Abstract
Previously unrecognized mRNAs originating from a dual promoter at the stat92E locus are described. One of these encodes a truncated protein, ΔNSTAT92E, that lacks the N-terminal 133 amino acids. Antibodies detect both the full-length and truncated molecules early in embryogenesis (1–5 h), and mRNA detection by specific RT-PCR reactions accords with the protein distribution. Given that the N termini of mammalian STATs are known to have positive functions in transcriptional activation, we explored the role of ΔNSTAT92E early in embryogenesis. By increasing the ΔNSTAT92E-to-STAT92E ratio in overexpression and RNAi experiments, we observe phenotypes compatible with suppression of wild-type STAT92E activity. We therefore conclude that the short form of STAT92E is a naturally occurring dominant-negative product that can be added to the growing list of negative regulators of STAT activity.
Keywords: Drosophila melanogaster, STAT92E, alternative promoters, differential splicing
In response to more than 40 different polypeptides, one or more of seven known mammalian STAT (signal transducers and activators of transcription) transcription factors becomes phosphorylated on tyrosine, dimerizes, and enters the nucleus (Darnell 1997; Stark et al. 1998; Levy and Darnell 2002). Transcriptional activation by the STATs affects growth, differentiation, and the immune response, as well as resistance to infection mediated by interferons (IFNs), through the study of which they were discovered (Darnell et al. 1994). How such a diversity of biological functions is achieved by a relatively small group of transcription factors is still unknown. Cooperation with other transcription factors (Look et al. 1995; Stocklin et al. 1996; Zhang et al. 1999; Levy and Darnell 2002) while bound at closely spaced sites on DNA in so-called enhanceosomes (Carey 1998) likely increases the transcriptional potential of activated STATs.
On the other hand, there are a series of proteins that have the capacity to act as inhibitors of STAT function and in so doing deliver a balanced amount of STAT-dependent transcriptional activation. The negative-acting proteins include cytoplasmic tyrosine phosphatases and proteins termed SOCS and CIS whose genes are induced by cytokines (Starr and Hilton 1999; Krebs and Hilton 2001). The induced SOCS and CIS proteins inhibit STAT tyrosine phosphorylation by binding to kinases or receptors, completing a negative feedback loop. In the nucleus there are tyrosine phosphatases (Haspel and Darnell 1999; ten Hoeve et al. 2002) and PIAS proteins (Shuai 2000) that negatively regulate already activated STATs by either removing the tyrosine phosphate or binding to phosphorylated STATs and blocking their DNA-binding.
An additional potential mechanism for regulating the transcriptional impact of seven chromosomal loci is the generation of multiple proteins from the differential processing of a primary transcript. In fact, the first described STAT family member, Stat1, is produced as a full-length protein (750 residues) called Stat1α and as a C-terminally truncated protein (712 residues) called Stat1β. The stat1β mRNA results from a differential poly(A) site choice, which dictates different 3′ splicing. The stat1α mRNA has a terminal exon (Schindler et al. 1992; Muller et al. 1993) encoding 38 amino acids that act as a transcriptional activation domain (TAD). Omission of these 38 amino acids in STAT1β cause it to fail in transcriptional activation. Shorter forms lacking the TAD of both Stat3 and Stat5 also exist (Schaefer et al. 1995, 1997; Wang et al. 1996). STAT1β, STAT3β, and STAT5β when overexpressed have a dominant-negative effect (Stark et al. 1998; Bromberg and Darnell 2000). Furthermore, mice lacking specifically STAT3β have inaccurate regulation of the acute-phase response in the liver (Yoo et al. 2002).
In Drosophila, one STAT gene locus, stat92E, has been identified from which two extremely similar alternative splicing products are known to arise; one, 761 amino acids in length, contains seven residues in the region upstream of the tyrosine phosphorylation site that the other lacks (Hou et al. 1996; Yan et al. 1996b). In our original description of the STAT92E protein, we observed, using Schneider cell extracts, not one but three inducible phosphotyrosine-containing DNA–protein complexes that cannot be accounted for by the seven-residue difference. The largest of these represented dimers of the full-length STAT92E protein. The nature of the two faster-migrating DNA–protein complexes was not clear. However, we suggested the possibility of a significantly shorter STAT molecule (∼70–80 kD) that could dimerize and bind DNA and also pair with the longer molecule to form a heterodimeric DNA-binding complex of intermediate mobility.
Here we describe mRNAs with different 5′ exons that arise from two different primary transcripts at the stat92E locus. When processed, these primary transcripts yield several different mRNAs, some of which encode a protein lacking the N-terminal STAT domain (amino acids 1–134). The existence of the shorter protein is shown with specific anti-sera. This shorter protein, when phosphorylated and bound to DNA, produces the faster-migrating DNA–protein complex that was observed earlier.
The N termini of mammalian STATs have several established roles in STAT transcriptional activation (Levy and Darnell 2002). Therefore, we tested the function of ΔNSTAT92E in two assays in early development. Overexpression of ΔNSTAT92E and specific suppression, by RNAi, of all stat92E mRNA or specifically mRNA encoding the full-length protein, both produce phenotypes compatible with a negative function for ΔNSTAT92E in development. Because of the variation of these isoforms during development, we propose that ΔNSTAT92E may act as a regulated damper on the transcriptional activator STAT92E.
Results
Western blotting and EMSA of S2 cell, embryo, and larvae nuclear extracts
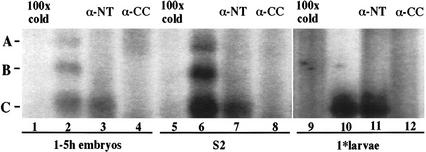
The existence of an alternative form of STAT92E was first suggested because nuclear extracts from vanadate-H2O2-treated Drosophila Schneider (S2) cells produced three DNA–protein complexes in electrophoretic mobility shift assays (EMSA; labeled A, B, and C, with A being the slowest-migrating band) using an optimized DNA-binding-site oligonucleotide probe (Yan et al. 1996b). The A and B complexes were increased upon transfection of the cells with a STAT92E-containing plasmid that incorporated a Flag epitope tag. Only complexes A and B were supershifted by the antibody to the epitope tag. These experiments established that the 86-kD protein encoded by the stat92E cDNA was responsible for complex A and possibly contributed, as a heterodimer with another STAT protein, to complex B. The nature of the C complex became the target of this study. (As is discussed below, the finding of three bands, A, B, and C, is repeated in Fig. 2, below, where Schneider cell extracts from nontransfected cells were used.)
Figure 2.
STAT92E-specific DNA–protein complexes in EMSA of extracts using the optimal STAT92E binding site oligonucleotide. Nuclear extract from 1–5-h embryos (lanes 1–4) and from S2 cells (lanes 5–8) and whole-cell extracts of 24–36-h first-instar larvae (lanes 9–12) were used. The extracts were incubated with either 100× cold oligonucleotide or the α-NT or α-CC antibodies. In 1–5-h embryos and S2 cells, the full-length and the N-terminally truncated forms of STAT92E are activated, bind DNA, and form the homo- and heterodimeric complexes A, B, C described in the text (lanes 2,6). In first-instar larvae only the short form is observed as complex C (lane 10). The antiserum targeting the N terminus supershifts complexes A and B but not C (lanes 3,7,11), whereas the antiserum targeting the coiled-coil region supershifts all three complexes (lanes 4,8,12).
