defined nucleus of the inferior pulvinar complex (O'Brien et al. 2001). As pointed out by Cusick et al. (1993), this nucleus (medial inferior pulvinar, or PIM) stands out from the rest of the pulvinar complex by virtue of having the expected neurochemical characteristics of a primary relay nucleus. Perhaps owing to the existence of this ‘lemniscal’ pathway, MT neurons respond with very short latencies in comparison with cells in all other areas except V1 (ffytche et al. 1995; Schmolesky et al. 1998; Raiguel et al. 1999), and many remain active even after extensive lesions of V1 (Rodman et al. 1989; Girard et al. 1992; Rosa et al. 2000b; Azzopardi et al. 2003). In fact, MT neurons can mediate conscious visual sensation even in the absence of V1, provided that stimuli of certain spatio-temporal characteristics are presented (Barbur et al. 1993; Sahraie et al. 1997; Zeki & ffytche 1998). Finally, MT is also unusual among extrastriate areas in forming a relatively simple and precise ‘first-order’ representation of the visual field (Allman & Kaas 1971; Fiorani et al. 1989; Xu et al. 2004), the significance of which will be discussed in §6, and in having sharply circumscribed histological borders, comparable in definition only with those of primary sensory areas such as A1 and S1 (figure 4; see also Tootell et al. 1985; Huffman & Krubitzer 2001; Sincich et al. 2003). In opposition to this body of evidence, the argument for MT as a higher-order area relies primarily on the study of laminar patterns of corticocortical connections (e.g. Felleman & Van Essen 1991). While we recognize that the quantitative analysis of these types of data usually reveals a good anatomical correlate of the probable levels of processing within the same hierarchical pathway (e.g. through the use of the ‘supragranular/infragranular index’; Barone et al. 2000; Vezoli et al. 2004), it is also the case that the relationship tends to break down when one analyses connections between areas that may not operate strictly in series. For example, contrary to the expectations of a hierarchical model, frontal lobe projections to visual cortex originate predominantly from supragranular layers (Shipp et al. 1998). Whereas the concept of two primary visual areas might sound strange to some, it would probably not cause much reaction among most auditory physiologists, who have grown accustomed, over a quarter of a century, to the idea of more than one auditory ‘core’ field (e.g. Reale & Imig 1980).
Figure 4.
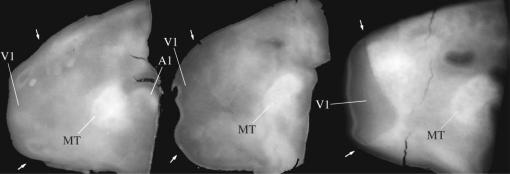
Vibratome slices through flat-mounted preparations of the posterior neocortex of three marmosets, showing the distinctive architecture of area MT. Caudal is towards the left, and dorsal towards the top of each panel. These slices have not been stained, and the borders of V1 (arrows) and MT are made visible only by differences in myelination (highly myelinated regions appearing lighter). The borders of MT are distinct throughout, while those of V1 are clearer in the deeper layers (right panel). The primary auditory cortex appears as another heavily myelinated region, just below the lip of the lateral sulcus (left panel). For details, see Rosa & Elston (1998).
It has long been known that primary sensory fields are among the first cortical areas to develop5, including achieving morphological/histological maturation and undergoing critical periods (Condé et al. 1996; Gogtay et al. 2004). In embryonic life, preplate regions that are destined to become primary areas also become committed relatively early by means of sharp, genetically regulated molecular specification steps (Polleux et al. 1997; Donoghue & Rakic 1999; Smart et al. 2002). In this context, it will be important to test whether a high level of molecular specification applies to MT, as predicted by the hypothesis outlined above. One would expect, for example, that MT-specific expression of the Eph receptors and their ligands (ephrins) may occur at some stages of corticogenesis, including a sharp molecular border similar to that demonstrated for developing V1 (Donoghue & Rakic 1999).
In summary, the unusual characteristics of area MT (among extrastriate areas) support the idea that the clear histological boundaries and the relative stability of the proportions between primary areas and ‘older’ cortices (such as the entorhinal area; see figure 3) are likely to result from these areas claiming defined territories of the protomap or preplate ahead of other areas, guided by precise molecular labels. As argued above, modulations of the duration of the cell cycle and the number of cell divisions, which form the probable evolutionary mechanism of cortical expansion (Kornack & Rakic 1998), would be more likely to affect the configurations of regions forming later in development, including the expansion of individual areas and the emergence of new fields. Late-maturing areas of the human brain, such as the prefrontal and inferior temporal cortices, are among those which have experienced relatively recent expansion and subdivision in primate evolution (Preuss & Goldman-Rakic 1991a,b; Condé et al. 1996; Woo et al. 1997; Cruz et al. 2003; Gogtay et al. 2004).
In theory, it is also the case that the later a given cortical region matures, the greater the chance that environmental or other epigenetic factors could play a role in defining its final configuration in the cortex. Yet although it is clear that adaptations to specific ecological niches correlate with changes in the sizes and configuration of areas, as well as their topographic maps (Huffman et al. 1999; Rosa & Krubitzer 1999), the exact manner in which different cortical phenotypes emerge in development, particularly among closely related species, remains for the most part unknown. Consistent correlations between cortical structure and habits have been difficult to establish among primates. However, homologues of V2 and V4 are smaller (relative to V1) in nocturnal primate species than would be expected by comparison with diurnal species of similar size (Rosa et al. 1997a,b; Rosa 2002).
6. ‘Core’ fields and the formation of sensorimotor maps
In this section, we examine the possible consequences of asynchrony in the formation and maturation of cortical areas for the establishment of topographic maps, with the focus on visual areas. To date, it remains unclear whether visuotopic maps have a function in facilitating certain types of neural computations (see Rosa 1997; Rosa & Tweedale 2004, for reviews). Nonetheless, they form one of the most important components of the currently accepted definition of what constitutes a visual area, and therefore understanding the relationship between maps and areas is of some practical importance. Despite this, there is little information available on the developmental steps responsible for the formation of topographies outside the primary sensory areas (Rosa 2002). This constitutes a major limitation to our understanding of cortical arealization, as the maps found in higher-order areas tend to be the ones that are coarser, topologically more complex and, according to some investigators, may even comprise incomplete representations of the sensory surface. Hence, their interpretation is more controversial, as demonstrated in §7 of the present paper.
In the absence of direct evidence, it has been instructive to consider to what extent the characteristics of adult cortical sensory maps may provide hints about the likely developmental mechanisms involved in their formation. Recently, we proposed that the observed asynchrony in cortical development, with the primary sensory areas leading the way, combined with an almost universal drive towards configurations that maximize overlap between the receptive fields of adjacent neurons, would have the potential to considerably simplify the formation of visual topographies similar to those observed in non-primary cortices of adult primates (Rosa 2002). The key features of this argument are summarized in figure 5. Briefly, we proposed that only the topographic organizations of ‘core’ areas need to be under a strict genetic control, such as that implied by the patterns of ligand expression during the phase of establishment of thalamocortical innervations (e.g. Vanderhaeghen et al. 2000). The precise maps generated in these early forming areas could then act as reference points, or ‘anchors’, for the specification of additional sensory maps, which would develop sequentially as a function of distance from the primary area; this gradient-like maturation is supported by studies in primates (Condé et al. 1996; Bourne et al. in press). A crucial prediction of this model has been confirmed recently in experiments involving ferret pups, when it was demonstrated that early lesions of V1 (thereby removing the main topographic ‘anchor’) have widespread consequences for the visuotopic organization of adult extrastriate cortex, including V2, V3, and temporal and parietal areas (Restrepo et al. 2003). Even though receptive fields of near-normal size were observed in the territories expected to correspond to these areas, and despite the preservation of local topographic continuity, the global visuotopic maps and patterns of callosal connections became disrupted beyond recognition.
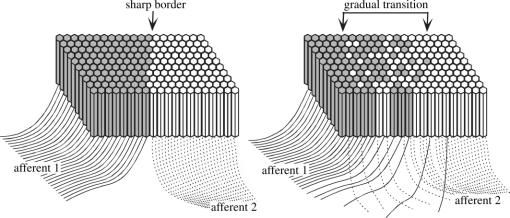
A process similar to the one depicted in figure 5 would require a hypothetical developmental mechanism that promotes local topographic continuity, with cells in adjacent columns having overlapping receptive fields, not only within the same area, but also across areas. Whereas the nature of the continuity-promoting mechanism remains unknown, the organization of adult cortices strongly implies that receptive field overlap of adjacent cells is a major constraint in topographic map development (Kaas & Catania 2002). Neurons in the early postnatal cortex undergo significant changes in their mode of intercellular communication, which could underlie the tendency to retain topographies consisting of gradual changes, and to ‘prune out’ those reflecting decorrelated activity in adjacent cells (reflecting innervations by disparate visual field loci). For example, late prenatal and early postnatal neurons communicate extensively through gap junctions, forming clusters of coupled cells that share intracellular messengers, and which tend to become active in synchrony (Montoro & Yuste in press). The reliance on gap junction signalling decreases gradually over the first few weeks of life, in an opposite relationship to the emergence of chemical synaptic activity. It has been suggested that the biophysical characteristics of the connexins expressed in different phases of postnatal maturation would dictate that only cells with synchronous depolarizations maintain their gap junction communication, particularly after the early postnatal period (Maxeiner et al. 2003). This could potentially provide a way of stabilizing maps that are based on a smooth progression of receptive field position.
According to the model illustrated in figure 5, the temporally graded development of extrastriate cortex around two primary nodes (V1 and MT) would have profound consequences for the functional organization of the adult primate brain (Rosa 2002). First, while the visual maps of these early forming, precisely specified areas could organize as simple isomorphs of the sensory surface (as predicted from modelling studies of spontaneous map formation within structures with sharply defined boundaries; Willshaw & Von der Malsburg 1976; figure 5, left column), those in areas which are formed at progressively later stages would tend to self-organize into second-order representations of the visual field, given the constraints placed by the pre-existing topography. Second, the precision of topographic maps would tend to deteriorate as a function of distance from the two primary areas. This would primarily be owing to later-forming cortices being furthest from the zones of strict molecular specification, allowing the refinement of their maps with increasingly greater degrees of freedom (figure 5, right column). For the same reason, one would also expect that fields located furthest from the primary areas would display more variability, not only between species but also between individuals. This is supported both by individual variability of the visuotopic configuration of the V3/V4 border in monkeys (Gattass et al. 1988; Rosa & Tweedale 2004; see figure 5, middle right), and by the difficulty in establishing a consistent visuotopic organization in the human region topologically equivalent to monkey V4 (Tootell & Hadjikhani 2001; see figure 5, bottom right). Finally, as indicated in figure 5, the more recent cortical fields, in terms of both evolution and development, would correspond to those added at the centre of the regions of expansion. As observed experimentally, they have the coarsest sensory representations, which may combine more than one modality (for example, the cortex between A1 and MT). This would also have implications in the search for homologous areas in different primate species: although it should be possible to establish one-to-one homologies among those areas that mature during earlier stages of development, the correspondence is likely to become increasingly blurred for ‘late’ areas.
7. How well do we know what we think we know? the case of the ‘third tier’ visual cortex in New World monkeys
The current ‘textbook’ view of visual cortex is that it consists of morphologically distinct, functionally dedicated and stereotypically connected cortical areas, each forming a topographic map of the visual field. In practice, however, more often than not the exact relationship between architectural fields, connectionally defined fields and topographic maps has proven harder to elucidate than suggested by this statement. Even today it is fair to say that there are only three areas (V1, V2 and MT) for which a precise correspondence has been demonstrated between delimitations based on such a wide correlation of criteria. Although many more areas have been proposed, they remain subjects of dispute. In this section, we highlight the pitfalls involved in mapping visual cortical subdivisions, and conclude that it is likely that much of the present uncertainty simply reflects the fragmentary nature of the experimental evidence so far obtained. We will do this by focusing in some detail on the history of the organization of the strip of cortex located immediately rostral to V2 (the ‘third tier’ extrastriate cortex) in New World monkeys. This region has been the focus of a long-standing controversy, and it is only very recently that a clearer picture of its organization has started to emerge.
