Abstract
Mouse chromosome 7F4/F5, where the imprinting domain is located, is syntenic to human 11p15.5, the locus for Beckwith-Wiedemann syndrome. The domain is thought to consist of the two subdomains Kip2 (p57kip2)/Lit1 and Igf2/H19. Because DNA methylation is believed to be a key factor in genomic imprinting, we performed large-scale DNA methylation analysis to identify the cis-element crucial for the regulation of the Kip2/Lit1 subdomain. Ten CpG islands (CGIs) were found, and these were located at the promoter sites, upstream of genes, and within intergenic regions. Bisulphite sequencing revealed that CGIs 4, 5, 8, and 10 were differentially methylated regions (DMRs). CGIs 4, 5, and 10 were methylated paternally in somatic tissues but not in germ cells. CGI8 was methylated in oocyte and maternally in somatic tissues during development. Parental-specific DNase I hypersensitive sites (HSSs) were found near CGI8. These data indicate that CGI8, called DMR-Lit1, is not only the region for gametic methylation but might also be the imprinting control region (ICR) of the subdomain.
DNA methylation is an epigenetic phenomenon in which cytosine is modified to 5-methyl cytosine. In the mammalian genome, DNA methylation has been shown to be associated with important phenomena such as tissue-specific gene expression, X-chromosome inactivation, carcinogenesis, and genomic imprinting (Razin and Cedar 1991; Avner and Heard 2001; Baylin et al. 2001; Reik and Walter 2001). Genomic imprinting is a specific example of the epigenetic phenomenon whereby gene expression is restricted to only one parental allele. Most imprinted genes exist in clusters, suggesting a coordinated regulation of imprinted genes (Ferguson-Smith and Surani 2001; Reik and Walter 2001). Establishment of the imprint must occur in gametogenesis because this is the only chance during development in which the male and female genomes are in distinct compartments (Tilghman 1999; Reik and Walter 2001). In fact, several imprinted genes have been reported in which methylation was inherited from gametes (Stöger et al. 1993; Tremblay et al. 1995; Shemer et al. 1997). This is called gametic methylation and is thought to be a gametic imprint.
One of the two major imprinting domains is mapped to human chromosome 11p15.5, a locus for Beckwith-Wiedemann syndrome (BWS). Its murine ortholog is on chromosome 7F4/F5 (Caspary et al. 1998; Paulsen et al. 1998). The gene organization of the region is highly conserved between human and mouse (Engemann et al. 2000; Onyango et al. 2000; Paulsen et al. 2000; Yatsuki et al. 2000). Transgenic mice, gene targeting, and translocation experiments have suggested that this domain may be separated into two subdomains (Sun et al. 1997; Zhang et al. 1997; Caspary et al. 1998; Cleary et al. 2001). One of these subdomains includes the Igf2 and H19 genes (Igf2/H19 subdomain). The expression of Igf2 and H19 is epigenetically controlled by an imprinting control region (ICR), which is located near a CpG island (CGI) upstream of H19 and is characterized by the differentially methylated region (DMR; Thorvaldsen et al. 1998). This subdomain, however, does not affect the imprinting of Kvlqt1 and p57Kip2, and vice versa (Sun et al. 1997; Caspary et al. 1998). These genes are several hundred kilobases away from the Igf2/H19 subdomain and possibly consist of another subdomain (the Kip2/Lit1 subdomain). Therefore, another ICR will be identified in this region (Fig. 1). The discovery of human LIT1(KCNQ1-AS, KCNQ1OT1), and the finding that loss of imprinting of this gene is independent of IGF2 imprinting, further support the above notion (Lee et al. 1999; Mitsuya et al. 1999; Smilinich et al. 1999; Feinberg 2000; Maher and Reik 2000). In fact, it was proposed that the CGI of Lit1 is a candidate for the ICR and may regulate p57Kip2 expression (Engemann et al. 2000; Horike et al. 2000).
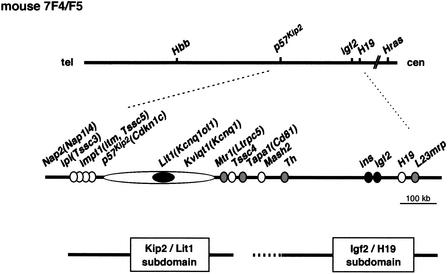
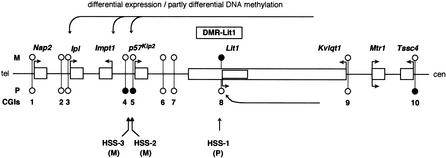
Figure 1.
Imprinting domain and a schematic physical map of mouse 7F4/F5. The relative position of the genes is based on previously published data. The imprinting status is shown by filled circles (paternally expressed), open circles (maternally expressed), and gray circles (biallelic expression). The locations of the Kip2/Lit1 and Igf2/H19 subdomains are shown below the map.
Accurate mapping and analysis of genomic DNA methylation at developmental stages are essential for understanding the regulation of the Kip2/Lit1 subdomain, because DNA methylation is important for the establishment and/or maintenance of imprinting. Although a candidate region has been identified, the underlying mechanism remains to be clarified. We sequenced approximately 500 kb of the region between Nap2(Nap1l4) and Tapa1 (Cd81) in mouse where eight genes were clustered (Fig. 1; Yatsuki et al. 2000). In this subdomain, Nap2 (Nap1l4), Ipl (Tssc3), Impt1 (Itm, Tssc5), p57Kip2(Cdknlc), Kvlqt1(Kcnq1), and Tssc4 were imprinted and expressed from maternal allele (Hatada and Mukai 1995; Qian et al. 1997; Dao et al. 1998; Gould and Pfeifer 1998; Morisaki et al. 1998; Engemann et al. 2000). Lit1(Kcnqt1-AS, Kcnq1ot1) was also imprinted but was expressed from paternal allele (Smilinich et al. 1999; Yatsuki et al. 2000). Mtr1(Ltrpc5) was not imprinted, and consequently both alleles were expressed (Fig. 1, top of Fig. 2; Paulsen et al. 1998; Yatsuki et al. 2000).
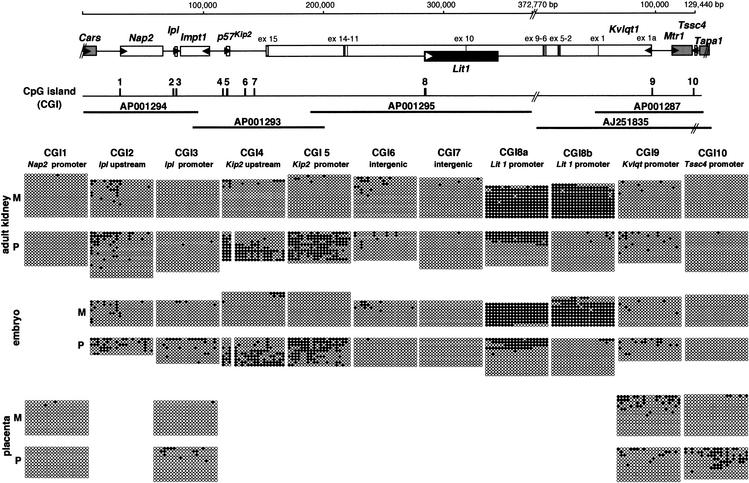
Figure 2.