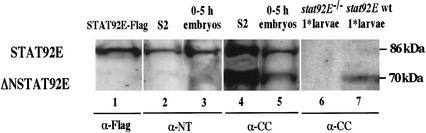
Antibodies were produced to several domains of the STAT92E protein, two of which were used in the Western blots in Figure 1. The antibody to the N terminus (residues 1–134, denoted α-NT) reacted with a protein the same size as the Flag-tagged STAT92E construct prepared in the original experiments. The antibody (labeled α-CC) against residues 135–338, the region of the STAT92E protein expected to lie in the coiled-coil domain of a generalized STAT structure (Becker et al. 1998; Chen et al. 1998), reacted with both the full-length 86-kD molecule and also with a shorter ∼70-kD molecule. Both the 86-kD and 70-kD molecules were present in S2 cell extracts and in extracts from 0–5-h embryos. Both proteins emanate from transcription of the stat92E gene, because neither protein was observed in first-instar larvae homozygous for the stat92E null allele, stat92E06346. This allele lacks stat92E mRNA because of a P-element insertion upstream of the gene (Hou et al. 1996). This result was made possible by creating the fly stock, stat92E06346/TM3, Act–GFP (for parental stocks and crosses, see Materials and Methods section) so that homozygous null larvae with the lowest maternal STAT92E levels could be selected late in development but before they die. The α-CC antibody only reacted with extract from wild-type or heterozygous larvae but not with extract prepared from homozygous stat92E−/− null first-instar larvae (Fig. 1, cf. lanes 6 and 7). The 70-kD band was much more prominent than the 86-kD band at first instar. We concluded that the 70-kD form was most likely an N-terminal truncation of STAT92E (ΔNSTAT92E) and not another STAT family member.
Figure 1.
Western blot of nuclear extracts from S2 cells, 1–5-h embryos, and first-instar larvae. The anti-serum used is indicated under each panel. S2 cells and 1–5-h embryos express both full-length and an N-terminally truncated form of STAT92E (lanes 2–5). In first-instar (1*) larvae that are stat92E−/− null mutants, no STAT92E is detected as compared with wild-type larvae, showing that the faster-migrating band is not caused by cross-reaction with a protein other than STAT92E (lane 6). Wild-type first-instar larvae predominantly express the N-terminally truncated form of STAT92E (lane 7). Flag-tagged recombinant STAT92E was transfected into S2 cells, and the extract was run simultaneously but blotted with Flag antibody separately, to indicate the position of full-length STAT92E (lane 1).
To correlate the existence of a shorter N-terminally truncated protein with the DNA-binding behavior observed earlier, gel shift assays were used. S2 cells were grown and treated with vanadate-peroxide for 15 min, which is known to cause accumulation of tyrosine-phosphorylated STAT92E molecules (Yan et al. 1996b). Extracts were then prepared from these cells, from 1–5-h embryos, and from first-instar larvae. The α-NT serum, the α-CC serum, or 100× cold oligonucleotide was added to test the reactivity of the various DNA:protein complexes (Fig. 2). In the S2 and embryo extracts, complexes A, B, and C were present (Fig. 2, lanes 2,6) and could be competed with 100× cold oligonucleotide (Fig. 2, lanes 1,5), indicating specificity for the STAT92E DNA-binding site. All three complexes could be supershifted with the α-CC serum (Fig. 2, lanes 4,8), whereas the α-NT serum supershifted complexes A and B but not C (Fig. 2, lanes 3,7). In extracts from first-instar larvae, only the C band could be detected, in agreement with the antibody identification of an N-terminal truncation of STAT92E (cf. Fig. 1, lane 7, and Fig. 2, lane 10). The α-CC serum supershifted this complex (Fig. 2, cf. lanes 11 and 12). Thus, the EMSA C complex appears to be a homodimer of the N-terminally truncated form of STAT92E, and the A complex a homodimer of full-length STAT92E, with the B complex representing a heterodimer of these two proteins.
BLAST sequence similarity searches identify STAT92E short-form clones
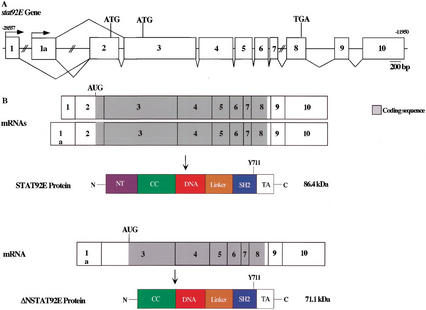
We next sought to establish the existence of sequences in the Drosophila genome that would give rise to the shorter form and to compare them to the pattern of exons for the full-length protein. The Drosophila EST database contained potential cDNA sequences (Rubin et al. 2000) that encoded a STAT92E protein lacking N-terminal residues. Using the sequence of the 5′-UTR and the nucleotides encoding the first 150 amino acids as input data, three cDNA clones possessing a sequence not in the original stat92E cDNA were identified (Table 1). This new sequence, which we called Exon 1a (for 1 alternate), was used subsequently as input data, and several more clones containing this sequence were defined. In clones LP02469 and LD47212, Exon 1a replaces Exons 1 and 2, and the mRNA sequence predicts a protein lacking the N-terminal domain with a molecular mass of 71 kD, translating from the first AUG with a Kozak sequence (Kozak 1991). In the other clones, Exon 1a replaces Exon 1 but retains Exon 2, and a full-length STAT92E protein is predicted from the longest open reading frame. LP02469 and LD47212 were sequenced, and it was determined that the former contained stat92E cDNA complete to the C terminus, including the seven-residue insert EPEPLVL at amino acid 698 (full-length STAT92E numbering).
Table 1.
BLAST sequence similarity searches
| Accession number
|
Clone ID
|
Library
|
EST exon structure
|
Input data
|
|---|---|---|---|---|
| AA948793 | LD27431 | Embryo | 1a/2 | 5′-UTR |
| AA439338 | LD13879 | Embryo | 1a/2 | 5′-UTR |
| AI259128 | LP02469 | Larval–early pupal | 1a/3 | aa 1–150 |
| AA816374 | LD01314 | Embryo | 1a/2 | Exon 1a |
| AI258551 | LP01773 | Larval–early pupal | 1a/unknown | Exon 1a |
| AI515398 | LD47212 | Embryo | 1a/3 | Exon 1a |
| AI514989 | LD46642 | Embryo | 1a/2 | Exon 1a |
| AI542017 | SD08040 | Schneider L2 cells | 1a/2 | Exon 1a |
Drosophila EST clones possessing novel sequence were identified in a series of search routines of the dbEST using the 5′-untranslated region (UTR) of stat92E, the sequence encoding amino acids (aa) 1–150 of STAT92E, and the novel sequence termed Exon 1a, as the input data.
The genomic location of Exons 1 and 1a relative to each other and to Exon 2 was revealed in a search of the Drosophila genomic sequence. The only matching STAT sequences were in a region embracing stat92E (GenBank accession no. AE003731), and the order, 5′ to 3′, of Exon 1 > Exon 1a > Exon 2 was established.