In a series of classical studies of the visual cortex of owl monkeys (a New World species), Allman & Kaas (1975) defined a series of ‘third tier’ areas, each forming a complete visuotopic map of the contralateral hemifield. Among these was the dorsomedial area (DM), which occupied the dorsal-most aspect of the occipital lobe and adjacent midline. According to the original description, DM included representations of the upper and lower visual quadrants that adjoined V2, and was distinct from all adjacent areas by virtue of being heavily myelinated, and (as demonstrated by subsequent investigations) by having stronger connections with V1 (Lin et al. 1982; Weller et al. 1991; Krubitzer & Kaas 1993). At the same time, studies of the third visual complex in Old World macaque monkeys described a different pattern of organization. In this genus, the cortex anterior to dorsal V2 only represented the lower visual quadrant, in a manner consistent with the hypothesis that this region was part of a more extensive third visual area (V3) that formed a mirror-image of V2 (Zeki 1969, 1978; Van Essen & Zeki 1978; Gattass et al. 1988). Although at first glance these data indicated that different species of monkey might have rather different patterns of areas, other studies also started to document intriguing similarities between the area(s) rostral to V2 in New World and Old World monkeys. For example, similar to New World monkeys, the parts of the macaque third complex adjacent to dorsal V2 were found to be more densely myelinated, and to receive much stronger projections from V1, than those adjacent to ventral V2 (Van Essen et al. 1986; Colby et al. 1988; Stepniewska & Kaas 1996; Felleman et al. 1997; Beck & Kaas 1999; Nakamura et al. 2004). These histological and connectional parallels have also suggested another possibility: that the reported differences could be more apparent than real, reflecting, at least in part, different criteria used by different studies to define the boundaries of areas in this region.
The possibility of errors in interpretation has been highlighted by the studies of Lyon & Kaas (2001, 2002), who reached the conclusion that there is not, in fact, an area DM in the dorsal cortex adjacent to V2 of New World monkeys (including marmosets, titis, owl monkeys and squirrel monkeys). Instead, on the basis of anatomical tracing, these authors proposed that the dorsal ‘third tier’ region in these species formed an elongated topographic map of the lower contralateral quadrant, which was part of a V3 similar to that described in Old World monkeys (figure 6a). According to this model, a smaller area DM, including representations of both quadrants, would be located entirely rostral to V3. This provided an attractive way of unifying data obtained in several primate taxa, by implying a one-to-one correspondence between the areas of Old World and New World monkeys: in both types of primate there would be area V3, forming a reduced mirror-image of V2, followed by another area (DM in New World monkeys, V3a in the macaque) restricted to the dorsal extrastriate cortex. However, this proposal also led to several important unanswered questions. For example, how could it be reconciled with the earlier electrophysiological evidence of an upper quadrant representation located immediately adjacent to dorsal V2 in New World monkeys (Allman & Kaas 1975)? The presence of such a region was a key piece of information leading to the original proposal of DM, which had since been independently confirmed by other studies (Krubitzer & Kaas 1993; Rosa & Schmid 1995; Sereno 1998). Moreover, what were the implications of the connectional and histological asymmetries between dorsal and ventral cortex, observed by earlier studies, in terms of the single V3 model? Clearly, major incompatibilities remained.
Figure 6.
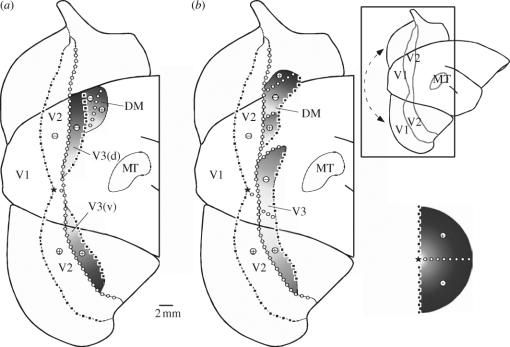
Models of the organization of the dorsal ‘third tier’ cortex in New World marmoset monkeys. (a) and (b) are schematic ‘unfolded’ views of the caudal neocortex showing the locations of well-defined areas (V1, V2 and MT), as well as other, more controversial subdivisions. The anatomical relationships in these diagrams are illustrated in the insert (top right), which includes a lateral view of the marmoset brain (right hemisphere) with ‘flaps’ of medial and ventral cortex unfolded to create a global view of the extrastriate cortex; the arrow indicates a discontinuity introduced along the long axis of V1. (a) The model advocated by Lyon & Kaas (2001) on the basis of anatomical tracing of V1 connections and histological examination of flat-mounted histological material stained for cytochrome oxidase. In this model, the dorsal third tier cortex is dominated by the lower quadrant representation of area V3, which also includes an upper quadrant representation in ventral cortex. A smaller area DM is located entirely rostral to V3. (b) The model proposed by Rosa & Schmid (1995) and Rosa & Tweedale (2000) on the basis of electrophysiological recordings and analysis of myelin-stained sections. Here, the third visual complex includes both a smaller V3, and area DM; the latter has representations of both the upper and lower quadrants adjacent to V2. The visuotopic organization is indicated according to the symbols summarized in the bottom right diagram: representations of the vertical meridian in black squares, representations of the horizontal meridian in white circles, representations of the upper quadrant in ‘+’ signs and representations of the lower quadrants in ‘−’ signs. Gradients of eccentricity are indicated by levels of grey (white, light grey: central representation; dark grey, black: peripheral representation). Abbreviations: V3(d), dorsal component of V3; V3(v), ventral component of V3.
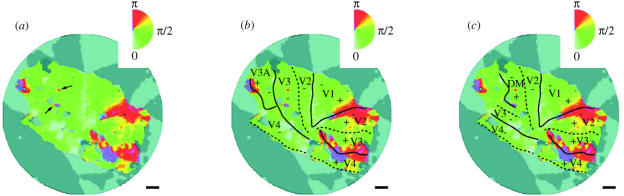
In recent studies, our laboratory revisited the organization of the New World monkey third visual complex through a comprehensive combination of anatomical tracing, fine-grained physiological recordings and histological techniques (Rosa & Tweedale 2000; Rosa et al. 2000a, 2005). The use of multiple, mutually reinforcing methods in the same experiments allowed us to clarify this situation substantially. As is often the case in science, the truth lay somewhere in between these two proposed models (figure 6b). On one hand, we confirmed that New World monkeys (marmosets in this case) do have a V3-like area, which forms a representation of the visual field including both upper and lower quadrants. This area is, however, not as extensive as implied by Lyon & Kaas (2001, 2002). Instead, near the dorsal midline we found evidence for a separate area, similar to DM described by Allman & Kaas (1975), in cortex immediately adjacent to V2 (figure 6b). As discussed in §8, these results have suggested a new interpretation of the homologies between New World and Old World monkeys, which in turn raises testable hypotheses that may lead to a refinement of the Old World monkey cortical map.
The key evidence regarding the presence of DM in the cortex rostral to V2 is summarized in figures 7 and 8. Sequences of receptive fields recorded at closely spaced sites across the most lateral part of the V2/DM border cross the horizontal meridian, and invade the upper visual quadrant, without any suggestion of a reversal that could indicate the presence of a V3-like field (figure 7). The point where the receptive fields invade the upper visual field corresponds to an architectural transition between V2, caudally, and a zone of dense myelination, rostrally; this architectural field is absent from the ventral cortex anterior to V2 (Rosa & Schmid 1995; Rosa et al. 2005).6 Injections of retrograde tracers throughout this ‘third tier’ densely myelinated zone revealed homotopic projections from V1, which originated in both the upper and lower quadrant representations (figure 8a), primarily from cells located at the level of the stria of Gennari (layer 3C; Brodmann's layer 4B) and slightly above. By contrast, injections located posterior to this zone (i.e. in V2) resulted in retrogradely labelled cells that were concentrated in more superficial sublayers of V1, while injections located entirely anterior to it revealed no V1 projections. Finally, based on extensive recordings covering the entire extent of the densely myelinated zone and adjacent areas (Rosa & Schmid 1995), we found that this architectural field formed a single, albeit complex map of the visual field. Consistent with the neuroanatomical data (figure 8b), this visuotopic map included a continuous representation of the lower visual field and a split representation of the upper visual field. Whereas this is an unusual topographic map by the standards of previous descriptions of visuotopy in primate visual areas, it comprises organizational features that are similar in complexity to those described in visual areas of cats (Palmer et al. 1978; Sherk & Mulligan 1993), such as a ‘field discontinuity’ that runs obliquely in relation to the horizontal and vertical meridians, and islands of different ‘field sign’ (Sereno et al. 1994; see Rosa & Schmid 1995; Rosa et al. 2005; for a more detailed discussion).
Figure 7.
Topographic transition between areas in the dorsal extrastriate cortex of a marmoset. This sequence of receptive fields and recording sites demonstrates the absence of a V3-like area inserted between V2 and the dorsomedial area (DM), at least in some species of New World monkey. Top left: parasagittal section (left hemisphere; rostral is to the left), showing electrode tracks and the region that is enlarged in the bottom left panel. Bottom left: location of recording sites, numbered consecutively according to their radial projection to layer four. Recording sites in V2 are shown by white circles, those in DM by black circles, and those in cortex rostral to DM (area DA and the posterior parietal cortex, as defined by Rosa & Schmid 1995) by white squares. Arrows point to the V1/V2 border, to the border between V2 and DM (evident by a sudden increase in myelination), and to the rostral border of DM (marked by an increased separation of the bands of Baillarger). Middle and right: receptive fields recorded in V2, DM and in the cortex rostral to DM. In V2, receptive fields corresponding to progressively more rostral sites move gradually from the vertical meridian towards the horizontal meridian. After the DM border is crossed, the receptive fields become larger, but do not revert towards the lower vertical meridian, as would be expected if V3 existed at this level; instead, they progress to invade the upper visual field.
Figure 8.
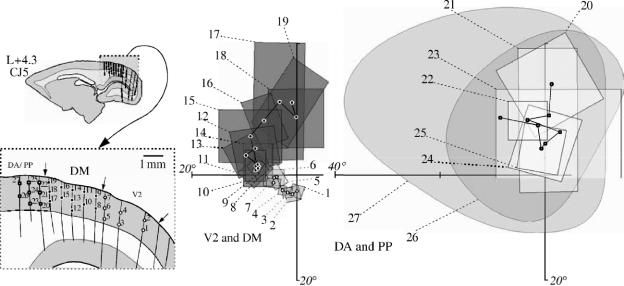
Visuotopic organization of DM, as revealed by (a) anatomical tracing and (b) electrophysiology. (a) Schematic view of the spatial relationship between the locations of injection sites of retrograde tracers in DM (numbered circles) and labelled patches in V1. The outlines of the areas correspond to the contours of V1 and DM in the various cases with injections, aligned and scaled to equal area. The locations of the representations of the fovea (star) and horizontal meridian (dotted line) in V1 are indicated. Injections near the caudal border of DM result in label including the horizontal meridian representation (light patches, 1–5). Injections in the lateral half of DM result in label in the upper visual field representation of V1 (dark grey patches, 6–9), while those in the medial half of DM result in label in the lower visual field representation (medium grey, 10–13). (b) Summaries of the visuotopic organizations of V1 and DM, each based on hundreds of recording sites (V1: redrawn from fig. 8 of Fritsches & Rosa 1996; DM: redrawn from fig. 17 of Rosa & Schmid 1995). The visual field representation is coded according to the diagram shown on the right: representations of the vertical meridian in black squares, representations of the horizontal meridian in white circles, 45° isopolar contours in solid line, isoeccentricity contours in dashed line, representations of the upper quadrant in ‘+’ signs, and representations of the lower quadrants in ‘−’ signs. The stars indicate a line of field discontinuity through the upper quadrant that characterizes the DM map.