Detailed methylation status of CpG islands (CGIs) in the Kip2/Lit1 subdomain. At the top is a schematic gene cluster based on the sequences obtained in the Kip2/Lit1 subdomain. The numbers above the line show a summation based on the sequences, which includes one gap. The imprinting status is shown by filled boxes (paternally expressed), open boxes (maternally expressed), and gray boxes (biallelic expression). Exons of Kvlqt1 are depicted as vertical bars. The transcriptional orientation of genes is indicated by horizontal arrowheads. Middle, a schematic CGI. According to sequencing results, a total of 10 CGIs (CGIs 1–10) were identified. The thick horizontal lines below the line indicate the sequenced regions, with GenBank accession nos. Bottom, the detailed methylation status of CGIs in somatic tissues. Each circle represents a CpG dinucleotide on the strand: (●) a methylated cytosine; (○) an unmethylated cytosine. Paternal and maternal alleles were distinguished by DNA polymorphism, as presented in Table 1. Bisulphite sequencing was performed in adult kidney, embryo (10.5 dpc), and placenta (8.5 dpc) DNAs. The placenta was used when it was proved the gene was expressed monoallelically in this tissue. In adult kidney, at least 10 clones were sequenced from each parental allele. Primers are described in Methods. The first 20 CpGs of the analyzed CGIs are presented and numbered from 1–20 from the left. F1 hybrid mice used were all BPF1. M, maternal; P, paternal.
Here we analyzed the methylation status of the mouse genomic region corresponding to the Kip2/Lit1 subdomain through germ cell, early embryo, and adult somatic tissue to find the DMRs and the regions for gametic methylation. We therefore performed sodium bisulphite-genomic sequencing of a total of 10 CGIs in this domain. From our results, CGI4 (p57Kip2 upstream), CGI5 (p57Kip2 promoter), CGI8 (Lit1 promoter), and CGI10 (Tssc4 promoter) were shown to be the DMR in somatic tissues. Furthermore, CGI8 and not CGIs 4, 5, or 10 was solely methylated in germ cells, suggesting that the CGI8 would be the ICR in this subdomain.
RESULTS
Identification of CGIs in Mouse Kip2/Lit1 Subdomain
We reported previously the nucleotide sequence of 390 kb between Kvlqt1 and Tapa1 (Yatsuki et al. 2000) and extended the sequencing up to 500 kb between Nap2 and TapaI (accession nos. AP001293 and AP001294), which covered an entire Kip2/Lit1 subdomain. It has been reported that many imprinted genes have been differentially methylated in their control regions (Thorvaldsen et al. 1998). The DMR usually exists in CGI. We have identified 10 CGIs based on sequence information around this region (Fig. 2). These CGIs were named CGIs 1 to 10 (Table 1). CGIs 1, 3, 5, 8, 9, and 10 were located in the promoter region of the genes (Table 1, Fig. 2). CGIs 2 and 4 were upstream of genes, and CGIs 6 and 7 existed in an intergenic region. To determine the methylation status of the parental alleles, we searched for nucleotide-sequence polymorphisms between two mouse strains, C57BL/6 and PWK, that would allow us to distinguish between two parental alleles in their F1 hybrid mice. Several polymorphisms were found and are shown in Table 1.
Table 1.
The Position of CGIs Based on Sequences and DNA Polymorphisms in Amplified Regions
| CGI | Databases | Position of CGI | Polymorphism (B6/PWK) | PCR- amplified region | Position of first presented CpG | Transcriptional start site near the CGI |
|---|---|---|---|---|---|---|
| 1 | AP001294 | 30585-31349 (765 bp) | 30704 A/T | 30480-30877 (25) | 30514 | Nap2 30826 (5′-3′) |
| 2 | AP001294 | 76525-76745 (221 bp) | 76541 T/G | 76469-76795 (20) | 76489 | |
| 3 | AP001294 | 77348-78008 (661 bp) | 77447 C/T | 77320-77980 (47) | 77342 | lpl 77376 (5′-3′) |
| 4 | AP001293 | 30370-30593 (224 bp) | 30214 G/A | 30134-30465 (26) | 30191 | |
| 5 | AP001293 | 33971-36417 (2447 bp) | 34012 T/G | 33926-34377 (53) | 33986 | p57Kip2 34481 (5′-3′) |
| 6 | AP001293 | 49478-50205 (728 bp) | 49380 G/A | 49346-49694 (29) | 49373 | |
| 7 | AP001293 | 57282-57713 (432 bp) | 57421 G/- | 57188-57705 (49) | 57322 | |
| 8 | AP001295 | 8a 101981-102270 (290 bp) | 101803 A/G | 101735-102282 (23) | 101956 | Lit1 101974 (5′-3′) |
| 8b 102713-103490 (778 bp) | 103181 G/C | 102639-103483 (22) | 103099 | |||
| 9 | AP001287 | 32410-33175 (766 bp) | 32315 G/A | 32256-32707 (34) | 32318 | Kvlqt1 32717 (3′-5′) |
| 10 | AP001287 | 70536-71088 (553 bp) | 70844 G/A | 70628-71113 (39) | 70648 | Tssc4 70393 (3′-5′) |
Numbers are derived from GenBank databases. Length of CGIs and analyzed numbers of CpGs are shown in parentheses in the columns labeled “position of CGI” and “PCR amplified region,” respectively. CGI, CpG island.
CGIs 4, 5, 8, and 10 Associated with p57Kip2, Lit1, and Tssc4 Carry the DMR in Somatic Tissues
To define the methylation status of all CGIs, we performed sodium bisulphite sequencing using BPF1 genomic DNA (Fig. 2). The first 20 CpGs of analyzed CGIs are presented. An adult kidney and whole embryo derived from 10.5 days postconception (dpc) were used as somatic tissues, because it is known that most genes in this region were expressed and imprinted in these tissues. The placenta was used when it was proven that the gene was expressed monoallelically in this tissue.
First, we analyzed CGIs 1, 3, 5, 8, 9, and 10, which exist in the promoter of genes. Among these CGIs, differential methylation was dominantly observed in CGIs 5 and 8. Paternal methylation was observed in CGI5, that is, p57Kip2 promoter, in adult kidney and embryo (Fig. 2). Most prominent methylation was observed in CGI8, located in the promoter region of Lit1. This CGI8 was separated into CGIs 8a and 8b (Fig. 2, Fig. 3C). Their regions were heavily methylated on the maternal allele in kidney and embryo. However, CGI8a was also methylated partly on the paternal allele. Methylation of CGI10 was observed partially but preferentially on the paternal allele in placenta, in which Tssc4 was expressed maternally. CGIs 1, 3, and 9 were not differentially methylated. CGI1 was not methylated in the tissues analyzed. Both alleles of CGIs 3 and 9 were partially but equivalently methylated in kidney, embryo, and placenta.
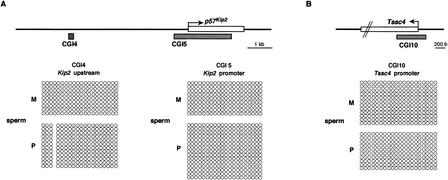
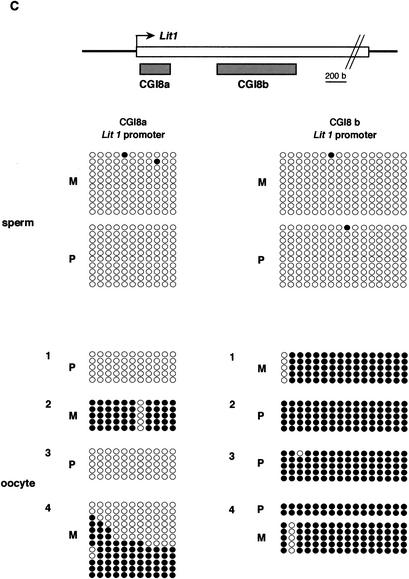
Figure 3.
Methylation status in gametes. (A) Nonparental methylation of CGIs 4 and 5 in sperm. (Top) Schematic structure of CGIs 4 and 5 in and around p57Kip2. The bent arrow indicates a transcriptional start site; the open box on the line represents p57Kip2. The gray boxes below the line represent CGIs 4 and 5. The methylation status of sperm genomic DNA is presented. (B) Nonparental methylation of CGI10 in sperm. The methylation status of sperm genomic DNA is presented in Tssc4 promoter. (C) Gametic methylation of CGI8 in oocyte but not in sperm. The methylation status of CGIs 8a and 8b is shown in sperm and oocyte. In oocyte, clones are grouped by separate PCRs. The schematic presentation is the same as shown in Fig. 2. However, the first 11 and first 16 CpGs are presented in CGI8a and CGI8b, respectively. These were analyzed by bisulphite sequencing in BPF1.