Expression of ΔNSTAT92E in cultured S2 cells
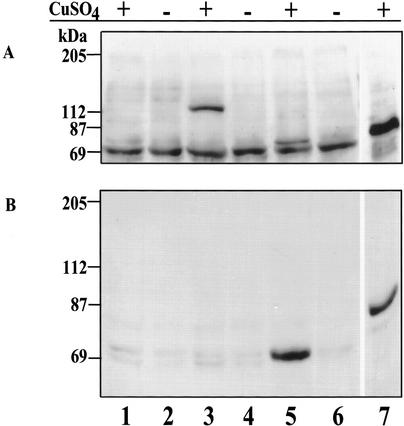
To show that the ΔNSTAT92E protein predicted by the cDNA of clone LP02469 can, indeed, be expressed from this cDNA, we transferred the entire clone to an S2 cell expression vector (pMT/V5-His C) that adds a His tag and is inducible by CuSO4. After transient transfection and induction, protein extracts were prepared and subjected to SDS-PAGE, followed by Western blotting with anti-His antibody. A CuSO4-inducible and His-tagged protein of molecular mass 73 kD was observed (Fig. 3A, lane 5; the V5-His tag adds 2.6 kD). The same extracts also reacted in a Western blot using α-CC serum (Fig. 3B). The inducible 73-kD protein reacted with the α-CC antibody, confirming that the ΔNSTAT92E protein is expressed stably in S2 cells from the cloned cDNA LP02469, where Exon 1a is joined to Exon 3, replacing Exons 1 and 2.
Figure 3.
Expression of ΔNSTAT92E from clone LP02469. (A) The Western blot was probed with anti-His antibody. Cells were induced or uninduced (+ or −) with copper sulfate. Untransfected S2 cells (lanes 1,2); S2 cells transfected with pMT/V5-His/lacZ (lanes 3,4); S2 cells transfected with pMT/V5-His/ΔNSTAT92E (lanes 5,6); and S2 cells transfected with pMT/V5-His/STAT92E-TAD (lane 7). (B) The blot was probed with α-CC serum. The lanes are the same as in A. Tagged ΔNSTAT92E, 73 kD; tagged β-galactosidase, 119 kD; tagged STAT92E-TAD, 82 kD.
Alternative promoter use and differential splicing generate several stat92E transcripts
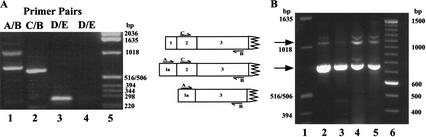
To determine independently that we could recover previously registered mRNAs and to support the antibody findings of Figures 1 and 2, RT-PCR was performed using poly(A)+ RNA from embryos, larvae, or adult flies as well as total RNA from S2 cells. Primers were designed to anneal to Exon 1a (Primer A), Exon 2 (Primer C), and Exon 3 (Primer B). The presence of the ΔNstat92E mRNA and of an mRNA encoding the 86-kD protein both originating from the second promoter between Exons 1 and 2 was indicated by the amplification of two products with the primer pair A/B, with sizes of 1047 bp and 734 bp (Fig. 4A, lane 1). The primer pair C/B was used as the positive control, and one product of 650 bp was observed as expected. (Only the gel containing embryo RNA reactions is shown, although identical results were observed for the other mRNA samples.) In Figure 4A, lanes 3 and 4 contain the +RT and −RT reactions using primers for Drosophila GAPDH, showing that the mRNA was not contaminated with genomic DNA. The RT-PCR products were further amplified in a standard PCR reaction (Fig. 4B) to generate sufficient material for DNA sequencing, which confirmed that in the 734-bp product Exon 1a is spliced to Exon 3, and in the 1047-bp product Exon 1a is spliced to Exon 2.
Figure 4.
RT-PCR of S2 cell, Drosophila embryo, larva, and adult RNA. (A) 1.5% agarose/TBE gel of RT-PCR reactions for embryo RNA. The primer locations are indicated. Primers D and E are to the Drosophila GAPDH sequence; (lane 4) the cDNA− reaction; (lane 5) 1-kb DNA ladder. Similar results were seen for larvae, adult fly, and S2 cell RNA. (B) Shows 1.5% agarose/TBE gel of PCR reactions performed on the RT-PCR reactions of primer pair A/B; (lane 1) 1-kb DNA ladder; (lane 2) S2 cell; (lane 3) larva; (lane 4) embryo; (lane 5) adult; (lane 6) 100-bp DNA ladder. The arrows indicate the bands that were gel-purified and directly sequenced.
Because mRNAs containing Exons 1 then 2, 1a then 2, and 1a then 3 had been identified, it seemed that two overlapping transcription units (beginning with either Exon 1 or 1a) existed in the stat92E locus. Therefore, we determined the start sites of the RNA transcripts using 5′-RACE (rapid amplification of cDNA ends) assays and DNA sequencing of several of the cDNA clones. In this manner we identified two different initiator sites (Fig. 5), proving the alternative use of two promoters. The first promoter possesses a potential TATA-box sequence at position −26. Although promoter 1a does not have a TATA-box sequence, its initiator site is followed by a downstream promoter element (DPE) at +28 (Burke and Kadonaga 1996, 1997). Promoter 1a has a C at +14 and a G at position +24, which increase DPE promoter activity (Kutach and Kadonaga 2000). Downstream promoter elements are found in ∼30%–40% of Drosophila promoters (Kutach and Kadonaga 2000) and are postulated to be functional counterparts to the TATA box.
Figure 5.
Two stat92E Promoters. (A) The transcription start sites are labeled +1 (>60% of the RACE-PCR clones started at these sites). Asterisks indicate the 5′- ends of other clones. The two highlighted exons are separated by 975 bp in the genomic sequence. A potential TATA box, an initiator, and a downstream promoter element are underlined. (B) Diagram showing the proper spacing between these elements and their Drosophila consensus sequences.
Therefore, alternative promoter usage defines the 5′-end of two mRNAs that encode 86-kD protein STAT92E and allows for differential splicing from transcripts originating from promoter 1A to generate the shorter form, ΔNSTAT92E (summarized in Fig. 6). We could find no evidence, by either RACE-PCR or by searching the EST database, of cDNAs wherein Exon 1 is alternatively spliced to Exon 3. Transcription from the two different promoters, 1 and 1a, may well be regulated differently based on the differences in their sequences.
Figure 6.
Gene, mRNA transcript, and protein structures of STAT92E. (A) Diagram of the exon structure of the stat92E gene. Exon 1a is 975 bp downstream of Exon 1 and 8.6 kb upstream of Exon 2 in the Drosophila genome (GenBank accession no. AE003731). Exons 7 and 8 are 1.8 kb apart. When aligned with the stat92E cDNA, the last exon identified by Adams et al. (2000) is split into two exons (Exons 9 and 10 here). (B) Three mRNA transcripts identified for stat92E. The first is the original stat92E message, which is transcribed starting at the upstream promoter, where the start codon AUG appears in the last third of Exon 2 and a full-length protein of 86 kD is translated. The seven-residue insert after residue 698 occurs via a differential splice between Exons 6 and 7 (Michael Melnick, pers. comm.). These two mRNAs are not distinguished here. The second message is the alternative start-site transcript, where Exon 1a precedes Exon 2 and the same start codon is used. The third is a splicing variant of this transcript, where Exon 1a is spliced to Exon 3 and a second start codon is used, truncating the N-terminal domain. The domain structure predicted for STAT92E is by analogy to the X-ray crystal structures of mammalian Stat1 and Stat3 (Becker et al. 1998; Chen et al. 1998).