For many years, the alternatives of having a V3-like area, homologous to area 19 of non-primates, or a string of ‘third tier’ areas have been promoted as mutually exclusive (e.g. Allman & Kaas 1975; Krubitzer & Kaas 1993; Stepniewska & Kaas 1996). One major contribution of our studies in the marmoset and capuchin has been to demonstrate that, at least in New World monkeys, this is not necessarily the case. As illustrated in figure 2, we found that the ventral cortex rostral to V2 forms a relatively simple, mirror-symmetrical representation of the upper visual quadrant, similar to that described for macaque ventral V3 (Gattass et al. 1988) or VP (Newsome et al. 1986). However, we also found that this area continued dorsally into the region between the foveal representation of V2 and area MT, where a complementary lower quadrant representation was found (figure 6b). Importantly, this lower quadrant representation is similar to ventral V3 in terms of receptive field size, myeloarchitecture and connections (Rosa & Tweedale 2000; Rosa et al. 2000a). The resulting ‘complete’ V3 does receive projections from V1, which nonetheless can be distinguished from V1-DM projections in terms of density and laminar origin (see also Nakamura et al. 2004, for similar evidence in the macaque).
In summary, recognizing the existence of a complete V3 in New World monkeys did not require negating the existence of other areas, such as DM, more dorsally in the third tier cortex. For the purposes of the present review, we find particular comfort in the fact that our results vindicated the concept of DM as a ‘third tier’ area, which corresponds well to a myeloarchitectural zone, contains a representation of the entire visual field and can be distinguished from all adjacent areas on the basis of its laminar pattern of connections with V1. While these characteristics are reminiscent of the ‘textbook’ definition of a visual area, it is also clear that attaining such a delimitation also means accepting that cortical topographic maps can be rather complex, even at such an ‘early’ level of processing. More generally, the long and convoluted story of the New World monkey third visual complex illustrates the fact that some of the present controversies are likely to be solved as more complete information becomes available.
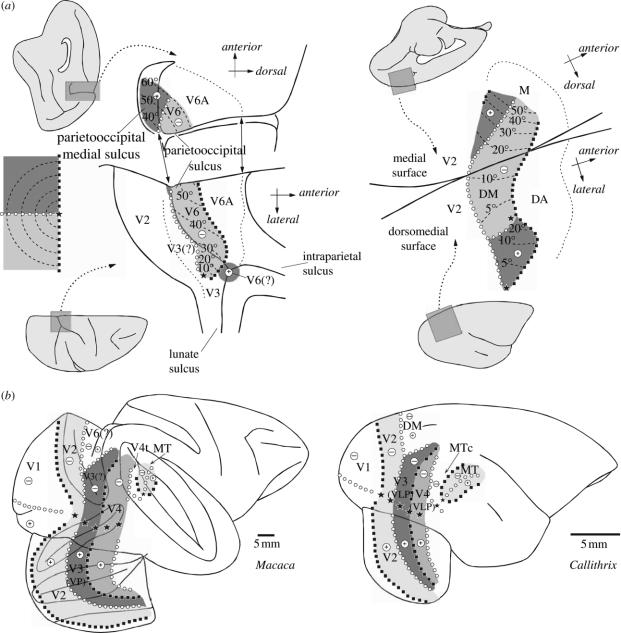
8. How similar can different species of primate be? Seeking the homologues of V3 and DM in Old World monkeys
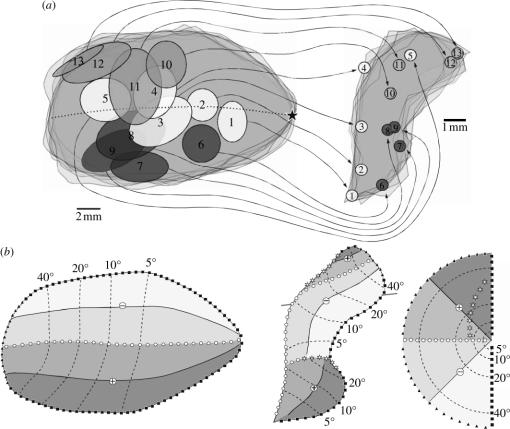
Do the New World monkey DM and V3 have clear-cut Old World monkey homologues and, if so, do they look exactly the same? These are not rhetorical questions, particularly if one assumes, as proposed above, that the formation and refinement of any given topographic map in development may be shaped by the configuration of adjacent maps. In this situation, it would be quite possible to have two distinct areas, originating from different evolutionary events yet in the same position relative to V2, sharing a similar general topographic organization. The argument for homology becomes stronger, however, if specific functional, connectional and neurochemical parallels can be traced between species, as it would be unlikely that all of these would have originated in parallel purely by chance. As outlined in more detail elsewhere (Rosa & Tweedale 2001), we feel that there is a strong case for homology between DM and the macaque's sixth visual area (V6), as recently redefined by Galletti et al. (1999). The visual topography of V6 is compatible with a DM-like model, on the basis of the relative positions of the representations of the visual meridians. In addition, V6 comprises a continuous lower quadrant representation that separates a peripheral upper quadrant representation, medially, from a possible central upper quadrant representation, laterally (figure 9a). Whereas the central upper quadrant representation was not actually mapped by Galletti et al. (1999), these authors pointed out that its existence was demonstrated by the results of Van Essen & Zeki (1978). If confirmed, this would indicate the existence of a visuotopic map in V6 that is uniquely similar to the one found in New World monkey DM (Rosa & Tweedale 2004). The origin of the laminar projections from V1 to V6 in the macaque (Galletti et al. 2001) is also exactly the same as that observed in the marmoset (Vogt-Weisenhorn et al. 1995; Rosa et al. 2005). Finally, both areas are part of relatively direct, oligosynaptic pathways to and from the premotor cortices, are characterized by heavy myelination, and have the same basic visual response properties (Rosa & Tweedale 2001; Galletti et al. 2004).
Figure 9.
Comparison of the organization of extrastriate areas in macaque (left) and marmoset (right) monkeys. (a) Comparison of the organization of the dorsal extrastriate cortex rostral to V2. In both panels, the representation of the lower quadrant (−) is shown in light grey, and that of the upper quadrant (+) in dark grey. In addition, foveal representations are indicated by stars, isoeccentricity lines by dashed lines, representations of the vertical meridian by black squares, and those of the horizontal meridian by circles (see insert at the left). (a) Left: the visuotopy of macaque area V6 is illustrated in dorsal (bottom) and medial (top) views of the brain, with the sulci partially unfolded (redrawn, with different symbols and orientation, from Galletti et al. 1999). The double-ended arrows join corresponding points of the rostral bank of the parietooccipital sulcus, so as to indicate the topological continuity between the medial and dorsal views illustrated. The lips and fundi of the sulci are shown as continuous lines, and the rostral border of V6A as a dotted line. The dark grey oval adjoining the lateral end of V6 indicates the likely location of the central upper quadrant representation in this area, which was not studied in detail by Galletti et al. (1999). The representation of the upper vertical meridian in V6 is hidden from view, being located near the fundi of the sulci. The boxed regions in the inserts (far left) show the approximate location of the illustrated regions in the intact macaque brain. (a) Right: visuotopy of area DM illustrated in dorsomedial (bottom) and medial (top) view of the marmoset brain (Rosa & Schmid 1995). The marmoset brain has no sulci in this region. The representation of the central upper quadrant in this species has been mapped in detail (dark grey region at the lateral extreme of DM), and occupies a region equivalent to that proposed for the equivalent region of V6. (b) Comparison of the visuotopic organization of lateral and ventral cortices, including a new hypothesis on the extent of dorsal V3. Left: lateral view of the right hemisphere of a macaque brain in which the sulci and the ventral surface were partially ‘unfolded’ to allow visualization of the cortical areas. The visuotopic organization is indicated according to the following symbols: the foveal representation is shown by stars, vertical meridian representations are indicated by black squares, horizontal meridian representations by white circles, upper quadrant representations by ‘+’ and lower quadrant representations by ‘−’. The visuotopic organizations of V1 and V2 are based on Gattass et al. (1981), and the organizations of MT, V4 and V4t are based on Gattass et al. (1988). The visuotopy of ventral V3 is also based on Gattass et al. (1988). However, the extent of dorsal V3 was redefined so that the vertical meridian representation crosses the prelunate gyrus, as proposed by Maguire & Baizer (1984) and Youakim et al. (2001). This redefinition of the extent of dorsal V3 results in an organization that strongly resembles the one found in New World monkeys (b) right, from Rosa & Tweedale 2000). See also figure 10.
The main difference between the macaque and the marmoset remains the fact that, in the former species, it is believed that a bridge of V3 interposes between V2 and V6 (figure 9a, left). One way to reconcile our observations in New World monkeys (figure 9a, right) with the data presently available on the macaque cortex is to propose that the position of V6/DM changes in different species, as a result of the addition of further areas in the larger species. Different final configurations of areas are possible in the context of the evolutionary/developmental hypothesis outlined in previous sections of this paper, particularly in regions not adjacent to the primary areas. However, it is also important to realize that the published evidence for a V3 that extends that far medially is not particularly strong (see Rosa & Tweedale 2001 for review). Area V6 (according to the definition of Galletti et al. 1999; see figure 9a) is similar to ‘dorsal V3’ of other studies (e.g. Felleman et al. 1997) in having a rostral border that corresponds to the lower vertical meridian, in being strongly myelinated, and in receiving projections that originate from V1 cells located predominantly at the level of the stria of Gennari. Thus, it is possible that parts of V6 may have been included in dorsal V3 in previous work, where the entire extent of the dorsal ‘third tier’ cortex was not explored with dense grids of penetrations. Whereas Galletti et al. (1999) recognize a thin V3 bridge inserted between V2 and V6, the illustrated evidence suggests that these recording sites may in fact have been located in V2 (see Rosa & Tweedale 2001; for discussion). The existence of V6 in the medial part of the ‘third tier’ cortex would explain why the myeloarchitecture of ‘V3’, as presently defined in species such as the macaque and the capuchin, changes dramatically at the level of the annectent gyrus (Rosa et al. 1993b), as well as the presence of certain unexpected features revealed by functional mapping studies of the macaque extrastriate cortex. These include the discontinuous nature of the lower vertical meridian representation that is taken to coincide with the rostral border of V3 (e.g. Gattass et al. 1988; see figure 10, left), and the presence of a sector of upper vertical meridian representation along the putative rostral border of dorsal V3, as revealed in recent fMRI studies (figure 11). As redefined in the New World monkey, V3 also becomes a more suitable candidate for being homologous to area 19 of non-primates, given its location (Rosa 1999), architecture (Price 1985), receptive field properties (Burkhalter & Van Essen 1986) and connections (Felleman et al. 1997), which, as in non-primates, suggest a role for V3 in the ‘ventral stream’ (Tanaka et al. 1987; Dinse & Kruger 1990; Dreher et al. 1996; Rosa & Manger in press).
Figure 10.