Second, CGIs 2 and 4, which lay upstream of genes, were analyzed. CGI2 was located upstream of Ipl and partially methylated in both alleles, but did not show any distinct bias of methylation. CGI4, located 4.1 kb upstream of p57Kip2, showed paternal methylation as well as CGI5, as mentioned above. This pattern of methylation was maintained in embryo as well.
Finally, intergenic CGIs 6 and 7 located between p57Kip2 and Kvlqt1 were analyzed. Their parental alleles were hypomethylated in kidney and embryo. The results were confirmed by analysis of the methylation status of some of these CGIs in the opposite cross, PBF1 mice.
As a result, DMRs were found in CGIs 4, 5, 8, and 10. Although CGIs 1, 3, and 9 existed near the promoter of the genes—Nap2, Ipl, and Kvlqt1, respectively, which have been reported as imprinted genes—these CGIs did not show any differential methylation in expressed tissues, but were hypomethylated in both alleles. Therefore, these CGIs located in p57Kip2 upstream, p57Kip2 promoter, Lit1 promoter, and Tssc4 promoter, respectively, were chosen for further analysis.
Only CGI8 Is Methylated in Gametic Tissue
We focused on the methylation status of CGIs 4, 5, 8, and 10 in gametes, which showed differential methylation in somatic tissues. To clarify whether CGIs 4, 5, and 10 are methylated in gametic tissue, we performed bisulphite sequencing using sperm DNA of BPF1 mice, because their paternal alleles were specifically methylated in somatic tissues. Parental alleles of CGIs 4, 5, and 10 were not methylated at all in sperm DNA (Fig. 3A,B). Therefore, we concluded that gametic methylation of these CGIs did not occur in sperm.
In contrast, CGI8, which was methylated maternally in somatic tissues, was analyzed in growing oocytes and in sperm (Fig. 3C). To analyze oocytes, several PCRs were carried out on bisulphite-treated DNA. Clones were prepared from each PCR and sequenced. Clones derived from separate PCRs were not combined, to show that the variability that may occur among PCRs from the sample is due to PCR bias (Warnecke et al. 1998). Although various patterns of methylation were obtained in CGI8a, depending on the experiment, full methylation was consistently obtained in CGI8b on both alleles. On the other hand, sperm was not methylated in this region at all. We confirmed that CGI8, particularly CGI8b, was fully methylated gametically, as reported previously (Engemann et al. 2000).
Overall analyses of 10 CGIs showed only one methylated region of CGI8 in gametes. As this region was a DMR and, in addition, had a gametic methylation, the CGI8 was designated as DMR-Lit1(DMR of Lit1) in the Kip2/Lit1 subdomain.
Differential Methylation of CGI8b Is Gradually Established in Early Preimplantation Embryo
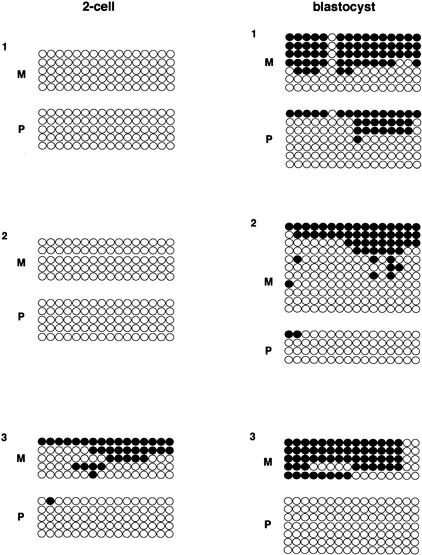
The methylation status of CGI8 was analyzed in early preimplantation embryo (Fig. 4). We expected maternal methylation because this region was gametically methylated in oocyte. However, CGI8a was hypomethylated on the maternal allele at this stage, including two-cell, morula, and blastocyst (data not shown). CGI8b again showed maternal hypomethylation in two-cell, but this CGI showed preferentially maternal methylation in blastocyst (Fig. 4). This maternal methylation, however, reappeared completely at 10.5 dpc embryo, as mentioned above (Fig. 2). Thus, maternal methylation was complete between 3.5 and 10.5 dpc.
Figure 4.
Methylation status of CGI8b in preimplantation embryos. The methylation status of two-cell and blastocyst DNAs was analyzed in CGI8b. The presentation is basically the same as in Fig. 3C. Clones are grouped by separate PCRs. Independent experiments were performed several times; representative results are shown. The primers are described in Methods. F1 hybrid embryos used here are all BPF1.
Parental Expression and Methylation of p57Kip2 and Lit1 Are Strictly Maintained Throughout Development
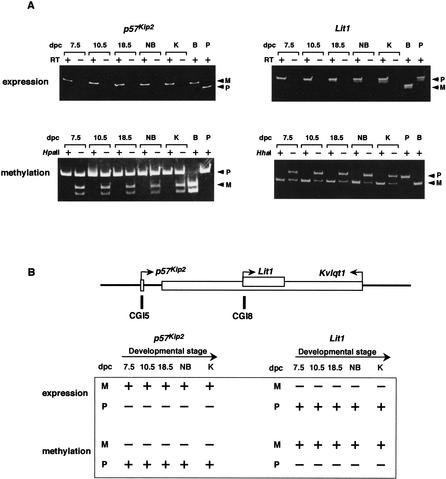
We analyzed the parental expression and methylation of p57Kip2 and Lit1 during development in somatic tissues, because it is likely that p57Kip2 is a target gene of DMR-Lit1 and Lit1 involves the DMR-Lit1. The search for methylation status showed the CGI5 (p57Kip2 promoter) to be the DMR in somatic cells (Fig. 2). To survey the expression and methylation status of p57Kip2 during the developmental stage, we performed RT-PCR and a methylation-sensitive enzyme assay. Both maternal expression and paternal methylation were tightly maintained throughout the stages of development (Fig. 5A, left).
Figure 5.
Parental expression and methylation of p57Kip2 and Lit1 during development. (A) Allelic expression and methylation of p57Kip2 and Lit1 in F1 mice. The mRNA and genomic DNA obtained from various tissues were used for RT-PCR and genomic PCR, respectively. HpaII and HhaI indicate restriction enzymes used for methylation-sensitive genomic PCR; +, digested; −, not digested. Tissues used for analyses were as follows: 7.5, 10.5, and 18.5 dpc embryos; NB, whole newborn; K, adult kidney; B, adult B6 kidney; P, adult PWK kidney. RT indicates reverse transcription; +, transcriptase added; −, no transcriptase. Primers are described in Methods. Two alleles were distinguished as described in Methods. F1 hybrid mice were all BPF1. (B) (Top) Schematic structure of p57Kip2, Lit1, and Kvlqt1. (Bottom) Schematic pattern of expression and methylation during development for p57Kip2 and Lit1, respectively. In expression, +, expressed; −, nonexpressed. In methylation, +, methylated; −, nonmethylated.
CGI8 (Lit1 promoter) is also a DMR. Lit1 was expressed paternally and methylated maternally in somatic tissues. This expression and methylation were opposite those of p57Kip2. We analyzed the expression and methylation status of Lit1 in CGI8b during the developmental stages. Paternal expression and maternal methylation of Lit1 were similarly maintained at all stages as well (Fig. 5A, right). A summary of the above results is depicted in Figure 5B. Allelic expression of Lit1 was further examined in many adult tissues, such as brain, skeletal muscle, spleen, heart, lung, liver, kidney, testis, and placenta. Lit1 was paternally expressed, except in testis, in which the gene was expressed biallelically (data not shown).