Distribution of stat92E mRNAs during development
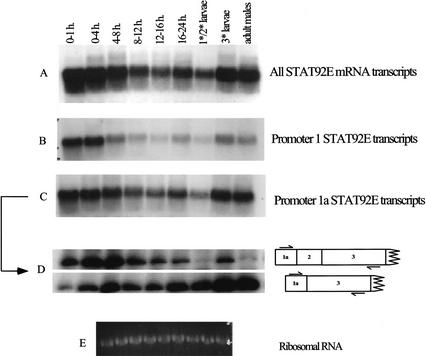
To determine whether the steady-state concentration of mRNAs transcribed from the two stat92E promoters changes throughout development, we first performed Northern blots on RNA extracted from different stages (Fig. 7). A probe (Exons 3 and 4) that reacted with all mRNAs (Fig. 7A) showed a large amount of maternal stat92E mRNA (Fig. 7A, 0–1-h lane) and decreasing amounts of mRNA later in embryogenesis, with stronger expression returning in third-instar larvae and adults. This same picture was mirrored in the mRNA that arises from promoter 1a (Fig. 7C). The later embryonic increase was not as prominent in mRNA arising from promoter 1 (Fig. 7B). Quantitative RT-PCR (Fig. 7D) to distinguish promoter 1a mRNAs encoding full-length or shorter protein revealed that the ΔNstat92E mRNA was largely responsible for the strong signal in larval and adult samples. These results are consistent with the protein expression shown in Figure 1, where ΔNSTAT92E predominates in first-instar larvae. Furthermore, the existence is indicated of a regulated alternative splicing mechanism for transcripts arising from promoter 1a, opening the possibility for different functions for the full-length STAT92E protein compared with the shorter ΔNSTAT92E protein.
Figure 7.
Staged Northern blot and RT-PCR. Total RNA was collected at various times during embryogenesis, from first- and second-instar larvae together, from third-instar larvae, and from adult males. (A) Probe specific for Exons 3 and 4. (B) Probe specific for Exon 1. (C) Probe specific for Exon 1a. (D) Quantitative RT-PCR to amplify the alternatively spliced transcripts from promoter 1a. Primer locations are indicated. (E) Ethidium-bromide-stained ribosomal RNA indicates loading of total RNA in each lane.
Expression of ΔNSTAT92E results in suppression of STAT signaling
We next tested the developmental effects of changing the ratio of STAT92E to ΔNSTAT92E in embryogenesis by overexpressing ΔNSTAT92E. The pair-rule gene even-skipped (eve) is expressed in seven stripes along the anterior/posterior axis of the 2–3-hour-old embryo. The expression pattern is under the control of modular enhancers that integrate spatial information of localized transcription factors to produce the seven-stripe pattern (Small et al. 1996). For instance, in loss-of-function (LOF) mutants of stat92E and hopscotch (the Drosophila JAK), eve stripes, most commonly stripes 3, 5, and 7, are variably reduced, whereas stripes 1 and 2 are usually unaffected (Binari and Perrimon 1994; Hou et al. 1996; Yan et al. 1996b). That activation of stripe 3 and 7 is the direct consequence of STAT92E binding to two neighboring STAT DNA-binding sites contained in the eve stripe 3 + 7 enhancer (3.5 kb upstream of the transcription start site) was shown by point mutagenesis and reporter gene assays in embryos (Yan et al. 1996b). Therefore, if ΔNSTAT92E had a dominant-negative function, overexpression would be expected to suppress production of EVE protein at STAT92E-dependent stripes but not at other eve stripes.
We used the binary UAS–Gal4 expression system for conditional overexpression of ΔNSTAT92E early in embryonal development (Brand et al. 1994). This system takes advantage of the ability of the yeast transcriptional activator GAL4 to function in Drosophila cells (Fischer et al. 1988). Drosophila strains that express GAL4 specifically at one stage of development or in a specific cell type can be mated to strains carrying a GAL4-responsive transgene that expresses any gene of interest. We chose NGT–Gal4 (nanos–GAL4–tubulin), which has been used to express GAL4 early in development during stripe formation of other pair-rule genes (Tracey et al. 2000). We assayed EVE expression with an anti-EVE antibody in stocks carrying both a Gal4-responsive UAS–ΔNstat92E transgene and NGT–Gal4 (Fig. 8). For comparison, we included a stat92E397 embryo (Fig. 8C) defective in STAT function because of a truncation at the SH2 domain (Silver and Montell 2001). We found that EVE production of stripes 3, 5, and 7 was variably reduced in 20%–40% of the embryos overexpressing ΔNSTAT92E (Fig. 8B). Control embryos with only NGT–GAL4 (Fig. 8A) or embryos overexpressing STAT92E (data not shown) displayed a wild-type seven-stripe pattern. In addition, as was previously shown for other STAT pathway mutants (Yan et al. 1996b), the stat92E397 embryos show reduction of stripe 3 and poor spreading so that stripe count was not possible. Taken together, these experiments suggest that ΔNSTAT92E can suppress transcriptional activation by STAT92E in vivo.
Figure 8.
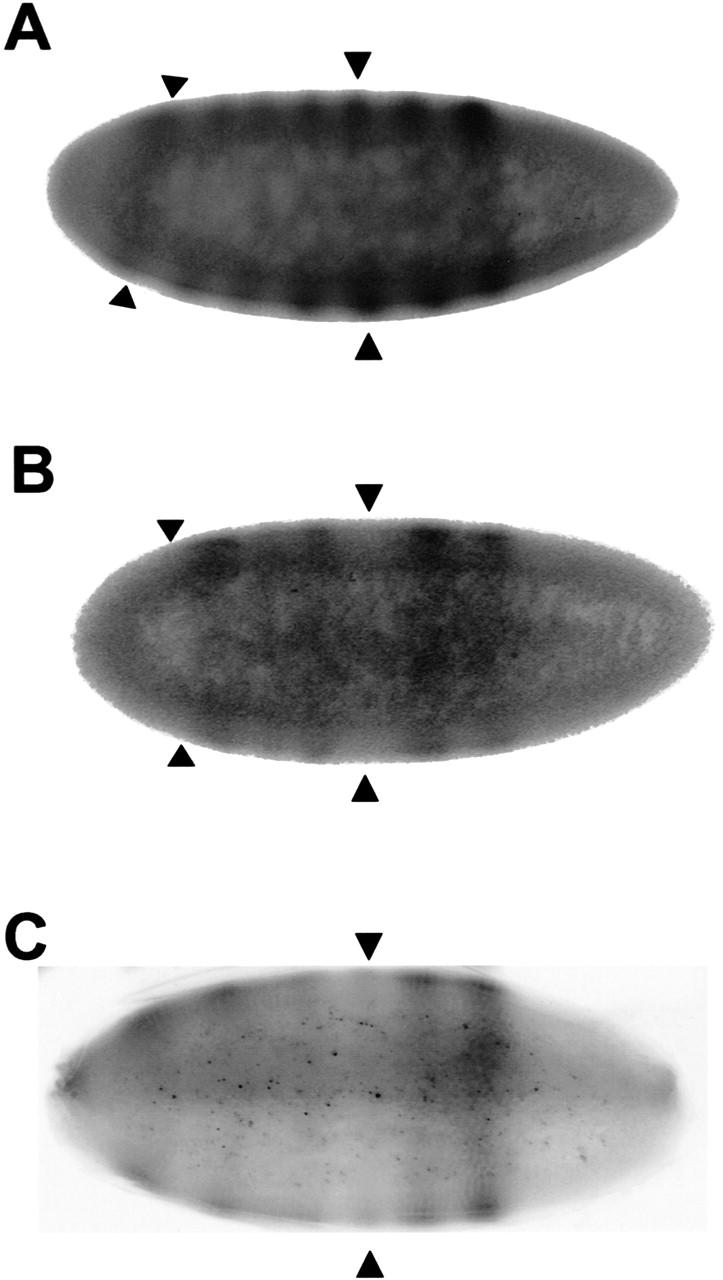
Overexpression of ΔNSTAT92E in 1–5-h old embryos suppresses activation of the STAT92E-dependent eve stripes 3, 5, and 7. (A) Seven eve stripes can be observed in NGT-Gal4 embryos representing the wild-type pattern. (B) In UAS–ΔNstat92E; NGT–Gal4 embryos, stripes 3 and 7 are clearly suppressed and stripe 5 to some degree. The position of the missing stripes 3 and 7 is marked by arrowheads. (C) stat92E397 germ-line clone (Silver and Montell 2001) with a strongly suppressed stripe 3 and poor spreading of other stripes. The anterior is to the right.