A new hypothesis on the organization of dorsal extrastriate cortex in Old World monkeys, based on our studies in New World monkeys. The key point here is to demonstrate that the raw data on which the currently accepted subdivision of the macaque cortex is based are equally compatible with another interpretation (illustrated in figure 9b). Left: original interpretation of boundaries of visual areas in the macaque dorsal cortex, based on Gattass et al. (1988) and Colby et al. (1988). This diagram represents a bidimensional reconstruction of the macaque caudal extrastriate cortex, with the visual topography of V2, V3, V3A, V4, V4t and PO indicated (dark area represents the central 1° of the visual field, dashed lines are isoeccentricity lines; see insert for other symbols). The fine dotted lines indicate boundaries of areas which were interpolated based on myeloarchitectural evidence. Redrawn from fig. 5 of Gattass et al. (1988), with the exception of the organization of V3A, which was based on figs. 3, 8, 11 and 13 of the same reference, and PO, which was based on Colby et al. (1988). Right: a re-interpretation of the same data, based on our studies of New World monkeys and on the studies of Maguire & Baizer (1984) in the macaque. In this model, a lower quadrant representation previously assigned to ‘V3A’ (corresponding to Maguirre and Baizer's area PM) forms the continuation of VP into the anterior bank of the lunate sulcus and prelunate gyrus. This would result in a ‘V3’ forming a complete representation of the visual field, similar to the New World monkey V3 (see figure 9b; right). The most medial part of the original ‘dorsal V3’, combined with area PO, forms the homologue of New World monkey DM (V6 of Galletti et al. 1999).
Figure 11.
Two views on the organization of the macaque third visual complex. (a) Topographic organization of the caudal cortex between V1 and V4, as revealed by fMRI data (Brewer et al. 2002). The representation of polar angles is indicated by different colours, according to the key illustrated in the inserts. The arrows point to ‘anomalous’ sectors of upper quadrant representation found in the middle of dorsal V3. (b) A ‘classical’ interpretation of these data, including a V3 that is mirror-symmetrical to V2 and another area, V3a, located entirely anterior to V3. (c) A re-interpretation of the same observations, according to the hypothesis illustrated in figures 9b and 10. We submit that the revised borders, which imply two areas (V3 and DM, or V6) adjacent to V2, provide a better fit to the raw data. Scale bar, 5 mm.
In summary, the medial border of V3 may also need to be reconsidered in Old World monkeys; perhaps, similar to in marmosets, this area does not extend as far medially, giving way to a dorsal stream-dominated, densely myelinated field with stronger V1 connections (V6). As illustrated in figures 10 and 11, the raw observations supporting the currently accepted areal subdivision of the macaque dorsal extrastriate cortex are actually very similar to our data in New World monkeys, with the major differences coming from the investigator's choice of placement of boundaries. We propose that a redefinition of the macaque cortex along the lines shown in figures 9b and 10 would result in subdivisions that better conform to the expected characteristics of what constitutes a visual area, in terms of anatomical and physiological homogeneity. For example, previous investigators have noted that the architecture and response properties of ventral V3 are more akin to those found in parts of the prelunate gyrus (Burkhalter & Van Essen 1986) than to those found in ‘dorsal V3’; to these similarities we add the fact that both regions receive weak or no connections from V1 (Felleman et al. 1997; Nakamura et al. 2004). Moreover, others have described the existence of significant functional and connectional differences between the posteromedial and anterolateral parts of the prelunate gyrus (Zeki 1983; Maguire & Baizer 1984; Stepniewska & Kaas 1996; Youakim et al. 2001). Thus, the proposal of a redefinition of V3 is, at least to a preliminary level, compatible with existing evidence.
Although it is early to propose an exact correspondence between areas in New World and Old World monkeys, our results raise this possibility. A better grasp of the factors underpinning the evolution of cortical representations in general, and the likely homologies of DM and V3 in particular, will require more high-resolution anatomical and physiological data from a wider range of species. Either way, the complicated history of the organization of the third visual complex raises the issue of the difficulty in interpreting areal organization on the basis of limited criteria. Whereas the current technology for non-invasive mapping is certainly adequate for detecting similarities (and differences) in visuotopic organization between human and monkey visual cortices (Brewer et al. 2002), it must be kept in mind that the relationship between maps and areas can be quite complex, and that a clear definition of cortical areas requires the parallel consideration of connectional and histological criteria.
9. Other points of contention: what, if anything, is a cortical area?
As detailed in the previous sections, it has become increasingly clear that the fields forming the New World monkey ‘third tier’ visual cortex more or less conform to the traditional view of what a visual area should be. They have distinct architectural characteristics and connections, contain neurons with relatively well-defined receptive fields, and form topographic maps of visual space. It may be sobering to realize that, despite the many years of investigation required, this is likely to have represented a relatively easy problem to solve, within the broader context of cortical mapping: on final analysis, the current generation of neuroscientists did have access to the methods and concepts needed to attack this problem logically, and the resolution basically depended on technical issues. Is it the case that all we need is time, and perhaps an even better suite of methods, so as to reach a map that shows a complete and accurate mosaic of cortical areas? Or do we need to adjust our expectations as we move further towards trying to understand the large mass of association isocortex that characterizes the primate brain, in search of the neural bases of cognition?
A tacit assumption underlying much of the neuroscience conducted over the last century has been that all cortical areas are well-defined entities. This is reflected, for example, in current illustrations showing the cortex as a patchwork of fields of different sizes and shapes. Essentially, areas have been seen as ‘organs’: physically segregated and functionally specialized entities that interact via ordered ‘feed-forward’ and ‘feedback’ connections. Specialists in cortical architectonics do know, of course, that the situation is nowhere near as clear-cut, and that the degree of confidence with which we can draw the lines that separate the areas vary enormously; this was recognized by both Campbell (1905) and Brodmann (1909), among many others. Nonetheless, for the most part this is seen as a temporary situation. The general expectation has been that, given better histological, functional and molecular biological mapping techniques, the exact configuration of areas will eventually be revealed across the entire human (or animal) cortex, with a degree of precision similar to the one with which we can now define the primary sensory areas. It is important to recognize, however, that this remains an article of faith. Here, we would like to argue that, while many of the current controversies will undoubtedly be solved by future studies, one must also be prepared to face the prospect that the cerebral cortex is not entirely formed by neatly defined, homogeneous fields with sharp boundaries. In particular, it is possible that an accurate map of regions of evolutionarily new sectors of cortex may require probabilistic descriptions.
Consider, for example, our current level of understanding of the inferior temporal visual cortex (for the purposes of the present argument, we will concentrate on the expansive field ‘TE’ of Von Bonin & Bailey 1947). It is clear that this region is functionally heterogeneous, given that recordings from different locations yield neurons with distinct response properties, and that different lesions result in distinct constellations of symptoms (Blake et al. 1977; Baylis et al. 1987; Kobatake & Tanaka 1994; Janssen et al. 2000; Cowey et al. 2001; Tamura & Tanaka 2001). Moreover, architectural studies in New World and Old World monkeys demonstrate a degree of structural heterogeneity (Seltzer & Pandya 1978, 1989; Weller & Steele 1992). However, there has been limited success in proceeding beyond this point, particularly if one operates under the assumption that, similar to the occipital visual cortex, the larger cytoarchitectural fields can be logically subdivided into a set of smaller, functionally specialized areas. The morphological transitions throughout the inferior temporal cortex are genuinely gradual.7 For example, while most investigators agree that there is a clear change in histological character between caudal and rostral parts of cytoarchitectural area TE, precise boundaries are difficult to draw, even with more modern techniques (Kondo et al. 1994; Xu et al. 2003; Komatsu et al. in press). Moreover, the laminar patterns of interconnections between putative TE subdivisions defy attempts at classification as ‘extrinsic’ (‘feed-forward’/‘feedback’) or ‘intrinsic’, according to traditional criteria (Seltzer & Pandya 1989; Suzuki et al. 2000). Finally, visuotopic representations can, at best, be viewed in terms of broad trends (Boussaoud et al. 1991; Hikosaka 1998; Rosa & Tweedale 2000). All present evidence considered, the organization of TE could be just as adequately described in terms of rostral–caudal or dorsal–ventral changes in the proportions of modules dedicated to different functions, without the implication of a mosaic of sharply defined sub-areas (figure 12). At this point, the definition of a cortical area would become, to some extent, a matter of semantics. Would TE be best seen as a very large area which changes in character gradually, or as a large number of smaller areas, each with indistinct boundaries? In the latter case, one might argue that there is no ‘final’, definitive cortical map: at a microscopic level, subdivisions of higher-order association cortices may need to be defined in probabilistic terms, depending on which functional property, afferent connection or molecule one is interested in charting.
Figure 12.
Hypothetical views of the functional and anatomical transitions at the boundary of two cortical areas. Each diagram represents a number of cellular columns spanning from layer one (top) to the white matter (bottom), and two populations of afferent axons. Left: in this case, the border is unambiguous—anatomical and functional definitions coincide. This is because during development the afferent connections have segregated along a precise border, creating a sharp functional transition between cells with one type of neural response (dark columns) and those with a different type of neural response (white columns). This could also result in sharp histological transitions (for example, if afferent 1 represented a highly myelinated, fast conducting pathway, and afferent 2 a less myelinated pathway). Right: because the proportions of cells with different response properties and connections change gradually, in this second case the border is ambiguous as far as can be assessed by any single criterion. For example, based on the pattern of connections, one may recognize two areas that gradually merge onto each other, or three areas defined by different combinations of afferents. The histological characteristics would also be expected to change gradually.
There is no question that our present understanding of cortical cartography will be greatly refined in the future, including the resolution of many present controversies. Yet one may also need to be prepared for the possibility that the exploration of the association cortex, particularly in those fields that experienced recent marked expansion during human evolution, will require more flexible mapping criteria. For example, while accepting that major architectural fields can be used as a frame of reference for analysis of functional data in both monkey and human (Pandya & Yeterian 1996; Petrides & Pandya 1999, 2002), it is possible that these do not each correspond to an ‘area’, at least in the same way that V1 or MT are sharply defined ‘areas’ that circumscribe a well-defined population of neurons with unique properties, distinct from those found in all surrounding areas.
10. Conclusions
Cortical areas vary in their degree of definition, from the pencil-sharp boundaries of primary sensory areas to the ill-defined transitions of most association fields. Rather than seeing this only as a result of methods and criteria that are presently inadequate, we believe, on the basis of our studies in New World monkeys, that this reflects genuine features of primate cortical organization. We propose that some of these variations can be explained on the basis of the mechanisms involved in the evolutionary and developmental formation of the cortex. According to our hypothesis, borders that are sharply defined and methodologically robust in adults are those which are specified by the sharpest transitions in the expression of cell surface molecules during development. These early specified boundaries are likely to characterize phylogenetically older, ‘core’ areas, which can be reliably identified across many species. In particular, our studies in the marmoset, together with anatomical and physiological evidence obtained by others, suggest that area MT is one of these core fields. Our results also support the view that the present uncertainty regarding the organization of extrastriate visual areas can, in part, be traced to methodological factors. For example, our studies of the third visual complex suggest that, upon closer scrutiny, New World and Old World monkeys may be more similar than previously recognized. Yet our theory also predicts that in evolutionarily newer cortices, less precise boundaries, more complex sensorimotor maps and larger variability are to be expected, between both individuals and species. Comparative analyses of cortical organization, together with clarification of the developmental mechanisms underlying the formation of cortical maps (in particular, the degree of genetic specification involved in the establishment of different areas), will have a significant impact on our future efforts in cortical mapping.
The gradual process of refinement of our understanding of the cerebral cortex will also raise the issue of nomenclature as a crucial limitation of our current approaches to brain mapping. As illustrated by the convoluted history of the third tier visual cortex, it is relatively common to find that areas that are probably homologous, or parts thereof, have been given several distinct names by different researchers. More worrying, however, is the fact that the same names may have been given to different areas, as this may imply false homologies or analogies, and the consequent creation of much confusion for scientists, clinicians and students alike. This situation is, in no small part, owing to a lack of consensus regarding how best to define a cortical area. We believe that only through a better understanding of the processes of formation of areas in development and evolution will objective criteria emerge to allow a sensible, systematic nomenclature of the primate cortical areas.
Acknowledgments
The authors thank Dr Paul Manger for insightful discussions and comments on the manuscript. This study was supported by project grants from the National Health and Medical Research Council and Australian Research Council, and by equipment grants from the Clive and Vera Ramaciotti Foundation and ANZ Charitable Trust.