Allelic Chromatin Conformation Around CGIs 4, 5, and 8
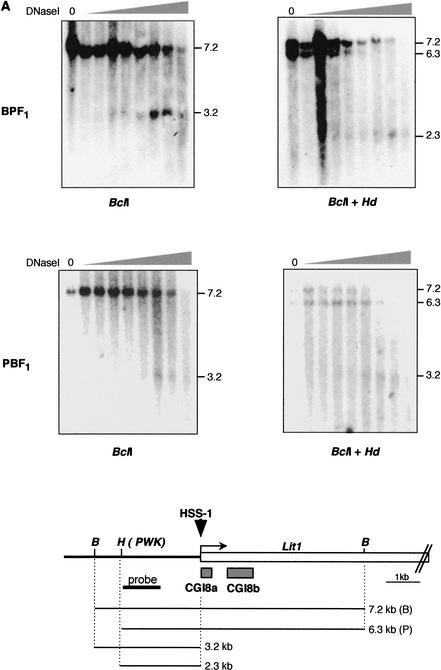
Because CGI8 is the DMR and the region for gametic methylation in the Kip2/Lit1 subdomain, it could be an ICR, as has been proposed by a number of groups (Lee et al. 1999; Mitsuya et al. 1999; Smilinich et al. 1999; Engemann et al. 2000). In some ICRs, their parental origin-specific chromatin structures were studied and nuclease-sensitive sites were identified (Hark and Tilghman 1998; Schweizer et al. 1999). To investigate the chromatin conformation around CGI8, we examined DNaseI hypersensitive sites (HSSs). To distinguish between the alleles, we used BPF1 hybrid mice and its reciprocal cross, PBF1. Nuclei from BPF1 and PBF1 primary fibroblasts were incubated with different amounts of DNase I. After purification, the DNA was digested with BclI and additionally with polymorphic HindIII. The sites of cleavage were detected with a hybridization probe at the 5′ end of a 6.3-kb HindIII/BclI fragment upstream of the LitI promoter. After digestion with BclI, a 3.2-kb fragment appeared. This length shifted to 2.3 kb by additional digestion with HindIII in BPF1 (Fig. 6A). This result indicated the presence of a hypersensitive site on the paternal chromosome at the 5′ border of CGI8a, because a polymorphic HindIII site was found in a genomic DNA of PWK mouse, from which the paternal allele had been derived. These results were confirmed by use of the reciprocal cross, PBF1 (Fig. 6A). Digestion with HindIII did not give rise to any change in the pattern, because the polymorphic HindIII site was not found in the genomic DNA of B6 mouse, from which the paternal allele was derived. This hypersensitive site was named HSS-1.
Figure 6.
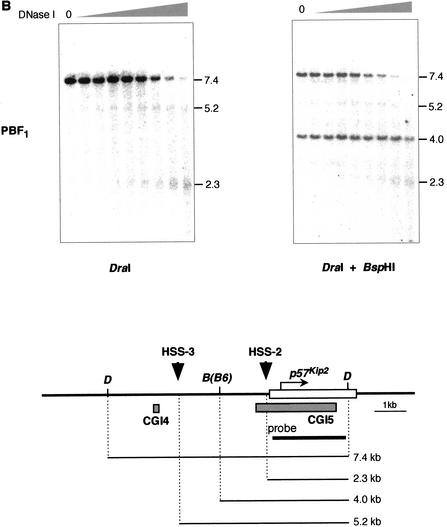
Parental-specific DNaseI HSSs near and around CGIs 4, 5, and 8. (A) DNaseI HSS on paternal allele near CGI8. Nuclei isolated from BPF1 or PBF1 primary fibroblast in mice were treated with an increasing amount of DNase I. After extraction, the DNA was digested with BclI or BclI and HindIII as indicated and subjected to Southern blotting analysis. The blots were probed with 1 kb of fragment amplified with the primer sets Lit-3 and Lit-5. The diagram shown below depicts Lit1 gene and CGI8 surrounded by restriction sites, with the start of transcription represented by the bent arrow; gray boxes below the gene represent CGI8a and 8b. Downward arrowhead indicates a nuclease HSS detected on the paternal chromosome, designated HSS-1. B, BclI; H, HindIII. HindIII is the polymorphic recognition site in PWK but not in B6 mouse. (B) HSS on maternal allele around CGIs 4 and 5. The same DNase I-treated DNA was used as above and treated similarly. DraI and BspH1 were used. BspH1 is the polymorphic restriction site in B6 mouse. The probe is a p57Kip2 cDNA. The HSSs obtained were designated HSS-2 and HSS-3. D, DraI; B, BspH1.
It is thought that p57Kip2 is one of the target genes in BWS (Reik and Maher 1997). Therefore, it is likely that this gene is under the control of DMR-Lit1. This gene has the DMR in CGIs 4 and 5. To understand the regulatory mechanism of this gene, we analyzed the chromatin structure in and around this gene. F1 genomic DNA was digested with DraI and subsequently by polymorphic BspH1, whose recognition site is in the DraI fragment from the B6 genome but not from the PWK genome. After digestion with DraI, 5.2- and 2.3-kb fragments appeared along with a 7.4-kb fragment in PBF1. Both the 5.2- and 2.3-kb fragments remained in the same position after additional BspH1 digestion, but a 7.4-kb fragment gradually disappeared with an increment of DNase I, although 4.0 kb remained. These results indicated that hypersensitive sites (HSS-2 and HSS-3) were derived from maternal chromosome. The HSS-2 existed just upstream of the transcription start site of this gene. The other HSS-3 was 3.0 kb further upstream of this gene. These data were confirmed by use of the probe, located between DraI and the HSS-3 site, and by use of the reciprocal cross, BPF1 (data not shown).
DISCUSSION
The methylation status of an imprinting Kip2/Lit1 subdomain spanning 500 kb on mouse chromosome 7F4/F5 was analyzed. All CGIs of the subdomain were analyzed by sodium bisulphite sequencing. Figure 7 summarizes the results of this study. On the maternal allele, DMR-Lit1 was methylated in oocyte, mid-embryo, and somatic tissues and Lit1 was not expressed, whereas flanking imprinted genes such as p57Kip2 were unmethylated and expressed. In contrast, on the paternal allele, DMR-Lit1 was demethylated from sperm to somatic tissues throughout development and Lit1 was expressed, whereas flanking imprinted genes were repressed in expression but not methylated except for p57Kip2 and Tssc4. Our results suggest that DMR-Lit1 is the ICR of this subdomain. The ICR is defined by the following characteristics: DMR, gametic methylation, and differential chromatin conformation (Ben-Porath and Cedar 2000). The disruption of this region would cause loss of imprinting. In fact, loss of imprinting of human LIT1 was observed in BWS as noted earlier (Lee et al. 1999; Mitsuya et al. 1999; Smilinich et al. 1999). This evidence, taken together with the presence of a paternally active chromatin structure near the DMR-Lit1, supports the idea that DMR-Lit1 is the ICR in the Kip2/Lit1 subdomain. The regulatory mechanism of this domain will be discussed in a later section.
Figure 7.
Imprinting regulation of Kip2/Lit1 subdomain by DMR-Lit1. Expression and DNA methylation status of imprinting cluster in this domain are depicted. The hypothetical mechanism of DMR-Lit-1 is described in the Discussion. Bent arrows indicate a parental-dependent transcription. Horizontal arrows pointing toward left show hypothetical enhancers that were suggested to exist experimentally (Cleary et al. 2001; John et al. 2001). Lollipops represent CGIs 1 to 10. Closed lollipops: methylated DNA; open lollipops: nonmethylated DNA. M, maternal allele; P, paternal allele. HSS-1, HSS-2, and HSS-3 show nuclease-hypersensitive sites near CGI8, CGI4, and CGI5, respectively. Not drawn to scale.