RNA interference
To remove specific forms of STAT92E for comparison with the overexpression results, RNAi experiments (Kennerdell and Carthew 1998; Clemens et al. 2000) involving dsSTAT-encoding sequences were performed. Prior to injecting dsRNAs (double-strand RNAs) corresponding to Exon 2 or Exons 3 and 4 into embryos, their efficacy in decreasing various stat92E mRNAs was characterized by RT-PCR in S2 cells in culture. dsRNA corresponding to Exons 3 and 4 reduced all stat92E mRNA and proteins, whereas dsRNA corresponding to Exon 2 specifically reduced the ΔNstat92E mRNA and protein with equal efficacy (Supplemental Fig. 1, in Supplementary Material at http://www.genesdev.org). Unfortunately, there is no exon combination that would leave stat92E mRNA unaffected and remove only the ΔNstat92E mRNA.
The JAK-STAT pathway not only affects stripe formation but also embryonic segmentation (Luo and Dearholf 2001), and defects in segmentation were used to score the effect of the injection of dsRNAs. Loss of maternal hopscotch, domeless (the pathway receptor), or stat92E activity often results in the deletion of the fourth and fifth abdominal segments, observed in cuticle preparations. Null embryos lacking both maternal and paternal contributions from these genes can have additional defects in the head and tail region (Hou et al. 1996; Yan et al. 1996b).
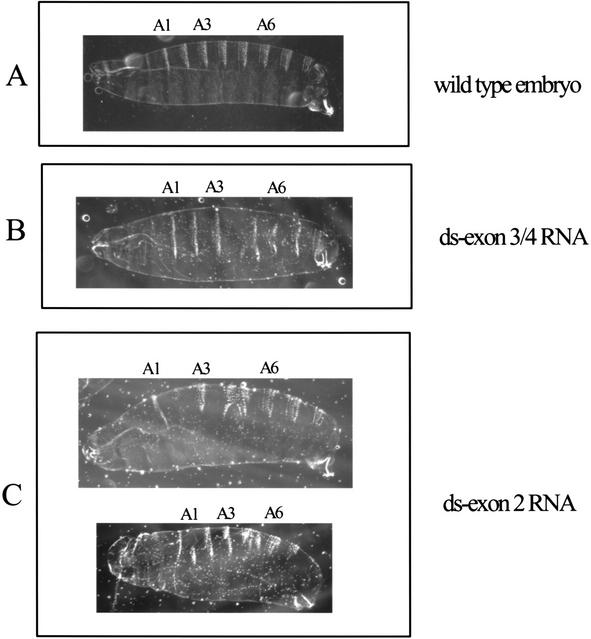
When embryos (0–1 h) were injected with ds-Exon3/4 RNA (Fig. 9B), which should lower the concentration of all possible stat92E mRNA, 7 of 103 surviving larvae had a loss and/or fusion of abdominal segments four and five. No defects in head or tail segments were seen. (Injection of buffer alone caused no defects in 105 animals.) Other embryos were injected with ds-Exon2 RNA (Fig. 9C), which would not affect the amount of ΔNstat92E mRNA but would decrease all the mRNA encoding full-length protein regardless of which promoter was used. In the surviving larvae, 16 of 153 had fused segments four and five, and defects in the head and tail were also observed in another 12 of the 153. Upon translation of the maternally deposited mRNA (plus any new mRNA synthesized very early in embryogenesis), these latter injected embryos would have a higher ratio of ΔNSTAT92E protein compared with full-length protein. That the more severe defective phenotype is seen in embryos where the ratio of ΔNSTAT92E-protein-to-full-length protein was increased shows that ΔNSTAT92E acts negatively. In contrast, when all the stat92E mRNAs are depleted, only the less severe defect is seen.
Figure 9.
RNA interference of stat92E activity in embryos. Cuticle preparations of embryos. (A) Wild-type embryo (buffer injection; 105 of 105), with abdominal segments 1, 3, and 6 labeled. (B) Injection of 5 μM ds-Exon3/4 RNA. The abdominal segments 4 and 5 are missing or fused (7 of 103). This phenotype is similar to paternally rescued hopscotch, stat92E, and domeless null embryos. (C) Injection of 5 μM ds-Exon2 RNA. Some embryos had the phenotype involving the abdominal segments 4 and 5 (16 of 153), whereas some had additional more severe segmentation defects involving the head and tail region (12 of 153). This phenotype is similar to parternally unrescued hopscotch, stat92E, and domeless null embryos. The images were scaled identically. Anterior is to the left.
Discussion
There are only seven STATs in mammals, whereas the proteins are activated by a very wide array of extracellular signaling proteins affecting events in a wide variety of cells. Even in Drosophila, where there is a single STAT gene, the pathway affects many different developmental events. This situation repeatedly raises the question of how specificity of cell responses is brought about.
The findings in the present work bear directly on the question of how a single gene or a limited set of genes affects a large number of pathways. The differential activity of negative-acting proteins (SOCS, PIAS, phosphatases) can obviously affect the duration and strength of a STAT gene activation. To this list of negative-acting proteins we can now add, at least in Drosophila, omission of the N-terminal STAT domain. Furthermore, differential appearance of functional forms of STAT92E in different cell types during development is possible with ΔNSTAT92E acting as a negative regulator of the full-length protein. This shorter protein could, as is the case in mammals, fail to activate genes as well as wild-type protein by failing to attract coactivators, for example, CBP/p300 (Zhang et al. 1996), or by failing to form tetramers when two STAT binding sites for dimers are close together (Xu et al. 1996; Vinkemeier et al. 1998). It is interaction between the N-terminal domains that allows tetramer formation, which is required in the activation of certain genes (Guyer et al. 1995; John et al. 1999).
The regulation of transcription from the two different promoters is obviously a possibility for controlling the effects of the full-length STAT protein. Use of only promoter 1 would produce only full-length protein. Transcription from promoter 1a would produce both full-length STAT92E and ΔNSTAT92E protein. Furthermore, in processing primary transcripts from promoter 1a, a choice of splice sites to include or exclude Exon 2 can occur. These choices are used, because the amount of stat92E and ΔNstat92E mRNA changes throughout development.
Overlapping transcription units were first discovered in the rat α-amylase gene, Amy-1a, where two different start sites produced liver or salivary gland mRNAs at vastly different rates but encoding the same protein (Schibler et al. 1983). Tissue-specific variation in start sites with resulting quantitative differences in protein formation has been described many times since, but in the case we describe here the utilization of the Exon 1a start site could have the effect of negatively regulating any protein arising from transcription using promoter 1, a situation most analogous to that described for the human CCAAT/enhancer-binding protein ɛ (C/EBPɛ) gene (Yamanaka et al. 1997). Alternative use of two promoters combined with differential splicing yields four C/EBPɛ mRNA isoforms, which in turn generate three proteins that differ in their N termini, with different transactivating properties.