Glossary
- DM
dorsomedial area
- MT
middle temporal area
Endnotes
‘In 1904 I pointed out in a publication that brains without sulci (lissencephalic) possessed great advantages over gyrencephalic ones for topical localisation, that is, the histological delimitation of cortical areas, for on the smooth surfaces of such hemispheres the shape, position and extent of the individual fields were more clearly visible than on folded surfaces, and that often it was only possible to understand the complex relationships of gyrencephalic hemispheres by reference to them.’ From Brodmann (1909; ch. IV).
It may be of historical interest to note, however, that the ubiquity of the use of macaques in studies of cortical organization was not nearly as prevalent at the time of Brodmann's and Campbell's investigations as it is today. Although this fact is not always recognized, Brodmann's most famous diagram representing the cytoarchitectural areas of the Old World monkey cerebral cortex is based on the study of a guenon (genus Cercopithecus). Campbell conducted parallel investigations on the cortex of various species of apes, using specimens obtained from Sherrington's laboratory following his studies on stimulation of motor cortex in these animals.
‘Function is no sure guide, for organs which are clearly homologous in two animals may be put to quite different uses. Observation shows that the shape, size or color of a structure gives little positive evidence of identity. Similarity in general anatomic position and relations to adjacent organs is a more useful clue to identification. Best of all is similarity in developmental history. Embryologic processes in vertebrates tend to be conservative, and organs which are quite different in the adult condition may reveal their homology through similarity in early embryonic stages.’ In Romer (1955; ch. 1).
‘The regions distinguished in man and the gyrencephalic monkeys are also found in their entirety in the marmoset, but usually in simplified form and arrangement and sometimes with a reduced number of areas.’ In Brodmann (1909; ch. IV).
‘The value of embryological researches has already been proved, at any rate in one direction, by taking advantage of the natural law that the maturation or myelinisation of the various tracts of fibres standing in relation with various functions does not occur coincidentally but follows a set sequence. Observing the tenets of this law the surface of the brain can be subdivided in accordance with the different times at which the medullated constituents become apparent… To exemplify my meaning I have merely to point to the visuo-sensory area; the calcarine cortex, in which the seeing function concentrates itself, is found to contain myelinised fibres at a relatively early date, certainly at a time when such fibres are absent from the surrounding field.’ From Campbell (1905; Introduction).
Myeloarchitectural evidence for an asymmetry between the dorsal and ventral extrastriate cortices anterior to V2 can be found as far back as Campbell's work, and Campbell may in fact have been the first person to correctly deduce the visual function of the DM cortex. Commenting on Flechsig's finding of a zone of heavy myelination at the posteromedial end of the parietal lobe (probably corresponding to the region that became Feld 15 in Flechsig's 1920 monography), Campbell states that ‘…in those instances in which the fibre supply is great, on tracing the area backward, it can always be found to be continuous with the field of cortex to which I have given the name “visuo-psychic,” indeed the type of arrangement it presents is actually the “visuo-psychic” type; hence I maintain that in these cases we have nerely to deal with a forward extension of the visual area’. From Campbell (1905; ch. VIII).
A typical statement to this effect can be found in Campbell (1905), who, referring to the ‘temporal field’, states that, ‘The various series of sections have been searched very carefully to ascertain whether this large area can be further split up on the basis of additional variations in the arrangement of cortical fibres but this has been found to be impossible, because although slight differences are noticeable these are merely undecided changes in density, and over all the absence of fibres of large calibre is the dominant feature and has been the main guide in settling the limits of the field…the topical variations in cell lamination are not equivalent in degree to the differences in fibre-arrangement, also the intervening gradations are by no means abrupt: hence the extent and limits of these types of lamination are by no means easy to define.’ From Campbell (1905; ch. VI).
One contribution of 12 to a Theme Issue ‘Cerebral cartography 1905–2005’.
References
- Adams D.L, Horton J.C. Capricious expression of cortical columns in the primate brain. Nature Neurosci. 2003;6:113–114. doi: 10.1038/nn1004. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. A representation of the visual field in the caudal third of the middle temporal gyrus of the owl monkey (Aotus trivirgatus) Brain Res. 1971;31:85–105. doi: 10.1016/0006-8993(71)90635-4. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. The dorsomedial cortical visual area: a third tier area in the occipital lobe of the owl monkey (Aotus trivirgatus) Brain Res. 1975;100:473–487. doi: 10.1016/0006-8993(75)90153-5. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb. Cortex. 2002;12:1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- Azzopardi P, Fallah M, Gross C.G, Rodman H.R. Response latencies of neurons in visual areas MT and MST of monkeys with striate cortex lesions. Neuropsychologia. 2003;41:1738–1756. doi: 10.1016/s0028-3932(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Barbur J.L, Watson J.D, Frackowiak R.S, Zeki S. Conscious visual perception without V1. Brain. 1993;116:1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- Barone P, Batardiere A, Knoblauch K, Kennedy H. Laminar distribution of neurons in extrastriate areas projecting to visual areas V1 and V4 correlates with the hierarchical rank and indicates the operation of a distance rule. J. Neurosci. 2000;20:3263–3281. doi: 10.1523/JNEUROSCI.20-09-03263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The architecture of the colour centre in the human visual brain: new results and a review. Eur. J. Neurosci. 2000;12:172–193. doi: 10.1046/j.1460-9568.2000.00905.x. [DOI] [PubMed] [Google Scholar]
- Baylis G.C, Rolls E.T, Leonard C.M. Functional subdivisions of the temporal lobe neocortex. J. Neurosci. 1987;7:330–342. doi: 10.1523/JNEUROSCI.07-02-00330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck P.D, Kaas J.H. Cortical connections of the dorsomedial visual area in Old World macaque monkeys. J. Comp. Neurol. 1999;406:487–502. [PubMed] [Google Scholar]
- Blake L, Jarvis C.D, Mishkin M. Pattern discrimination thresholds after partial inferior temporal or lateral striate lesions in monkeys. Brain Res. 1977;120:209–220. doi: 10.1016/0006-8993(77)90901-5. [DOI] [PubMed] [Google Scholar]
- Bourne J.A, Warner C.E, Rosa M.G.P. Postnatal maturation of marmoset monkey visual cortical areas: MT leads the way. Soc. Neurosci. Abstr. 2004:839.3. 2004. [Google Scholar]
- Bourne J.A, Warner C.E, Rosa M.G.P. Topographic and laminar maturation of striate cortex in early postnatal marmoset monkeys, as revealed by neurofilament immunohistochemistry. Cereb. Cortex. In press doi: 10.1093/cercor/bhh175. doi:10.1093/cercor/bhh175 [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Desimone R, Ungerleider L.G. Visual topography of area TEO in the macaque. J. Comp. Neurol. 1991;306:554–575. doi: 10.1002/cne.903060403. [DOI] [PubMed] [Google Scholar]
- Brewer A.A, Press W.A, Logothetis N.K, Wandell B.A. Visual areas in macaque cortex measured using functional magnetic resonance imaging. J. Neurosci. 2002;22:10 416–10 426. doi: 10.1523/JNEUROSCI.22-23-10416.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. Erste Mitteilung: Die Regio Rolandica. J. Psychol. Neurol. 1903a;2:79–107. [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. Zweite Mitteilung: Der Calcarinatypus. J. Psychol. Neurol. 1903b;2:133–159. [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. Dritte Mitteilung: Die Rindenfelder der niederen Affen. J. Psychol. Neurol. 1905a;4:177–226. [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. Vierte Mitteilung: Die Riesenpyramidentypus und sein Verhalten zu den Furchen bei den Karnivoren. J. Psychol. Neurol. 1905b;6:108–120. [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. Fünfte Mitteilung: über den allgemeinen Bauplan des Cortex pallii bei den Mammalieren und zwei homologe Rindenfelder im besonderen. Zugleich ein Beitrag zur Furchenlehre. J. Psychol. Neurol. 1906;6:275–400. [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. VI. Mitteilung: die Cortexgliederung des Menschen. J. Psychol. Neurol. 1908a;10:231–246. [Google Scholar]
- Brodmann K. Beiträge zur histologischen Lokalisation der Grosshirnrinde. VII. Mitteilung: die cytoarchitektonische Cortexgliederung der Halbaffen (Lemuriden) J. Psychol. Neurol. 1908b;10:287–334. [Google Scholar]
- Brodmann K. J. A. Barth; Leipzig: 1909. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. [Transl. by Garey, L. J. 1994 As localisation in the cerebral cortex. London: Smith-Gordon. [Google Scholar]
- Burke W, Dreher B, Wang C. Selective block of conduction in Y optic nerve fibres: significance for the concept of parallel processing. Eur. J. Neurosci. 1998;10:8–19. doi: 10.1046/j.1460-9568.1998.00025.x. [DOI] [PubMed] [Google Scholar]
- Burkhalter A, Van Essen D.C. Processing of color, form and disparity information in visual areas VP and V2 of ventral extrastriate cortex in the macaque monkey. J. Neurosci. 1986;6:2327–2351. doi: 10.1523/JNEUROSCI.06-08-02327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A.W. Cambridge University Press; Cambridge: 1905. Histological studies on the localisation of cerebral function. [Google Scholar]
- Campbell R, Zihl J, Massaro D, Munhall K, Cohen M.M. Speechreading in the akinetopsic patient, LM. Brain. 1997;120:1793–1803. doi: 10.1093/brain/120.10.1793. [DOI] [PubMed] [Google Scholar]
- Caviness V.S, Goto, Jr T, Tarui T, Takahashi T, Bhide P.G, Nowakowski R.S. Cell output, cell cycle duration and neuronal specification: a model of integrated mechanisms of the neocortical proliferative process. Cereb. Cortex. 2003;13:592–598. doi: 10.1093/cercor/13.6.592. [DOI] [PubMed] [Google Scholar]
- Colby C.L, Gattass R, Olson C.R, Gross C.G. Topographical organization of cortical afferents to extrastriate visual area PO in the macaque: a dual tracer study. J. Comp. Neurol. 1988;269:392–413. doi: 10.1002/cne.902690307. [DOI] [PubMed] [Google Scholar]
- Condé F, Lund J.S, Lewis D.A. The hierarchical development of monkey visual cortical regions as revealed by the maturation of parvalbumin-immunoreactive neurons. Brain Res. Dev. Brain Res. 1996;96:261–276. doi: 10.1016/0165-3806(96)00126-5. [DOI] [PubMed] [Google Scholar]
- Cowey A, Heywood C.A, Irving-Bell L. The regional cortical basis of achromatopsia: a study on macaque monkeys and an achromatopsic patient. Eur. J. Neurosci. 2001;14:1555–1566. doi: 10.1046/j.0953-816x.2001.01774.x. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O.D. Extrageniculo-striate visual mechanisms: compartmentalization of visual functions. Prog. Brain Res. 1988;75:307–320. doi: 10.1016/s0079-6123(08)60488-4. [DOI] [PubMed] [Google Scholar]