How is this subdomain regulated? It would be useful to clarify the regulatory mechanism, particularly of p57Kip2, in an imprinting cluster because this gene is directly responsible for BWS (Hatada et al. 1996; Lee et al. 1997; O'keefe et al. 1997). Chromosomal translocation within KvLQT1 has also caused this syndrome. It is thought that the primary cause of BWS in this case may be the loss of p57KIP2 expression. It was experimentally proven that the regulation of imprinted genes was affected by targeted chromosomal translocation (Cleary et al. 2001). Using mice carrying a site-specific translocation separating p57Kip2 and Kvlqt1, expression and imprinting of telomeric genes, including p57Kip2, were greatly influenced. This result strongly indicated that the translocation physically separated the telomeric genes from enhancer elements; thus, the ICR would exist at a distance from these genes. At the very least these elements must be in the centromeric as opposed to the telomeric region. These results are compatible with our results because we suspected that the ICR in this subdomain would be the DMR-Lit1, that is, 160 kb apart from p57Kip2 and located at the centromeric region.
On the other hand, the insulator model has been established in the imprinting of the Igf2/H19 subdomain (Thorvaldsen et al. 1998), and the same model was proposed in this Kip2/Lit1 subdomain (Horike et al. 2000; Cleary et al. 2001). Kanduri et al. (2002) showed that this DMR-Lit1 functions as an insulator. We also confirmed this result, but in our case, this DMR-Lit1 functioned bidirectionally (K. Joh and T. Mukai, unpubl.). This result is compatible with the proposed insular model, if we assume that the enhancer is in a more centromeric region than is the DMR-Lit1 (Fig. 7). Our present results suggest that methylated DMR-Lit1 would lose enhancer-blocking function on the maternal chromosome and therefore allow p57Kip2 to contact the enhancers, whereas the demethylated DMR-Lit1 could function as an enhancer-blocker on the paternal chromosome and hence disturb the interaction between enhancers and p57Kip2. Furthermore, the finding that p57Kip2 (Cdkn1c) and Ipl again failed to express, when maternal methylation of the DMR-Lit1 was impaired by targeting of Dnmt3L (Bourchis et al. 2001), clearly indicates a close relationship between the DMR-Lit1 function and the expression of the clustered imprinted genes. However, the transgenic experiment conducted by John et al. (2001) gave more complicated results. The BAC transgene, which spans 315 kb and includes this DMR-Lit1 and p57Kip2, showed enhanced expression of p57Kip2, but failed to show an imprinted expression of this gene. This result suggests that the existence of DMR-Lit1 is not sufficient for the imprinted expression of p57Kip2, that additional imprinting elements are required for regulation of this subdomain, and that these elements must be extremely distant from p57Kip2.
In general, it is thought that an imprinted gene has the DMR in its control region or at some other location which allows it to be expressed differentially (Ben-Porath and Cedar 2000). We analyzed all CGIs by bisulphite sequencing to search for the DMR in the mouse 7F4/F5 subdomain. To our knowledge, there have been no other attempts to examine the methylation status of one domain over 500 kb by bisulphite sequencing. We identified seven imprinted genes out of eight in the Kip2/Lit1 subdomain (Figs. 1,7). Six of seven imprinted genes had CGIs in their promoters, except for Impt1 (Table 1, Fig. 7). Three genes—p57Kip2, Lit1, and Tssc4—out of six CGI-associated genes had the DMR. However, the other three genes—Nap2, Ipl, and Kvlqt1—had mostly no methylation in their own CGIs, even though these three genes were examined at the time when they showed a monoallelic expression in placenta. It was reported that these genes showed imprinted expression in placenta at 12.5–16.5 dpc depending on the gene analyzed (Qian et al. 1997; Caspary et al. 1998; Engemann et al. 2000), and we analyzed the placenta at 8.5 dpc (Fig. 2) and 13.5 dpc (data not shown); the same results were obtained. These results indicate that imprinted genes are not always associated with the DMR. This implies that there may be a critical CpG other than the CGIs or some other mechanism such as acetylation/deacetylation and methylation in histone protein. Alternatively, it is possible that the DMR is not required in each gene if the DMR-Lit1 controls this domain en bloc.
CGI8 was solely methylated in germ cells. CGI8b was fully methylated in growing oocyte on both alleles, but CGI8a was partially methylated. We believe that the primary imprint exists in CGI8b and that this methylation reaches CGI8a later on, after fertilization, because CGI8a finally acquires the full methylation in mid-embryo, as shown in Figure 2.
When we analyzed CpG methylation in CGI8b by bisulphite sequencing in early embryo, maternal hypomethylation was observed in two-cell, but preferentially maternal methylation reappeared in blastocyst. It is thought that once methylation is acquired in germ cells, it is usually resistant to genome-wide demethylation during the preimplantation embryo in the ICR, as indicated by H19 in mice (Warnecke et al. 1998). In our case, CGI8b was demethylated in two-cell. There are two possible explanations: CGI8b is hypomethylated everywhere, or the methylated region moved elsewhere. With regard to the first possibility, mouse Ndn was demonstrated to be demethylated at the promoter region in blastocyst, although the allelic methylation was maintained through the germ cell, two-cell, four-cell, and morula stages (Hanel and Wevrick 2001). With regard to the second possibility, sites 3 and 4 of region 2 in mouse Igf2r were methylated in two-cell. However, site 3 was demethylated, whereas site 4 was maintained the methylated state in four-cell, implying that the methylated site moved from site 3 to site 4 at this stage (Shemer et al. 1996). Together these results indicate that differential methylation is not yet established during preimplantation embryo, as was the case with our results.
Engemann et al. (2000) analyzed Lit1-related CGI corresponding to CGI8b in early embryo by bisulphite analysis. Their results showed differential methylation in zygote. We analyzed two-cell and blastocyst, but not zygote. Our results showed hypomethylation in two-cell but differential methylation in blastocyst. When all of the results are combined, it turns out that CGI8b is methylated in oocyte, and after fertilization, maternal methylation is maintained in the zygote but disappears in two-cell and gradually reappears in blastocyst. We do not know at present why the pattern of methylation drastically changes during this short period. The region we analyzed was close to the 3′ end of CGI8b, but that examined by Engemann et al. is probably located at the 5′ end of CGI8b.
METHODS
Collection of Tissues and Isolation of DNA and RNA
Mice gametes, early embryos, and adult tissues were collected. Six-week-old females were superovulated by human chorionic gonadotropin (HCG) and mated. Oocytes, two-cell, morula, and blastocysts were collected from the oviducts or uterus by flushing. Oocytes were collected 12 h post-HCG and treated with hyaluronidase in 1 × PBS to remove any remaining cumulus cells, then washed in acidic Tyrodes solution to dissolve the zona pellucida. Oocytes were then repeatedly washed in 1 × PBS. Consequently, the contamination of other diploid cells was nearly zero in oocyte under microscopic observation. Two-cell, morula, and blastocysts were collected around 1.5, 2.5, and 3.5 dpc.
To prepare oocyte DNA, 40–45 pooled oocytes were resuspended in 160 μL of 10 mM Tris-HCl (pH 8.0), 10 mM EDTA, 150 mM NaCl, 0.1% SDS, 2 μg of λDNA, and 40 μg of proteinase K and incubated for 3 h at 55°C, then extracted with phenol-chloroform and precipitated with ethanol. Two-cell, morula, and blastocyst DNAs were prepared with the same procedure from 25, 15, and three embryos, respectively. Sperm DNA was isolated by the two-digestion method as described (Yoshida et al. 1995). Kidney, embryo, and placenta DNAs were isolated using the QIAamp DNA mini Kit (QIAGEN). RNA from mouse tissues was extracted using ISOGEN (NIPPON GENE) according to the manufacturer's instructions.
Computational Sequence Data Analysis
DNA sequence data were derived from AP001293, AP001294, AP001295, AP001287, and AJ251835 (Paulsen et al. 2000; Yatsuki et al. 2000). To identify CGI, GRAIL and CpG percent analyses were performed (http://compbio.ornl.gov/Grail-1.3/, http://www.nih.go.jp/yoken/genebank/cpg_per.html).