Finally, the present findings in Drosophila suggest the possibility in mammalian cells of tissue-specific transcription initiation sites and variations in N-terminal protein sequences of the STATs. It seems unlikely that such potentially valuable variations in gene regulation would be discarded in evolution.
Materials and methods
Cell culture
Schneider 2 (S2) cells were maintained in Shields and Sang M3 Insect Media (Sigma) supplemented with 10% heat-inactivated fetal bovine serum (Gemini Bio-Products) at 25°C.
Plasmids
Drosophila cDNA clones from the Berkeley Drosophila Genome Project were purchased from Research Genetics. DNA sequencing was performed at the Rockefeller University Protein DNA Technology Center. The cDNA from clone LP02469 was amplified by PCR using Vent polymerase (New England BioLabs) with primers annealing to the multiple cloning site of the pOT2a vector just upstream of the EcoRI site and to the C terminus of STAT92E, but with a stop-codon mutation to allow for histidine tagging. The PCR product was subcloned into the NotI site of pMT/V5-His C (Invitrogen). The insert of the LP02469 pMT/V5-His C plasmid was sequenced entirely, and a G → T point mutation was discovered that resulted in an Ochre termination signal. The same mutation was found in the original LP02469 cDNA clone. The point mutation was repaired using the QuikChange Mutagenesis Kit (Stratagene). The control expression vector pMT/V5-His/lacZ was from Invitrogen, and pMT/V5-His/STAT92E-TAD was prepared from the original stat92E cDNA by PCR-based subcloning into the EcoRI and XbaI sites of pMT/V5-His A such that the putative transactivation domain was removed (residues 694–754).
STAT92E antibody production
Two PCR fragments were generated corresponding to amino acids 1–134 and amino acids 135–338, the putative N-terminal and coiled-coil domains of STAT92E, with the forward primer containing an EcoRI site and the reverse primer an NotI site. Both fragments were cloned into the pGEX vector (Stratagene) to create glutathione S-transferase (GST) fusion proteins. The resulting clones were sequenced, and protein was expressed according to the manufacturer's protocol. The protein was purified on glutathione-coupled beads and injected into rats nine times at 4-wk intervals (Covance). In the first four injections, 100 μg of protein was injected, and in the later injections 50 μg per injection was used. The sera from the third bleed onward were tested on Western blots and in EMSA supershifts.
Western blots
Extracts were prepared from S2 cells, embryos, or larvae according to Betz et al. (2001). The Flag-tagged STAT92E construct and antibody used are described in Yan et al. (1996b). Protein concentrations were measured using the Bio-Rad protein assay, and 15 μg of total protein per lane was subjected to SDS-PAGE and immunoblotted using standard methods. Anti-STAT92E amino acids 1–134 of the N-terminal domain (α-NT) and anti-STAT92E amino acids 135–338 of the coiled-coil domain (α-CC) were used at a 1:10,000 and 1:20,000 dilution, respectively, in 5% non-fat milk dissolved in Tris-buffered saline containing Tween-20 (TBST). An anti-rat horseradish peroxidase (HRP) antibody (Jackson ImmunoResearch Laboratory) was used as the secondary antibody at the recommended concentration. The blots were developed with Renaissance chemiluminescence reagent (NEN).
EMSA
Electrophoretic mobility shift assays (EMSAs) were performed as previously described (Vinkemeier et al. 1996). The protocol was adapted for Drosophila embryos and larvae, and the signal-to-noise ratio was improved by the following modifications: staged Drosophila embryos were grown and collected from apple juice agar plates (20% agar, 3.3% sucrose, 25% apple juice), dechorionated for 2 min in 50% bleach, washed with PBS, and homogenized in low salt extraction buffer (Yan et al. 1996b) in 1.75-mL Eppendorf tubes using a minipestle (Kontes). After centrifugation the pellet was resuspended in high-salt nuclear extraction buffer and incubated on ice for 30 min. The samples were microfuged for 5 min, and 2 μL of the supernatant was used in the EMSA. The probe was described in Yan et al. (1996b). Alternatively, the nuclear extract of 1–5-h embryos and larvae was concentrated ∼10-fold in spin columns (Microcon 3000) before being applied to the EMSAs.
Fly stocks and crosses
To select stat92E06346 homozygous first-instar larvae, a stock was created that allowed for selection by the absence of the larval marker, actin green fluorescence protein (Act-GFP) located on the TM3 balancer chromosome. To this end, Sb/TM3, Act-GFP (B#4534) was crossed to stat92E06346/TM3 (B#1681), a lethal P-element insertion line. stat92E06346/TM3, Act-GFP larvae were selected, the F1 generation was selfed, and a stock was established. stat92E06346 homozygous larvae were identified by selecting colorless larvae under a dissecting microscope equipped with a fluorescent light source. Larvae of this stock were grown on apple juice/agar plates, and 24–36-h first-instar larvae were collected according to the absence of GFP using a Leica dissecting microscope with a green light source. Wild-type (Oregon R) flies were grown and selected in parallel. All stocks were from Bloomington, Indiana. The NGT line was kindly provided by J. Peter Gergen (State University of New York at Stony Brook, Stony Brook, NY). Four independent transgenic lines carrying UAS–ΔNstat92E were generated by P-element-mediated transformation. stat92E397 flies were kindly provided by the Montell lab (Silver and Montell 2001). Adults of a transgenic hs-stat92E stock expressing only full-length STAT92E were heat-shocked daily, and embryos were collected. EVE stripes in these embryos were wild type. That this stock actually expresses STAT92E is indicated by the fact that it can partially rescue the viability of stat92E06346 homozygotes (data not shown) and also rescues the wing vein abnormality of statHJ mutants (Yan et al. 1996a).
BLAST sequence similarity searches
Clones possessing novel sequence were identified in a series of search routines of the National Center for Biotechnology Information (NCBI) expressed sequence tagged database (dbEST) using the 5′-untranslated region (5′-UTR) of stat92E, the sequence encoding amino acids 1–150 of STAT92E, and the novel sequence termed exon 1a, as the input data (Altschul et al. 1990). The relative positions of Exons 1, 1a, and 2 in the genomic sequence were determined by a BLAST sequence similarity search of the NCBI Drosophila genome (Adams et al. 2000).
Expression of ΔNSTAT92E
Transient transfections were performed with the GIBCO-BRL Calcium Phosphate Transfection Kit. Recombinant protein expression was according to the Drosophila Expression System protocol (Invitrogen). Then 30 μg of total protein was subjected to SDS-PAGE and immunoblotted using standard methods.The expression of ΔNSTAT92E and the control, β-galactosidase, was confirmed using monoclonal Anti-His (C-term) Antibody (Invitrogen) at a 1:5000 dilution in 1% bovine serum albumin (BSA) dissolved in TBST. The secondary antibody, peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG (Jackson ImmunoResearch Laboratories), was used at the same dilution. The blot was developed with Renaissance chemiluminescence reagent (NEN). A second gel was immunoblotted and probed with the α-CC antibody as described above.