- Cruz D.A, Eggan S.M, Lewis D.A. Postnatal development of pre- and postsynaptic GABA markers at chandelier cell connections with pyramidal neurons in monkey prefrontal cortex. J. Comp. Neurol. 2003;465:385–400. doi: 10.1002/cne.10833. [DOI] [PubMed] [Google Scholar]
- Culham J.C, Kanwisher N.G. Neuroimaging of cognitive functions in human parietal cortex. Curr. Opin. Neurobiol. 2001;11:157–163. doi: 10.1016/s0959-4388(00)00191-4. [DOI] [PubMed] [Google Scholar]
- Cusick C.G, Scripter J.L, Darensbourg J.G, Weber J.T. Chemoarchitectonic subdivisions of the visual pulvinar in monkeys and their connectional relations with the middle temporal and rostral dorsolateral visual areas, MT and DLr. J. Comp. Neurol. 1993;336:1–30. doi: 10.1002/cne.903360102. [DOI] [PubMed] [Google Scholar]
- Daniel P.M, Whitteridge D. The representation of the visual field on the cerebral cortex in monkeys. J. Physiol. (Lond.) 1961;159:203–221. doi: 10.1113/jphysiol.1961.sp006803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R.B, Dunlop S.A, Finlay B.L. Neural development in metatherian and eutherian mammals: variation and constraint. J. Comp. Neurol. 1999;411:359–368. [PubMed] [Google Scholar]
- Desimone R, Ungerleider L.G. Multiple visual areas in the caudal superior temporal sulcus of the macaque. J. Comp. Neurol. 1986;248:164–189. doi: 10.1002/cne.902480203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Winter W, Oxnard C.E. Evolutionary radiations and convergences in the structural organization of mammalian brains. Nature. 2001;409:710–714. doi: 10.1038/35055547. [DOI] [PubMed] [Google Scholar]
- Dinse H.R, Kruger K. Contribution of area 19 to the foreground-background-interaction of the cat: an analysis based on single cell recordings and behavioural experiments. Exp. Brain Res. 1990;82:107–122. doi: 10.1007/BF00230843. [DOI] [PubMed] [Google Scholar]
- Dobkins K.R, Fine I, Hsueh A.C, Vitten C. Pattern motion integration in infants. J. Vis. 2004;4:144–155. doi: 10.1167/4.3.2. [DOI] [PubMed] [Google Scholar]
- Donoghue M.J, Rakic P. Molecular gradients and compartments in the embryonic primate cerebral cortex. Cereb. Cortex. 1999;9:586–600. doi: 10.1093/cercor/9.6.586. [DOI] [PubMed] [Google Scholar]
- Dougherty R.F, Koch V.M, Brewer A.A, Fischer B, Modersitzki J, Wandell B.A. Visual field representations and locations of visual areas V1/2/3 in human visual cortex. J. Vis. 2003;3:586–598. doi: 10.1167/3.10.1. [DOI] [PubMed] [Google Scholar]
- Dreher B, Wang C, Burke W. Limits of parallel processing: excitatory convergence of different information channels on single neurons in striate and extrastriate visual cortices. Clin. Exp. Pharmacol. Physiol. 1996;23:913–925. doi: 10.1111/j.1440-1681.1996.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Dubner R, Zeki S.M. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 1971;35:528–532. doi: 10.1016/0006-8993(71)90494-x. [DOI] [PubMed] [Google Scholar]
- Eadie M.J. Alfred Walter Campbell (1868–1937) J. Neurol. 2003;250:249–250. doi: 10.1007/s00415-003-0899-1. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Van Essen D.C. Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Felleman D.J, Burkhalter A, Van Essen D.C. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J. Comp. Neurol. 1997;379:21–47. doi: 10.1002/(sici)1096-9861(19970303)379:1<21::aid-cne3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Ferber S, Humphrey G.K, Vilis T. The lateral occipital complex subserves the perceptual persistence of motion-defined groupings. Cereb. Cortex. 2003;13:716–721. doi: 10.1093/cercor/13.7.716. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Guy C.N, Zeki S. The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain. 1995;118:1375–1394. doi: 10.1093/brain/118.6.1375. [DOI] [PubMed] [Google Scholar]
- Fine I, Wade A.R, Brewer A.A, May M.G, Goodman D.F, Boynton G.M, Wandell B.A, MacLeod D.I. Long-term deprivation affects visual perception and cortex. Nature Neurosci. 2003;6:915–916. doi: 10.1038/nn1102. [DOI] [PubMed] [Google Scholar]
- Finlay B.L, Darlington R.B. Linked regularities in the development and evolution of mammalian brains. Science. 1995;268:1578–1584. doi: 10.1126/science.7777856. [DOI] [PubMed] [Google Scholar]
- Finlay B.L, Darlington R.B, Nicastro N. Developmental structure in brain evolution. Behav. Brain Sci. 2001;24:263–278. [PubMed] [Google Scholar]
- Fiorani Jr M, Gattass R, Rosa M.G.P, Sousa A.P.B. Visual area MT in the Cebus monkey: location, visuotopic organization, and variability. J. Comp. Neurol. 1989;287:98–118. doi: 10.1002/cne.902870108. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Thieme; Leipzig: 1920. Anatomie des menschlichen Gehirns und Rückenmarks. [Google Scholar]
- Fritsches K.A, Rosa M.G.P. Visuotopic organisation of striate cortex in the marmoset monkey (Callithrix jacchus) J. Comp. Neurol. 1996;372:264–282. doi: 10.1002/(SICI)1096-9861(19960819)372:2<264::AID-CNE8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Gamberini M, Kutz D.F. The cortical visual area V6: brain location and visual topography. Eur. J. Neurosci. 1999;11:3922–3936. doi: 10.1046/j.1460-9568.1999.00817.x. [DOI] [PubMed] [Google Scholar]
- Galletti C, Gamberini M, Kutz D.F, Fattori P, Luppino G, Matelli M. The cortical connections of area V6: an occipito-parietal network processing visual information. Eur. J. Neurosci. 2001;13:1572–1588. doi: 10.1046/j.0953-816x.2001.01538.x. [DOI] [PubMed] [Google Scholar]
- Galletti C, Fattori P, Gamberini M, Kutz D.F. The most direct visual pathway to the frontal cortex. Cortex. 2004;40:216–217. doi: 10.1016/s0010-9452(08)70956-0. [DOI] [PubMed] [Google Scholar]
- Gattass R, Gross C.G, Sandell J.H. Visual topography of V2 in the macaque. J. Comp. Neurol. 1981;201:519–539. doi: 10.1002/cne.902010405. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa A.P.B, Gross C.G. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard P, Salin P.A, Bullier J. Response selectivity of neurons in area MT of the macaque monkey during reversible inactivation of area V1. J. Neurophysiol. 1992;67:1437–1446. doi: 10.1152/jn.1992.67.6.1437. [DOI] [PubMed] [Google Scholar]
- Gitton Y, Cohen-Tannoudji M, Wassef M. Role of thalamic axons in the expression of H-2Z1, a mouse somatosensory cortex specific marker. Cereb. Cortex. 1999;9:611–620. doi: 10.1093/cercor/9.6.611. [DOI] [PubMed] [Google Scholar]
- Glazko G.V, Nei M. Estimation of divergence times for major lineages of primate species. Mol. Biol. Evol. 2003;20:424–434. doi: 10.1093/molbev/msg050. [DOI] [PubMed] [Google Scholar]
- Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl Acad. Sci. USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Itzchak Y, Malach R. Cue-invariant activation in object-related areas of the human occipital lobe. Neuron. 1998;21:191–202. doi: 10.1016/s0896-6273(00)80526-7. [DOI] [PubMed] [Google Scholar]
- Hackett T.A, Stepniewska I, Kaas J.H. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J. Comp. Neurol. 1998;394:475–495. doi: 10.1002/(sici)1096-9861(19980518)394:4<475::aid-cne6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hackett T.A, Preuss T.M, Kaas J.H. Architectonic identification of the core region in auditory cortex of macaques, chimpanzees, and humans. J. Comp. Neurol. 2001;441:197–222. doi: 10.1002/cne.1407. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. Representation of foveal visual fields in the ventral bank of the superior temporal sulcus in the posterior inferotemporal cortex of the macaque monkey. Behav. Brain Res. 1998;96:101–113. doi: 10.1016/s0166-4328(97)00202-7. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. Receptive fields and functional architecture of monkey striate cortex. J. Physiol. (Lond.) 1968;195:215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman K.J, Krubitzer L. Area 3a: topographic organization and cortical connections in marmoset monkeys. Cereb. Cortex. 2001;11:849–867. doi: 10.1093/cercor/11.9.849. [DOI] [PubMed] [Google Scholar]
- Huffman K.J, Nelson J, Clarey J, Krubitzer L. Organization of somatosensory cortex in three species of marsupials, Dasyurus hallucatus, Dactylopsila trivirgata, and Monodelphis domestica: neural correlates of morphological specializations. J. Comp. Neurol. 1999;403:5–32. doi: 10.1002/(sici)1096-9861(19990105)403:1<5::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Janssen P, Vogels R, Orban G.A. Selectivity for 3D shape that reveals distinct areas within macaque inferior temporal cortex. Science. 2000;288:2054–2056. doi: 10.1126/science.288.5473.2054. [DOI] [PubMed] [Google Scholar]
- Kaas J. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav. Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- Kaas J.H, Catania K.C. How do features of sensory representations develop? Bioessays. 2002;24:334–343. doi: 10.1002/bies.10076. [DOI] [PubMed] [Google Scholar]
- Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J. Neurophysiol. 1994;71:856–867. doi: 10.1152/jn.1994.71.3.856. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral D.G. Macaque monkey retrosplenial cortex. I. Three-dimensional and cytoarchitectonic organization. J. Comp. Neurol. 2000;426:339–365. doi: 10.1002/1096-9861(20001023)426:3<339::aid-cne1>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral D.G. Macaque monkey retrosplenial cortex. II. Cortical afferents. J. Comp. Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Komatsu Y, Watakabe A, Hashikawa T, Tochitani S, Yamamori T. Retinol-binding protein gene is highly expressed in higher-order association areas of the primate neocortex. Cereb. Cortex. In press doi: 10.1093/cercor/bhh112. [DOI] [PubMed] [Google Scholar]
- Kondo H, Hashikawa T, Tanaka K, Jones E.G. Neurochemical gradient along the monkey occipito–temporal cortical pathway. Neuroreport. 1994;5:613–616. doi: 10.1097/00001756-199401000-00020. [DOI] [PubMed] [Google Scholar]
- Kornack D.R. Neurogenesis and the evolution of cortical diversity: mode, tempo, and partitioning during development and persistence in adulthood. Brain Behav. Evol. 2000;55:336–344. doi: 10.1159/000006668. [DOI] [PubMed] [Google Scholar]
- Kornack D.R, Rakic P. Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc. Natl Acad. Sci. USA. 1998;95:1242–1246. doi: 10.1073/pnas.95.3.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Erb M, Grodd W, Bulthoff H.H. Representation of the perceived 3-D object shape in the human lateral occipital complex. Cereb. Cortex. 2003;13:911–920. doi: 10.1093/cercor/13.9.911. [DOI] [PubMed] [Google Scholar]
- Krubitzer L.A, Kaas J.H. The organization and connections of somatosensory cortex in marmosets. J. Neurosci. 1990a;10:952–974. doi: 10.1523/JNEUROSCI.10-03-00952.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer L.A, Kaas J.H. Cortical connections of MT in four species of primates: areal, modular, and retinotopic patterns. Vis. Neurosci. 1990b;5:165–204. doi: 10.1017/s0952523800000213. [DOI] [PubMed] [Google Scholar]
- Krubitzer L.A, Kaas J.H. The dorsomedial visual area of owl monkeys: connections, myeloarchitecture, and homologies in other primates. J. Comp. Neurol. 1993;334:497–528. doi: 10.1002/cne.903340402. [DOI] [PubMed] [Google Scholar]