Genotyping and Allele Usage
DNA polymorphisms were used to discriminate one allele from another. For this purpose, a laboratory mouse C57BL/6 (B) and a wild mouse PWK (P) were mated, and DNA or RNA from F1 mouse ([C57BL/6 × PWK] F1) (BPF1) was used for the experiment. The opposite cross ([PWK × C57BL/6] F1) (PBF1) was also employed to confirm the experiment.
Sodium Bisulphite Treatment and Sequencing
Sodium bisulphite treatment was carried out as described (Paulin et al. 1998), with some modifications. Two micrograms of DNA samples were denatured in 0.3 M NaOH at 37°C for 30 min. To all samples, 208 μL of 6.2 M urea/bisulphite (pH 5) and 12 μL of 10 mM hydroquinone were added. The samples were incubated at 55°C for 16 h. The treated DNA was purified using GENECLEAN (Bio 101) and eluted with 100 μL of H2O. For DNA desulfonation, 11 μL of 3M NaOH was added, and samples were incubated at 37°C for 15 min. Two micrograms of glycogen were added to the DNA solution, precipitated with 166 μL of 5 M ammonium acetate and three volumes of ethanol, and resuspended in 30 μL of H2O. Oocyte, two-cell, morula, and blastocyst DNAs were finally resuspended in 10 μL of H2O.
PCRs were carried out using bisulphite-treated DNA and each primer set. The following primer pairs were used for amplification, with annealing temperatures shown in parentheses. Materials used for this analysis were somatic tissues such as adult kidney, embryo, placenta, and sperm, except for oocyte and early embryo. For CGI1, CN-BS1/CN-BS2 followed by CN-BS2/CN-BS5 (58.5°C); for CGI2, NI-BS2/NI-BS3 followed by NI-BS1/NI-BS2 (58°C); for CGI3, IP-BS1/IP-BS2 followed by IP-BS1/IP-BS3 (58°C); for CGI4, BS-1F/BS-1R (57°C); for CGI5, KP-BS1/KP-BS2 followed by KP-BS1/KP-BS3 (57°C); for CGI6, CG6-BS2/CG6-BS3 (57.5°C); for CGI7, CG7-BS3/CG7-BS5 followed by CG7-BS2/CG7-BS4 (57°C); for CGI8a, Lit-BS9/Lit-BS10 followed by Lit-BS12/Lit-BS9 (57.5°C); for CGI8b, Lit-BS1/Lit-BS2 followed by Lit-BS4/Lit-BS2 (58°C); for CGI9, Kv-BS1/Kv-BS5 followed by Kv-BS1/Kv-BS2 (58.5°C); for CGI10, ST-BS1/ST-BS2 followed by ST-BS1/ST-BS3 (58.5°C). In particular, analyses of CGI8a and 8b in oocytes and early embryos were carried out as follows. For CGI8a, Lit-BS10/Lit-BS16 was followed by Lit-BS12/Lit-BS16 (56°C); for CGI8b, Lit-BS2/Lit-BS4 was followed by Lit-BS4/Lit-BS21 (57°C). Primer sequences are as follows.
CN-BS1 5′-GTAGATAGAGGGTTAGAAGG-3′,
CN-BS2 5′-CCAACAAAACCACCTACCAT-3′,
CN-BS5 5′-AGAAGGTTTTGAGAAGTAGG-3′,
NI-BS1 5′-TGGGAATTTTGGAGGAGTTG-3′,
NI-BS2 5′-ACCCTACAATACTCAACCAC-3′,
NI-BS3 5′-TAGTAGGGGATTTTTGGGGT-3′,
IP-BS1 5′-ATGGGTAAGGGGTAGTTTGG-3′,
IP-BS2 5′-ACATTCCAAATCCCCTCTCC-3′,
IP-BS3 5′-CATTCACTTTCCCCATACCC-3′,
BS-1F 5′-GTGGTCTTGGACTTCTAGAACACT-3′,
BS-1R 5′-CTATTCTTAAAACCACTACCAAA-3′,
KP-BS1 5′-AGGATTTAGTTGGTAGTAGT-3′,
KP-BS2 5′-TATCCTATCCAACTTAAACC-3′,
KP-BS3 5′-TTTTCAATTTCAACAACACC-3′,
CG6-BS2 5′-ACAACCCCATTATAAAACCC-3′,
CG6-BS3 5′-TGATTTTGGTGGGTTAGAAG-3′,
CG7-BS2 5′-CCTAACCTATACTAAAAACC-3′,
CG7-BS3 5′-AGTTTGTTTGGAGAAGAAAG-3′,
CG7-BS4 5′-GTTGGGGGGTGGGGTAGTTT-3′,
CG7-BS5 5′-ATCCCAAACCAACCCCTATA-3′,
Lit-BS1 5′-GTGTGATTTTATTTGGAGAG-3′,
Lit-BS2 5′-AATCCCCCACACCTAAATTC-3′,
Lit-BS4 5′-TAAGGTGAGTGGTTTAGGAT-3′,
Lit-BS9 5′-CTAACTAATATAACCTCACC-3′,
Lit-BS10 5′-GGTTTAGTTAGGAAGGGATG-3′,
Lit-BS12 5′-GGATGAGGAAGGTAGGTTTT-3′,
Lit-BS16 5′-AACCAAAATACACCATCATA-3′,
Lit-BS21 5′-CCACTATAAACCCACACATA-3′,
Kv-BS1 5′-GGTTGGTGTATTGTAAGTGT-3′,
Kv-BS2 5′-CCCTTCTCACTAAAACTAAC-3′,
Kv-BS5 5′-ACTAACAACAATAACTACCC-3′,
ST-BS1 5′-TTAGGGAGGTTAGTGTTTAT-3′,
ST-BS2 5′-AAACACAAAACCCACACCAC-3′,
ST-BS3 5′-AACTACAAACCTCAACCCTT-3′.
The standard reaction mixture included 1 μL of bisulphite-treated DNA, 2.0 mM MgCl2, 0.2 mM dNTPs, 0.5 μM primers, and 1 unit of LA Taq polymerase (Takara Shuzo) in 10 μL. The amplification protocol was as follows: denaturation at 96°C for 2 min, followed by 45 cycles at 96°C for 30 sec, each annealing temperature for 30 sec, 72°C for 45 sec, and final elongation at 72°C for 3 min with a UNOII thermocycler (Whatman Biometra). The second PCR was performed in 30 cycles. To confirm the bisulphite reaction, the DMR upstream of H19 was used as a control. The methylated and unmethylated CpGs were almost equivalent in kidney, and full methylation was observed in sperm, as has been described (Warnecke et al. 1998).
PCR products were cloned into pT-7-blue vector, and each cloned PCR product was amplified by colony-PCR using a Takara Ex Taq kit (Takara Shuzo) and the following primers: primer1 5′-TCCGGCTCGTATGTTGTGTGGA-3′, primer2 5′-GTGCTGCAAGGCGATTAAGTTGG-3′. The amplified products were treated with exonuclease I and shrimp alkaline phosphatase (Amersham Pharmacia Biotech) and subjected to sequencing reaction using BigDye (Applied Biosystems) and DYEnamicTM ET terminator kits (Amersham Pharmacia Biotech).
RT-PCR and Methylation-Sensitive Enzyme Assay
RNA PCR kit (Takara Shuzo) was used for reverse transcriptase-based cDNA preparation. The following primers were used for the RT-PCR assay: for p57Kip2, KipBF/KipBR (60°C); for Lit1, LchipF/LchipR (64°C). BPF1 DNA was digested with either HpaII or HhaI and then subjected to the methylation-sensitive PCR assay. The following primers were used: for p57Kip2, Kip2aF/Kip2aR (64°C); for Lit1, LchipF/LchipR (64°C).