RT-PCR and 5′-RACE-PCR
Poly(A)+ RNA (20 ng) from Drosophila embryo, larvae, or adult (Clontech) was used with the ProSTAR HF Single-Tube RT-PCR System (Stratagene) to amplify stat92E mRNA possessing Exon 1a. The cDNA synthesis was performed at 42°C for 1 h, and the annealing temperature was lowered to 50°C. Primer pairs are indicated in Figure 4. Forward primer A, 5′-CAAAT GCGCAACCAGTTGAATTC-3′; reverse primer B, 5′-GGCAA TGCCTGTATTTTGCACA-3′; forward primer C, 5′-TCC GAGCCAGAATCAGAAACAC-3′; forward primer D, 5′-ACC GTCGACGGTCCCTCT-3′; reverse primer E, 5′-GTGTAGC CCAGGATTCCCT-3′. Total RNA from S2 cells was isolated using TRIzol Reagent (GIBCO-BRL), and the RT-PCR was performed as previously described without the use of radioactivity (Yang et al. 1999). Then 10% of the RT-PCR reactions was run on a 1.5% agarose/TBE gel. To improve the specificity and further amplify the products for direct sequencing, 1 μL of each of the four Primer A/B RT-PCR reactions was subjected to standard PCR (anneal at 52°C) and run on a preparative 1.5% agarose/TBE gel. The indicated bands were gel-purified and sequenced. The PCR reactions were not performed under quantitative conditions (i.e., low number of cycles); therefore, no interpretation as to the relative amounts of mRNA where Exon 1a is spliced to Exon 2 versus to Exon 3 can be made. To identify the 5′-ends for the S2 and embryo RNA transcripts, Clontech's SMART RACE cDNA amplification kit was used as directed by the manufacturer's protocol. The stat92E specific primers from Exon 1a, 5′-CACACACGGCTGGCTGGTATCGG-3′; Exon 2, 5′-CAAGCTCATGCTCTTGCTCTACCCG-3′; and Exon 3, 5′-GCGTGTACAGCTCAACGGCGGAG-3′, were used in Touchdown PCR, and the various products were gel-purified and subcloned into pCR2.1 vector (Invitrogen) to generate clones for sequencing. In all, 45 clones were sequenced.
Northern blotting
Total RNA from different stages of Drosophila was extracted using RNeasy (QIAGEN). Each lane contained 10 μg of total RNA separated and blotted (Hybond-N, Amersham) using standard procedures. Probes were radiolabeled using Prime-It RmT kit (Stratagene). The blot was hybridized (1 × 106 cpm/mL, 1% BSA Fraction V, 7% SDS, 0.5 M NaH2PO4 at pH 7, 1 mM EDTA at pH 8) overnight at 68°C, washed twice with 2× SSC, 0.5% SDS at 56°C for 10 min, then twice with 0.1× SSC, 0.1% SDS at 56°C for 30 min. Stripping was performed using the latter wash buffer at 70°C for 2 h. The quantitative RT-PCR reactions (400 ng of total RNA) shown were performed according to Yang et al. (1999) and run on a 5% agarose gel in TBE buffer.
Immunohistochemistry
Embryos at 1–5 h were fixed and stained according to standard procedures. The stat92E397 stock was a gift of Denise Montell (Johns Hopkins School of Medicine, Baltimore, MD) and has the point mutation Trp594STOP. The anti-EVE antibody, kindly provided by David Kosman (New York University), was used at a 1:500 dilution. HRP-conjugated secondary antibody (Jackson ImmunoResearch Labs) was used at a 1:1000 dilution. The HRP substrate was from Boehringer.
RNAi
RNAi in S2 cells was performed as described in Clemens et al. (2000). Cells were collected at 72 h for Western blotting or RT-PCR to determine the efficacy of the dsRNAs used in embryo injections (Supplemental Fig. 1, in Supplementary Material at http://www.genesdev.org). Preparation of dsRNA, injections of w− embryos, and cuticle analysis were performed as described in Kennerdell and Carthew (1998). ds-Exon2 RNA was prepared with T7-tagged primers, 5′-TTTCCTCCTTCTAACCATATTAA ATTC-3′ and 5′-ATTATCCTGTCTTCGATCCA-3′. ds-Exon3/4 RNA was complementary to Exon 3 and a portion of Exon 4 and was prepared with T7-tagged primers 5′-GTCCGAACAAATA ACGCC-3′ and 5′-ATAGGAAATCAAGGTTATCAAT-3′.
Acknowledgments
We thank Michael Melnick for providing the original genomic sequence of stat92E and Lois Cousseau for preparing the manuscript. We also thank Mike Young and Ulrike Gaul and their lab members for technical advice and sharing fly-related material and equipment. We thank Peter Gergen for providing the NGT-GAL4 stock; Denise Montell for the stat92E397 stock; Charles Dearolf for the hs-STAT92E fly stock; and Steven Hou for anti-STAT92E antibody. M.A.H. was a Cancer Research Institute Postdoctoral Fellow. This work was supported by NIH grants AI32489 and AI34420 to J.E.D.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL darnell@rockvax.rockefeller.edu; FAX (212) 327-8801.
Article and publication are at http://www.genesdev.org/cgi/doi/10.1101/gad.1020702.
References
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3β homodimer bound to DNA. Nature. 1998;394:145–151. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- Betz A, Lampen N, Martinek S, Young M, Darnell JJ. A Drosophila PIAS homologue negatively regulates stat92E. Proc Natl Acad Sci. 2001;98:9563–9568. doi: 10.1073/pnas.171302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binari R, Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes & Dev. 1994;8:300–312. doi: 10.1101/gad.8.3.300. [DOI] [PubMed] [Google Scholar]
- Brand A, Manoukian A, Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- Bromberg J, Darnell JJ. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- Burke TW, Kadonaga JT. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes & Dev. 1996;10:711–724. doi: 10.1101/gad.10.6.711. [DOI] [PubMed] [Google Scholar]
- ————— The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes & Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell JE, Jr, Kuriyan J. Crystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNA. Cell. 1998;93:827–839. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- Clemens JC, Worby CA, Simonson-Leff N, Muda M, Maehama T, Hemmings BA, Dixon JE. Use of double-stranded RNA interference in Drosophila cell lines to dissect signal transduction pathways. Proc Natl Acad Sci. 2000;97:6499–6503. doi: 10.1073/pnas.110149597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE. Stats and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Fischer J, Giniger E, Maniatis T, Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988;332:853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- Guyer NB, Severns CW, Wong P, Feghali CA, Wright TM. IFN-γ induces a p91/Stat1a-related transcription factor with distinct activation and binding properties. J Immunol. 1995;155:3472–3480. [PubMed] [Google Scholar]
- Haspel R, Darnell JJ. A nuclear protein tyrosine phosphatase is required for the inactivation of Stat1. Proc Natl Acad Sci. 1999;96:10188–10193. doi: 10.1073/pnas.96.18.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou XS, Melnick MB, Perrimon N. Marelle acts downstream of the Drosophila HOP/JAK kinase and encodes a protein similar to the mammalian STATs. Cell. 1996;84:411–419. doi: 10.1016/s0092-8674(00)81286-6. [DOI] [PubMed] [Google Scholar]