- Kuypers H.G, Szwarcbart M.K, Mishkin M, Rosvold H.E. Occipitotemporal corticocortical connections in the rhesus monkey. Exp. Neurol. 1965;11:245–262. doi: 10.1016/0014-4886(65)90016-6. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark W.E. Edinburgh University Press; Edinburgh: 1959. The antecedents of man: an introduction to the evolution of the primates. [Google Scholar]
- Levitt P. A monoclonal antibody to limbic system neurons. Science. 1984;223:299–301. doi: 10.1126/science.6199842. [DOI] [PubMed] [Google Scholar]
- Lewis J.W, Van Essen D.C. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto–occipital cortex. J. Comp. Neurol. 2000;428:79–111. doi: 10.1002/1096-9861(20001204)428:1<79::aid-cne7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lin C.S, Weller R.E, Kaas J.H. Cortical connections of striate cortex in the owl monkey. J. Comp. Neurol. 1982;211:165–176. doi: 10.1002/cne.902110206. [DOI] [PubMed] [Google Scholar]
- Luethke L.E, Krubitzer L.A, Kaas J.H. Connections of primary auditory cortex in the New World monkey, Saguinus. J. Comp. Neurol. 1989;285:487–513. doi: 10.1002/cne.902850406. [DOI] [PubMed] [Google Scholar]
- Lyon D.C, Kaas J.H. Connectional and architectonic evidence for dorsal and ventral V3, and dorsomedial area in marmoset monkeys. J. Neurosci. 2001;21:249–261. doi: 10.1523/JNEUROSCI.21-01-00249.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D.C, Kaas J.H. Evidence from V1 connections for both dorsal and ventral subdivisions of V3 in three species of New World monkeys. J. Comp. Neurol. 2002;449:281–297. doi: 10.1002/cne.10297. [DOI] [PubMed] [Google Scholar]
- Maguire W.M, Baizer J.S. Visuotopic organization of the prelunate gyrus in rhesus monkey. J. Neurosci. 1984;4:1690–1704. doi: 10.1523/JNEUROSCI.04-07-01690.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger P.R, Masiello I, Innocenti G.M. Areal organization of the posterior parietal cortex of the ferret (Mustela putorius) Cereb. Cortex. 2002;12:1280–1297. doi: 10.1093/cercor/12.12.1280. [DOI] [PubMed] [Google Scholar]
- Markstahler U, Bach M, Spatz W.B. Transient molecular visualization of ocular dominance columns (ODCs) in normal adult marmosets despite the desegregated termination of the retino–geniculo–cortical pathways. J. Comp. Neurol. 1998;393:118–134. [PubMed] [Google Scholar]
- Maxeiner S, Kruger O, Schilling K, Traub O, Urschel S, Willecke K. Spatiotemporal transcription of connexin45 during brain development results in neuronal expression in adult mice. Neuroscience. 2003;119:689–700. doi: 10.1016/s0306-4522(03)00077-0. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Adams R, Blakemore C. Mechanisms underlying the early establishment of thalamocortical connections in the rat. J. Neurosci. 1998;18:5723–5745. doi: 10.1523/JNEUROSCI.18-15-05723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro R.J, Yuste R. Gap junctions in developing neocortex: a review. Brain Res. Brain Res. Rev. In press doi: 10.1016/j.brainresrev.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Le W.R, Wakita M, Mikami A, Itoh K. Projections from the cytochrome oxidase modules of visual area V2 to the ventral posterior area in the macaque. Exp. Brain Res. 2004;155:102–110. doi: 10.1007/s00221-003-1698-8. [DOI] [PubMed] [Google Scholar]
- Newsome W.T, Maunsell J.H, Van Essen D.C. Ventral posterior visual area of the macaque: visual topography and areal boundaries. J. Comp. Neurol. 1986;252:139–153. doi: 10.1002/cne.902520202. [DOI] [PubMed] [Google Scholar]
- O'Brien B.J, Abel P.L, Olavarria J.F. The retinal input to calbindin-D28k-defined subdivisions in macaque inferior pulvinar. Neurosci. Lett. 2001;312:145–148. doi: 10.1016/s0304-3940(01)02220-0. [DOI] [PubMed] [Google Scholar]
- O'Leary D.D, Yates P.A, McLaughlin T. Molecular development of sensory maps: representing sights and smells in the brain. Cell. 1999;96:255–269. doi: 10.1016/s0092-8674(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Padberg J, Seltzer B, Cusick C.G. Architectonics and cortical connections of the upper bank of the superior temporal sulcus in the rhesus monkey: an analysis in the tangential plane. J. Comp. Neurol. 2003;467:418–434. doi: 10.1002/cne.10932. [DOI] [PubMed] [Google Scholar]
- Palmer L.A, Rosenquist A.C, Tusa R.J. The retinotopic organization of lateral suprasylvian visual areas in the cat. J. Comp. Neurol. 1978;177:237–256. doi: 10.1002/cne.901770205. [DOI] [PubMed] [Google Scholar]
- Pandya D.N, Yeterian E.H. Comparison of prefrontal architecture and connections. Phil. Trans. R. Soc. B. 1996;351:1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Paolini M, Sereno M.I. Direction selectivity in the middle lateral and lateral (ML and L) visual areas in the California ground squirrel. Cereb. Cortex. 1998;8:362–371. doi: 10.1093/cercor/8.4.362. [DOI] [PubMed] [Google Scholar]
- Payne B.R. Evidence for visual cortical area homologs in cat and macaque monkey. Cereb. Cortex. 1993;3:1–25. doi: 10.1093/cercor/3.1.1. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D.N. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur. J. Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya D.N. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- Pimenta A.F, Reinoso B.S, Levitt P. Expression of the mRNAs encoding the limbic system-associated membrane protein (LAMP). II. Fetal rat brain. J. Comp. Neurol. 1996;375:289–302. doi: 10.1002/(SICI)1096-9861(19961111)375:2<289::AID-CNE8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Piñon M.C.G.P, Gattass R, Sousa A.P.B. Area V4 in Cebus monkey: extent and visuotopic organization. Cereb. Cortex. 1998;8:685–701. doi: 10.1093/cercor/8.8.685. [DOI] [PubMed] [Google Scholar]
- Polleux F, Dehay C, Kennedy H. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J. Comp. Neurol. 1997;385:95–116. [PubMed] [Google Scholar]
- Preuss T.M. Specializations of the human visual system: the monkey model meets human reality. In: Kaas J.H, Collins C.E, editors. The primate visual system. CRC Press; Boca Raton: 2004. pp. 231–259. [Google Scholar]
- Preuss T.M, Goldman-Rakic P.S. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J. Comp. Neurol. 2001a;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Preuss T.M, Goldman-Rakic P.S. Architectonics of the parietal and temporal association cortex in the strepsirhine primate Galago compared to the anthropoid primate Macaca. J. Comp. Neurol. 2001b;310:475–506. doi: 10.1002/cne.903100403. [DOI] [PubMed] [Google Scholar]
- Preuss T.M, Qi H, Kaas J.H. Distinctive compartmental organization of human primary visual cortex. Proc. Natl Acad. Sci. USA. 1999;96:11 601–11 606. doi: 10.1073/pnas.96.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price D.J. Patterns of cytochrome oxidase activity in areas 17, 18 and 19 of the visual cortex of cats and kittens. Exp. Brain Res. 1985;58:125–133. doi: 10.1007/BF00238960. [DOI] [PubMed] [Google Scholar]
- Puce A, Perrett D. Electrophysiology and brain imaging of biological motion. Phil. Trans. R. Soc. B. 2003;358:435–445. doi: 10.1098/rstb.2002.1221. (doi: 10.1098/rstb.2002.1221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiguel S.E, Xiao D.K, Marcar V.L, Orban G.A. Response latency of macaque area MT/V5 neurons and its relationship to stimulus parameters. J. Neurophysiol. 1999;82:1944–1956. doi: 10.1152/jn.1999.82.4.1944. [DOI] [PubMed] [Google Scholar]
- Reale R.A, Imig T.J. Tonotopic organization in auditory cortex of the cat. J. Comp. Neurol. 1980;192:265–291. doi: 10.1002/cne.901920207. [DOI] [PubMed] [Google Scholar]
- Restrepo C.E, Manger P.R, Spenger C, Innocenti G.M. Immature cortex lesions alter retinotopic maps and interhemispheric connections. Ann. Neurol. 2003;54:51–65. doi: 10.1002/ana.10591. [DOI] [PubMed] [Google Scholar]
- Rodman H.R, Gross C.G, Albright T.D. Afferent basis of visual response properties in area MT of the macaque. I. Effects of striate cortex removal. J. Neurosci. 1989;9:2033–2050. doi: 10.1523/JNEUROSCI.09-06-02033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer A.S. 2nd edn. Saunders; Philadelphia: 1955. The vertebrate body. [Google Scholar]
- Rosa M.G.P. Visuotopic organization of primate extrastriate cortex. In: Rockland K.S, Kaas J.H, Peters A, editors. Cerebral Cortex, vol. 12: extrastriate cortex in primates. Plenum Press; New York: 1997. pp. 127–203. [Google Scholar]
- Rosa M.G.P. Topographic organisation of extrastriate areas in the flying fox: implications for the evolution of mammalian visual cortex. J. Comp. Neurol. 1999;411:503–523. doi: 10.1002/(sici)1096-9861(19990830)411:3<503::aid-cne12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P. Visual maps in the adult primate cerebral cortex: some implications for brain development and evolution. Braz. J. Med. Biol. Res. 2002;35:1485–1498. doi: 10.1590/s0100-879x2002001200008. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Elston G.N. Visuotopic organisation and neuronal response selectivity for direction of motion in visual areas of the caudal temporal lobe of the marmoset monkey (Callithrix jacchus): middle temporal area, middle temporal crescent, and surrounding cortex. J. Comp. Neurol. 1998;393:505–527. [PubMed] [Google Scholar]
- Rosa M.G.P, Krubitzer L.A. The evolution of visual cortex: where is V2? Trends Neurosci. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Schmid L.M. Visual areas in the dorsal and medial extrastriate cortices of the marmoset. J. Comp. Neurol. 1995;359:272–299. doi: 10.1002/cne.903590207. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Tweedale R. Visual areas in lateral and ventral extrastriate cortices of the marmoset monkey. J. Comp. Neurol. 2000;422:621–651. doi: 10.1002/1096-9861(20000710)422:4<621::aid-cne10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Tweedale R. The dorsomedial visual areas in New World and Old World monkeys: homology and function. Eur. J. Neurosci. 2001;13:421–427. doi: 10.1046/j.0953-816x.2000.01414.x. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Tweedale R. Maps of the visual field in the cerebral cortex of primates: functional organization and significance. In: Kaas J.H, Collins C.E, editors. The primate visual system. CRC Press; Boca Raton: 2004. pp. 261–288. [Google Scholar]
- Rosa M.G.P, Manger P.R. Clarifying homologies in the mammalian cerebral cortex: the case of the third visual area (V3) Clin. Exp. Pharmacol. Physiol. In press doi: 10.1111/j.1440-1681.2005.04192.x. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Gattass R, Fiorani Jr M. Complete pattern of ocular dominance stripes in V1 of a New World monkey, Cebus apella. Exp. Brain Res. 1988;72:645–648. doi: 10.1007/BF00250609. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Gattass R, Fiorani Jr M, Soares J.G.M. Laminar, columnar and topographic aspects of ocular dominance in the primary visual cortex of Cebus monkeys. Exp. Brain Res. 1992;88:249–264. doi: 10.1007/BF02259100. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Schmid L.M, Krubitzer L.A, Pettigrew J.D. Retinotopic organization of the primary visual cortex of flying foxes (Pteropus poliocephalus and Pteropus scapulatus) J. Comp. Neurol. 1993;335:55–72. doi: 10.1002/cne.903350105. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Soares J.G.M, Fiorani Jr M, Gattass R. Cortical afferents of visual area MT in the Cebus monkey: possible homologies between New and Old World monkeys. Vis. Neurosci. 1993;10:827–855. doi: 10.1017/s0952523800006064. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Casagrande V.A, Preuss T, Kaas J.H. Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti) J. Neurophysiol. 1997;77:3193–3217. doi: 10.1152/jn.1997.77.6.3193. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Fritsches K.A, Elston G.N. The second visual area in the marmoset monkey: visuotopic organisation, magnification factors, architectonical boundaries, and modularity. J. Comp. Neurol. 1997;387:547–567. doi: 10.1002/(sici)1096-9861(19971103)387:4<547::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Piñon M.C, Gattass R, Sousa A.P.B. ‘Third tier’ ventral extrastriate cortex in the New World monkey, Cebus apella. Exp. Brain Res. 2000;132:287–305. doi: 10.1007/s002210000344. [DOI] [PubMed] [Google Scholar]