KipBF 5′-CCGGGTGATGAGCTGGGAAC-3′,
KipBR 5′-AGAGAGGCTGGTCCTTCAGC-3′,
LchipF 5′-GCCGAGTCAGAACGCACTGG-3′,
LchipR 5′-TTCCCAATCCCCCACACCTG-3′,
Kip2aF 5′-GCGTCGCGGTGTCACGTTAC-3′,
Kip2aR 5′-TCGGAGCTTGCCTGCCTGTT-3′.
PCRs were performed using an LA Taq PCR system (Takara Shuzo). To distinguish between two alleles of p57Kip2, 24 bp of deletion and Eco109I RFLP between B6 and PWK were used for the expression assay and the methylation assay, respectively. NciI RFLP was used for Lit1.
Mapping DNase I-Hypersensitive Sites
Preparation of nuclei, DNase I treatment, and purification of the DNA were carried out exactly as described (Sambrook and Russell 2001). Approximately 108 fibroblast cells were used to collect nuclei from the cell (Hu et al. 1997). The primary fibroblast was established from newborn mice of PBF1 or BPF1. We confirmed that at the least, the expression and methylation of Lit1 and p57Kip2 were maintained the same as those of endogenous genes in this system. The DNase I used was RQ1 RNase-free DNase (Promega). The primers used for probe preparation of Southern blotting analyses in CGI8 were the following.
Lit-3 5′-CAGACCTTTGGGAAACCCAC-3′
Lit-5 5′-CTCAGAAGCTACTCCTAGCT-3′.
The probe for the Southern analysis in CGIs 4 and 5 was p57Kip2 cDNA.
WEB SITE REFERENCES
http://compbio.ornl.gov/Grail-1.3/; Gene Recognition and Assembly Internet Link (Version 1.3).
http://www.nih.go.jp/yoken/genebank/cpg_per.html; test program for C+G % + CpG graph.
Acknowledgments
We thank all of the members of the Department of Biochemistry at Saga Medical School. This work was supported by grants-in-aid for Scientific Research on Priority Areas and for JSPS Fellows from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and from the Uehara Memorial Foundation.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL mukait@post.saga-med.ac.jp; FAX 81-952-34-2067.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.110702.
REFERENCES
- Avner P, Heard E. X-chromosome inactivation: Counting, choice and initiation. Nat Rev Genet. 2001;2:59–67. doi: 10.1038/35047580. [DOI] [PubMed] [Google Scholar]
- Baylin SB, Esteller M, Rountree MR, Bachman KE, Schuebel K, Herman JG. Aberrant patterns of DNA methylation, chromatin formation and gene expression in cancer. Hum Mol Genet. 2001;10:687–692. doi: 10.1093/hmg/10.7.687. [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Cedar H. Imprinting: Focusing on the center. Curr Opin Genet Dev. 2000;10:550–554. doi: 10.1016/s0959-437x(00)00126-x. [DOI] [PubMed] [Google Scholar]
- Bourchis D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–2539. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- Caspary T, Cleary MA, Baker CC, Guan XJ, Tilghman SM. Multiple mechanisms regulate imprinting of the mouse distal chromosome 7 gene cluster. Mol Cell Biol. 1998;18:3466–3474. doi: 10.1128/mcb.18.6.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary MA, van Raamsdonk CD, Levorse J, Zheng B, Bradley A, Tilghman SM. Disruption of an imprinted gene cluster by a targeted chromosomal translocation in mice. Nat Genet. 2001;29:78–82. doi: 10.1038/ng715. [DOI] [PubMed] [Google Scholar]
- Dao D, Frank D, Qian N, O'Keefe D, Vosatka RJ, Walsh CP, Tycko B. IMPT1, an imprinted gene similar to polyspecific transporter and multi-drug resistance genes. Hum Mol Genet. 1998;7:597–608. doi: 10.1093/hmg/7.4.597. [DOI] [PubMed] [Google Scholar]
- Engemann S, Strodicke M, Paulsen M, Franck O, Reinhardt R, Lane N, Reik W, Walter J. Sequence and functional comparison in the Beckwith-Wiedemann region: Implications for a novel imprinting centre and extended imprinting. Hum Mol Genet. 2000;9:2691–2706. doi: 10.1093/hmg/9.18.2691. [DOI] [PubMed] [Google Scholar]
- Feinberg AP. The two-domain hypothesis in Beckwith-Wiedemann syndrome. J Clin Invest. 2000;106:739–740. doi: 10.1172/JCI10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith AC, Surani MA. Imprinting and the epigenetic asymmetry between parental genomes. Science. 2001;293:1086–1089. doi: 10.1126/science.1064020. [DOI] [PubMed] [Google Scholar]
- Gould TD, Pfeifer K. Imprinting of mouse Kvlqt1 is developmentally regulated. Hum Mol Genet. 1998;7:483–487. doi: 10.1093/hmg/7.3.483. [DOI] [PubMed] [Google Scholar]
- Hanel ML, Wevrick R. Establishment and maintenance of DNA methylation patterns in mouse Ndn: Implications for maintenance of imprinting in target genes of the imprinting center. Mol Cell Biol. 2001;21:2384–2392. doi: 10.1128/MCB.21.7.2384-2392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hark AT, Tilghman SM. Chromatin conformation of the H19 epigenetic mark. Hum Mol Genet. 1998;7:1979–1985. doi: 10.1093/hmg/7.12.1979. [DOI] [PubMed] [Google Scholar]
- Hatada I, Mukai T. Genomic imprinting of p57KIP2, a cyclin-dependent kinase inhibitor, in mouse. Nat Genet. 1995;11:204–206. doi: 10.1038/ng1095-204. [DOI] [PubMed] [Google Scholar]
- Hatada I, Ohashi H, Fukushima Y, Kaneko Y, Inoue M, Komoto Y, Okada A, Ohishi S, Nabetani A, Morisaki H, et al. An imprinted gene p57KIP2is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996;14:171–173. doi: 10.1038/ng1096-171. [DOI] [PubMed] [Google Scholar]
- Horike S, Mitsuya K, Meguro M, Kotobuki N, Kashiwagi A, Notsu T, Schulz TC, Shirayoshi Y, Oshimura M. Targeted disruption of the human LIT1 locus defines a putative imprinting control element playing an essential role in Beckwith-Wiedemann syndrome. Hum Mol Genet. 2000;9:2075–2083. doi: 10.1093/hmg/9.14.2075. [DOI] [PubMed] [Google Scholar]
- Hu JF, Vu TH, Hoffman AR. Genomic deletion of an imprint maintenance element abolishes imprinting of both insulin-like growth factor II and H19. J Biol Chem. 1997;272:20715–20720. doi: 10.1074/jbc.272.33.20715. [DOI] [PubMed] [Google Scholar]
- John RM, Ainscough JF, Barton SC, Surani MA. Distant cis-elements regulate imprinted expression of the mouse p57Kip2 (Cdkn1c) gene: Implications for the human disorder, Beckwith-Wiedemann syndrome. Hum Mol Genet. 2001;10:1601–1609. doi: 10.1093/hmg/10.15.1601. [DOI] [PubMed] [Google Scholar]
- Kanduri C, Fitzpatrick G, Mukhopadhyay R, Kanduri M, Lobanenkov V, Higgins M, Ohlsson R. A differentially methylated imprinting control region within the Kcnq1 locus harbors a methylation-sensitive chromatin insulator. J Biol Chem. 2002;277:18106–18110. doi: 10.1074/jbc.M200031200. [DOI] [PubMed] [Google Scholar]