- John S, Vinkemeier U, Soldaini E, Darnell JJ, Leonard W. The significance of tetramerization in promoter recruitment by Stat5. Mol Cell Biol. 1999;19:1910–1918. doi: 10.1128/mcb.19.3.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell JR, Carthew RW. Use of dsRNA-mediated genetic interference to demonstrate that frizzled and frizzled 2 act in the wingless pathway. Cell. 1998;95:1017–1026. doi: 10.1016/s0092-8674(00)81725-0. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of vertebrate mRNA sequences: Intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs DL, Hilton DJ. SOCS proteins: Negative regulators of cytokine signaling. Stem Cells. 2001;19:378–387. doi: 10.1634/stemcells.19-5-378. [DOI] [PubMed] [Google Scholar]
- Kutach A, Kadonaga J. The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol Cell Biol. 2000;20:4754–4764. doi: 10.1128/mcb.20.13.4754-4764.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, D. and Darnell, J.E., Jr. 2002. STATs: Transcriptional control and biologic impact. Nat. Rev. Mol. Cell Biol. (in press). [DOI] [PubMed]
- Look DC, Pelletier MR, Tidwell RM, Roswit WT, Holtzman MJ. Stat1 depends on transcriptional synergy with Sp1. J Biol Chem. 1995;270:30264–30267. doi: 10.1074/jbc.270.51.30264. [DOI] [PubMed] [Google Scholar]
- Luo H, Dearholf C. The JAK/STAT pathway and Drosophila development. Bioessays. 2001;23:1138–1147. doi: 10.1002/bies.10016. [DOI] [PubMed] [Google Scholar]
- Muller M, Laxton C, Briscoe J, Schindler C, Improta T, Darnell JE, Jr, Stark GR, Kerr IM. Complementation of a mutant cell line: Central role of the 91 kDa polypeptide of ISGF3 in the interferon-α and -γ signal transduction pathways. EMBO J. 1993;12:4221–4228. doi: 10.1002/j.1460-2075.1993.tb06106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G, Hong L, Brokstein P, Evans-Holm M, Frise E, Stapleton M, Harvey D. A Drosophila complementary DNA resource. Science. 2000;287:2222–2224. doi: 10.1126/science.287.5461.2222. [DOI] [PubMed] [Google Scholar]
- Schaefer TS, Sanders LK, Nathans D. Cooperative transcriptional activity of Jun and Stat3β, a short form of Stat3. Proc Natl Acad Sci. 1995;92:9097–9101. doi: 10.1073/pnas.92.20.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer TS, Sanders LK, Park OK, Nathans D. Functional differences between Stat3α and Stat3β. Mol Cell Biol. 1997;17:5307–5316. doi: 10.1128/mcb.17.9.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U, Hagenbuchle O, Wellauer PK, Pittet AC. Two promoters of different strengths control the transcription of the mouse α-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983;33:501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Schindler C, Fu XY, Improta T, Aebersold R, Darnell JE., Jr Proteins of transcription factor ISGF-3: One gene encodes the 91- and 84-kDa ISGF-3 proteins that are activated by interferon α. Proc Natl Acad Sci. 1992;89:7836–7839. doi: 10.1073/pnas.89.16.7836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K. Modulation of STAT signaling by STAT-interacting proteins. Oncogene. 2000;19:2638–2644. doi: 10.1038/sj.onc.1203522. [DOI] [PubMed] [Google Scholar]
- Silver DL, Montell DJ. Paracrine signaling through the JAK/STAT pathway activates invasive behavior of ovarian epithelial cells in Drosophila. Cell. 2001;107:831–841. doi: 10.1016/s0092-8674(01)00607-9. [DOI] [PubMed] [Google Scholar]
- Small S, Blair A, Levine M. Regulation of two pair-rule stripes by a single enhancer in the Drosophila embryo. Dev Biol. 1996;175:314–324. doi: 10.1006/dbio.1996.0117. [DOI] [PubMed] [Google Scholar]
- Stark G, Kerr I, Williams B, Silverman R, Schreiber R. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- Starr R, Hilton D. Negative regulation of the JAK/STAT pathway. Bioessays. 1999;21:47–52. doi: 10.1002/(SICI)1521-1878(199901)21:1<47::AID-BIES6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Stocklin E, Wissler M, Gouilleux F, Groner B. Functional interactions between Stat5 and the glucocorticoid receptor. Nature. 1996;383:726–728. doi: 10.1038/383726a0. [DOI] [PubMed] [Google Scholar]
- ten Hoeve J, Ibarra-Sanchez M, Fu Y, Zhu W, Tremblay M, David M, Shuai K. Identification of a nuclear Stat1 protein tyrosine phosphatase. Mol Cell Biol. 2002;22:5662–5668. doi: 10.1128/MCB.22.16.5662-5668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WJ, Ning X, Klingler M, Kramer S, Gergen J. Quantitative analysis of gene function in the Drosophila embryo. Genetics. 2000;154:273–284. doi: 10.1093/genetics/154.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkemeier U, Cohen SL, Moarefi I, Chait B T, Kuriyan J, Darnell JE., Jr DNA binding of in vitro activated Stat1 α, Stat1 β and truncated Stat1: Interaction between NH2-terminal domains stabilizes binding of two dimers to tandem DNA sites. EMBO J. 1996;15:5616–5626. [PMC free article] [PubMed] [Google Scholar]
- Vinkemeier U, Moarefi I, Darnell JE, Jr, Kuriyan J. Structure of the amino-terminal protein interaction domain of STAT-4. Science. 1998;279:1048–1052. doi: 10.1126/science.279.5353.1048. [DOI] [PubMed] [Google Scholar]
- Wang D, Stravopodis D, Teglund S, Kitazawa J, Ihle JN. Naturally occurring dominant negative variants of Stat5. Mol Cell Biol. 1996;16:6141–6148. doi: 10.1128/mcb.16.11.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Ya-Lin S, Hoey T. Cooperative DNA binding and sequence selective recognition conferred by the Stat amino terminal domain. Science. 1996;273:794–797. doi: 10.1126/science.273.5276.794. [DOI] [PubMed] [Google Scholar]
- Yamanaka R, Kim GD, Radomska HS, Lekstrom-Himes J, Smith LT, Antonson P, Tenen DG, Xanthopoulos K G. CCAAT/enhancer binding protein ɛ is preferentially up-regulated during granulocytic differentiation and its functional versatility is determined by alternative use of promoters and differential splicing. Proc Natl Acad Sci. 1997;94:6462–6467. doi: 10.1073/pnas.94.12.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Lou H, Darnell JE, Jr, Dearolf CR. A JAK-STAT pathway regulates wing vein formation in Drosophila. Proc Natl Acad Sci. 1996a;93:5842–5847. doi: 10.1073/pnas.93.12.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Small S, Desplan C, Dearolf CR, Darnell JE., Jr Identification of a Stat gene that functions in Drosophila development. Cell. 1996b;84:421–430. doi: 10.1016/s0092-8674(00)81287-8. [DOI] [PubMed] [Google Scholar]
- Yang E, Wen Z, Haspel RL, Zhang JJ, Darnell JE., Jr The linker domain of Stat1 is required for γ interferon-driven transcription. Mol Cell Biol. 1999;19:5106–5112. doi: 10.1128/mcb.19.7.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JY, Huso DL, Nathans D, Desiderio S. Specific ablation of STAT3β distorts the pattern of Stat3-responsive gene expression and impairs recovery from endotoxic shock. Cell. 2002;108:331–344. doi: 10.1016/s0092-8674(02)00636-0. [DOI] [PubMed] [Google Scholar]
- Zhang JJ, Vinkemeier U, Gu W, Chakravarti D, Horvath CM, Darnell JE., Jr Two contact regions between Stat1 and CBP/p300 in interferon γ signaling. Proc Natl Acad Sci. 1996;93:15092–15096. doi: 10.1073/pnas.93.26.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wrzeszczynska MH, Horvath CM, Darnell JE., Jr Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation. Mol Cell Biol. 1999;19:7138–7146. doi: 10.1128/mcb.19.10.7138. [DOI] [PMC free article] [PubMed] [Google Scholar]