- Rosa M.G.P, Tweedale R, Elston G.N. Visual responses of neurons in the middle temporal area of New World monkeys after lesions of striate cortex. J. Neurosci. 2000;20:5552–5563. doi: 10.1523/JNEUROSCI.20-14-05552.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M.G.P, Palmer S.M, Gamberini M, Tweedale R, Piñon M.C, Bourne J.A. Resolving the organization of the New World monkey third visual complex: The dorsal extrastriate cortex of the marmoset (Callithrix jacchus) J. Comp. Neurol. 2005;483:166–191. doi: 10.1002/cne.20412. [DOI] [PubMed] [Google Scholar]
- Sahraie A, Weiskrantz L, Barbur J.L, Simmons A, Williams S.C, Brammer M.J. Pattern of neuronal activity associated with conscious and unconscious processing of visual signals. Proc. Natl Acad. Sci. USA. 1997;94:9406–9411. doi: 10.1073/pnas.94.17.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky M.T, Wang Y, Hanes D.P, Thompson K.G, Leutgeb S, Schall J.D, Leventhal A.G. Signal timing across the macaque visual system. J. Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schrago C.G, Russo C.A. Timing the origin of New World monkeys. Mol. Biol. Evol. 2003;20:1620–1625. doi: 10.1093/molbev/msg172. [DOI] [PubMed] [Google Scholar]
- Schroeder C.E, Foxe J.J. The timing and laminar profile of converging inputs to multisensory areas of the macaque neocortex. Brain Res. Cogn. Brain Res. 2002;14:187–198. doi: 10.1016/s0926-6410(02)00073-3. [DOI] [PubMed] [Google Scholar]
- Sekiyama K, Kanno I, Miura S, Sugita Y. Auditory-visual speech perception examined by fMRI and PET. Neurosci. Res. 2003;47:277–287. doi: 10.1016/s0168-0102(03)00214-1. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya D.N. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya D.N. Intrinsic connections and architectonics of the superior temporal sulcus in the rhesus monkey. J. Comp. Neurol. 1989;290:451–471. doi: 10.1002/cne.902900402. [DOI] [PubMed] [Google Scholar]
- Sereno M.I. Brain mapping in animals and humans. Curr. Opin. Neurobiol. 1998;8:188–194. doi: 10.1016/s0959-4388(98)80139-6. [DOI] [PubMed] [Google Scholar]
- Sereno M.I, McDonald C.T, Allman J.M. Analysis of retinotopic maps in extrastriate cortex. Cereb. Cortex. 1994;4:601–620. doi: 10.1093/cercor/4.6.601. [DOI] [PubMed] [Google Scholar]
- Sereno M.I, Dale A.M, Reppas J.B, Kwong K.K, Belliveau J.W, Brady T.J, Rosen B.R, Tootell R.B.H. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Sherk H, Mulligan K.A. A reassessment of the lower visual field map in striate-recipient lateral suprasylvian cortex. Vis. Neurosci. 1993;10:131–158. doi: 10.1017/s0952523800003278. [DOI] [PubMed] [Google Scholar]
- Shipp S, Blanton M, Zeki S. A visuo-somatomotor pathway through superior parietal cortex in the macaque monkey: cortical connections of areas V6 and V6A. Eur. J. Neurosci. 1998;10:3171–3193. doi: 10.1046/j.1460-9568.1998.00327.x. [DOI] [PubMed] [Google Scholar]
- Sincich L.C, Adams D.L, Horton J.C. Complete flatmounting of the macaque cerebral cortex. Vis. Neurosci. 2003;20:663–686. doi: 10.1017/s0952523803206088. [DOI] [PubMed] [Google Scholar]
- Smart I.H, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley D.A, Rubin N. fMRI activation in response to illusory contours and salient regions in the human lateral occipital complex. Neuron. 2003;37:323–331. doi: 10.1016/s0896-6273(02)01148-0. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Kaas J.H. Topographic patterns of V2 cortical connections in macaque monkeys. J. Comp. Neurol. 1996;371:129–152. doi: 10.1002/(SICI)1096-9861(19960715)371:1<129::AID-CNE8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Striedter G.F. Stepping into the same river twice: homologues as recurring attractors in epigenetic landscapes. Brain Behav. Evol. 1998;52:218–231. doi: 10.1159/000006565. [DOI] [PubMed] [Google Scholar]
- Suzuki W.A, Amaral D.G. Perirhinal and parahippocampal cortices of the macaque monkey: cytoarchitectonic and chemoarchitectonic organization. J. Comp. Neurol. 2003;463:67–91. doi: 10.1002/cne.10744. [DOI] [PubMed] [Google Scholar]
- Suzuki W, Saleem K.S, Tanaka K. Divergent backward projections from the anterior part of the inferotemporal cortex (area TE) in the macaque. J. Comp. Neurol. 2000;422:206–228. doi: 10.1002/(sici)1096-9861(20000626)422:2<206::aid-cne5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Tamura H, Tanaka K. Visual response properties of cells in the ventral and dorsal parts of the macaque inferotemporal cortex. Cereb. Cortex. 2001;11:384–399. doi: 10.1093/cercor/11.5.384. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Ohzawa I, Ramoa A.S, Freeman R.D. Receptive field properties of cells in area 19 of the cat. Exp. Brain Res. 1987;65:549–558. doi: 10.1007/BF00235978. [DOI] [PubMed] [Google Scholar]
- Tootell R.B.H, Hadjikhani N. Where is ‘dorsal V4’ in human visual cortex? Retinotopic, topographic and functional evidence. Cereb. Cortex. 2001;11:298–311. doi: 10.1093/cercor/11.4.298. [DOI] [PubMed] [Google Scholar]
- Tootell R.B.H, Hamilton S.L, Silverman M.S. Topography of cytochrome oxidase activity in owl monkey cortex. J. Neurosci. 1985;5:2786–2800. doi: 10.1523/JNEUROSCI.05-10-02786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R.B.H, Mendola J.D, Hadjikhani N.K, Liu A.K, Dale A.M. The representation of the ipsilateral visual field in human cerebral cortex. Proc. Natl Acad. Sci. USA. 1998;95:818–824. doi: 10.1073/pnas.95.3.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell R.B.H, Tsao D, Vanduffel W. Neuroimaging weighs in: humans meet macaques in ‘primate’ visual cortex. J. Neurosci. 2003;23:3981–3989. doi: 10.1523/JNEUROSCI.23-10-03981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhaeghen P, Lu Q, Prakash N, Frisen J, Walsh C.A, Frostig R.D, Flanagan J.G. A mapping label required for normal scale of body representation in the cortex. Nature Neurosci. 2000;3:358–365. doi: 10.1038/73929. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C. Organization of visual areas in macaque and human cerebral cortex. In: Chalupa L, Werner J.S, editors. The visual neurosciences. MIT Press; Cambridge, MA: 2004. pp. 507–521. [Google Scholar]
- Van Essen D.C, Maunsell J.H. Two-dimensional maps of the cerebral cortex. J. Comp. Neurol. 1980;191:255–281. doi: 10.1002/cne.901910208. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C, Zeki S.M. The topographic organization of rhesus monkey prestriate cortex. J. Physiol. (Lond.) 1978;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen D.C, Newsome W.T, Maunsell J.H, Bixby J.L. The projections from striate cortex (V1) to areas V2 and V3 in the macaque monkey: asymmetries, areal boundaries, and patchy connections. J. Comp. Neurol. 1986;244:451–480. doi: 10.1002/cne.902440405. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C, Lewis J.W, Drury H.A, Hadjikhani N, Tootell R.B.H, Bakircioglu M, Miller M.I. Mapping visual cortex in monkeys and humans using surface-based atlases. Vis. Res. 2001;41:1359–1378. doi: 10.1016/s0042-6989(01)00045-1. [DOI] [PubMed] [Google Scholar]
- Vezoli J, Falchier A, Jouve B, Knoblauch K, Young M, Kennedy H. Quantitative analysis of connectivity in the visual cortex: extracting function from structure. Neuroscientist. 2004;10:476–482. doi: 10.1177/1073858404268478. [DOI] [PubMed] [Google Scholar]
- Vogt-Weisenhorn D.M, Illing R.B, Spatz W.B. Morphology and connections of neurons in area 17 projecting to the extrastriate areas MT and 19DM and to the superior colliculus in the monkey Callithrix jacchus. J. Comp. Neurol. 1995;362:233–255. doi: 10.1002/cne.903620207. [DOI] [PubMed] [Google Scholar]
- Von Bonin G. Walter Campbell (1868–1937) In: Haymaker W, Schiller F, editors. The founders of neurology. Thomas; Springfield: 1970. pp. 102–104. [Google Scholar]
- Von Bonin G, Bailey P. University of Illinois Press; Urbana: 1947. The neocortex of Macaca mulatta. [Google Scholar]
- Waleszczyk W.J, Wang C, Young J.M, Burke W, Calford M.B, Dreher B. Laminar differences in plasticity in area 17 following retinal lesions in kittens or adult cats. Eur. J. Neurosci. 2003;17:2351–2368. doi: 10.1046/j.1460-9568.2003.02674.x. [DOI] [PubMed] [Google Scholar]
- Watson J.D, Myers R, Frackowiak R.S, Hajnal J.V, Woods R.P, Mazziotta J.C, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb. Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Weller R.E, Steele G.E. Cortical connections of subdivisions of inferior temporal cortex in squirrel monkeys. J. Comp. Neurol. 1992;324:37–66. doi: 10.1002/cne.903240105. [DOI] [PubMed] [Google Scholar]
- Weller R.E, Steele G.E, Cusick C.G. Cortical connections of dorsal cortex rostral to V II in squirrel monkeys. J. Comp. Neurol. 1991;306:521–537. doi: 10.1002/cne.903060313. [DOI] [PubMed] [Google Scholar]
- Willshaw D.J, von der Malsburg C. How patterned neural connections can be set up by self-organization. Proc. R. Soc. B. 1976;194:431–445. doi: 10.1098/rspb.1976.0087. [DOI] [PubMed] [Google Scholar]
- Woo T.U, Pucak M.L, Kye C.H, Matus C.V, Lewis D.A. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- Xu L, Tanigawa H, Fujita I. Distribution of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate-type glutamate receptor subunits (GluR2/3) along the ventral visual pathway in the monkey. J. Comp. Neurol. 2003;456:396–407. doi: 10.1002/cne.10538. [DOI] [PubMed] [Google Scholar]
- Xu X, Collins C.E, Kaskan P.M, Khaytin I, Kaas J.H, Casagrande V.A. Optical imaging of visually evoked responses in prosimian primates reveals conserved features of the middle temporal visual area. Proc. Natl Acad. Sci. USA. 2004;101:2566–2571. doi: 10.1073/pnas.0308745101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youakim M, Bender D.B, Baizer J.S. Vertical meridian representation on the prelunate gyrus in area V4 of macaque. Brain Res. Bull. 2001;56:93–100. doi: 10.1016/s0361-9230(01)00608-6. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 1969;14:271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. The third visual complex of rhesus monkey prestriate cortex. J. Physiol. 1978;277:245–272. doi: 10.1113/jphysiol.1978.sp012271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. The distribution of wavelength and orientation selective cells in different areas of monkey visual cortex. Proc. R. Soc. B. 1983;217:449–470. doi: 10.1098/rspb.1983.0020. [DOI] [PubMed] [Google Scholar]
- Zeki S, ffytche D.H. The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain. 1998;121:25–45. doi: 10.1093/brain/121.1.25. [DOI] [PubMed] [Google Scholar]