- Lee MP, DeBaun M, Randhawa G, Reichard BA, Elledge SJ, Feinberg AP. Low frequency of p57KIP2mutation in Beckwith-Wiedemann syndrome. Am J Hum Genet. 1997;61:304–309. doi: 10.1086/514858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MP, DeBaun MR, Mitsuya K, Galonek HL, Brandenburg S, Oshimura M, Feinberg AP. Loss of imprinting of a paternally expressed transcript, with antisense orientation to KVLQT1, occurs frequently in Beckwith-Wiedemann syndrome and is independent of insulin-like growth factor II imprinting. Proc Natl Acad Sci. 1999;96:5203–5208. doi: 10.1073/pnas.96.9.5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Reik W. Beckwith-Wiedemann syndrome: Imprinting in clusters revisited. J Clin Invest. 2000;105:247–252. doi: 10.1172/JCI9340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuya K, Meguro M, Lee MP, Katoh M, Schulz TC, Kugoh H, Yoshida MA, Niikawa N, Feinberg AP, Oshimura M. LIT1, an imprinted antisense RNA in the human KvLQT1 locus identified by screening for differentially expressed transcripts using monochromosomal hybrids. Hum Mol Genet. 1999;8:1209–1217. doi: 10.1093/hmg/8.7.1209. [DOI] [PubMed] [Google Scholar]
- Morisaki H, Hatada I, Morisaki T, Mukai T. A novel gene, ITM, located between p57Kip2 and IPL, is imprinted in mice. DNA Res. 1998;5:235–240. doi: 10.1093/dnares/5.4.235. [DOI] [PubMed] [Google Scholar]
- O'Keefe D, Dao D, Zhao L, Sanderson R, Warburton D, Weiss L, Anyane-Yeboa K, Tycko B. Coding mutations in p57KIP2 are present in some cases of Beckwith-Wiedemann syndrome but are rare or absent in Wilms tumors. Am J Hum Genet. 1997;61:295–303. doi: 10.1086/514854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango P, Miller W, Lehoczky J, Leung CT, Birren B, Wheelan S, Dewar K, Feinberg AP. Sequence and comparative analysis of the mouse 1-megabase region orthologous to the human 11p15 imprinted domain. Genome Res. 2000;10:1697–1710. doi: 10.1101/gr.161800. [DOI] [PubMed] [Google Scholar]
- Paulin R, Grigg GW, Davey MW, Piper AA. Urea improves efficiency of bisulphite-mediated sequencing of 5′-methylcytosine in genomic DNA. Nucleic Acids Res. 1998;26:5009–5010. doi: 10.1093/nar/26.21.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen M, Davies KR, Bowden LM, Villar AJ, Franck O, Fuermann M, Dean WL, Moore TF, Rodrigues N, Davies KE, et al. Syntenic organization of the mouse distal chromosome 7 imprinting cluster and the Beckwith-Wiedemann syndrome region in chromosome 11p15.5. Hum Mol Genet. 1998;7:1149–1159. doi: 10.1093/hmg/7.7.1149. [DOI] [PubMed] [Google Scholar]
- Paulsen M, El-Maarri O, Engemann S, Strodicke M, Franck O, Davies K, Reinhardt R, Reik W, Walter J. Sequence conservation and variability of imprinting in the Beckwith-Wiedemann syndrome gene cluster in human and mouse. Hum Mol Genet. 2000;9:1829–1841. doi: 10.1093/hmg/9.12.1829. [DOI] [PubMed] [Google Scholar]
- Qian N, Frank D, O'Keefe D, Dao D, Zhao L, Yuan L, Wang Q, Keating M, Walsh C, Tycko B. The IPL gene on chromosome 11p15.5 is imprinted in humans and mice and is similar to TDAG51, implicated in Fas expression and apoptosis. Hum Mol Genet. 1997;6:2021–2029. doi: 10.1093/hmg/6.12.2021. [DOI] [PubMed] [Google Scholar]
- Razin A, Cedar H. DNA methylation and gene expression. Microbiol Rev. 1991;55:451–458. doi: 10.1128/mr.55.3.451-458.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reik W, Maher ER. Imprinting in clusters: Lessons from Beckwith-Wiedemann syndrome. Trends Genet. 1997;13:330–334. doi: 10.1016/s0168-9525(97)01200-6. [DOI] [PubMed] [Google Scholar]
- Reik W, Walter J. Genomic imprinting: Parental influence on the genome. Nat Rev Genet. 2001;2:21–32. doi: 10.1038/35047554. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: A laboratory manual, 3rd ed., chapter 17. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schweizer J, Zynger D, Francke U. In vivo nuclease hypersensitivity studies reveal multiple sites of parental origin-dependent differential chromatin conformation in the 150 kb SNRPN transcription unit. Hum Mol Genet. 1999;8:555–566. doi: 10.1093/hmg/8.4.555. [DOI] [PubMed] [Google Scholar]
- Shemer R, Birger Y, Dean WL, Reik W, Riggs AD, Razin A. Dynamic methylation adjustment and counting as part of imprinting mechanisms. Proc Natl Acad Sci. 1996;93:6371–6376. doi: 10.1073/pnas.93.13.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemer R, Birger Y, Riggs AD, Razin A. Structure of the imprinted mouse Snrpn gene and establishment of its parental-specific methylation pattern. Proc Natl Acad Sci. 1997;94:10267–10272. doi: 10.1073/pnas.94.19.10267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilinich NJ, Day CD, Fitzpatrick GV, Caldwell GM, Lossie AC, Cooper PR, Smallwood AC, Joyce JA, Schofield PN, Reik W, et al. A maternally methylated CpG island in KvLQT1 is associated with an antisense paternal transcript and loss of imprinting in Beckwith-Wiedemann syndrome. Proc Natl Acad Sci. 1999;96:8064–8069. doi: 10.1073/pnas.96.14.8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöger R, Kubicka P, Liu CG, Kafri T, Razin A, Cedar H, Barlow DP. Maternal-specific methylation of the imprinted mouse Igf2r locus identifies the expressed locus as carrying the imprinting signal. Cell. 1993;73:61–71. doi: 10.1016/0092-8674(93)90160-r. [DOI] [PubMed] [Google Scholar]
- Sun FL, Dean WL, Kelsey G, Allen ND, Reik W. Transactivation of Igf2 in a mouse model of Beckwith-Wiedemann syndrome. Nature. 1997;389:809–815. doi: 10.1038/39797. [DOI] [PubMed] [Google Scholar]
- Thorvaldsen JL, Duran KL, Bartolomei MS. Deletion of the H19 differentially methylated domain results in loss of imprinted expression of H19 and Igf2. Genes & Dev. 1998;12:3693–3702. doi: 10.1101/gad.12.23.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman SM. The sins of the fathers and mothers: Genomic imprinting in mammalian development. Cell. 1999;96:185–193. doi: 10.1016/s0092-8674(00)80559-0. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Saam JR, Ingram RS, Tilghman SM, Bartolomei MS. A paternal-specific methylation imprint marks the alleles of the mouse H19 gene. Nat Genet. 1995;9:407–413. doi: 10.1038/ng0495-407. [DOI] [PubMed] [Google Scholar]
- Warnecke PM, Mann JR, Frommer M, Clark SJ. Bisulphite sequencing in preimplantation embryos: DNA methylation profile of the upstream region of the mouse imprinted H19 gene. Genomics. 1998;51:182–190. doi: 10.1006/geno.1998.5371. [DOI] [PubMed] [Google Scholar]
- Yatsuki H, Watanabe H, Hattori M, Joh K, Soejima H, Komoda H, Xin Z, Zhu X, Higashimoto K, Nishimura M, et al. Sequence-based structural features between Kvlqt1 and Tapa1 on mouse chromosome 7F4/F5 corresponding to the Beckwith-Wiedemann syndrome region on human 11p15.5: Long-stretches of unusually well conserved intronic sequences of kvlqt1 between mouse and human. DNA Res. 2000;7:195–206. doi: 10.1093/dnares/7.3.195. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Sekiguchi K, Mizuno N, Kasai K, Sakai I, Sato H, Seta S. The modified method of two-step differential extraction of sperm and vaginal epithelial cell DNA from vaginal fluid mixed with semen. Forensic Sci Int. 1995;72:25–33. doi: 10.1016/0379-0738(94)01668-u. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. Altered cell differentiation and proliferation in mice lacking p57Kip2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]