Abstract
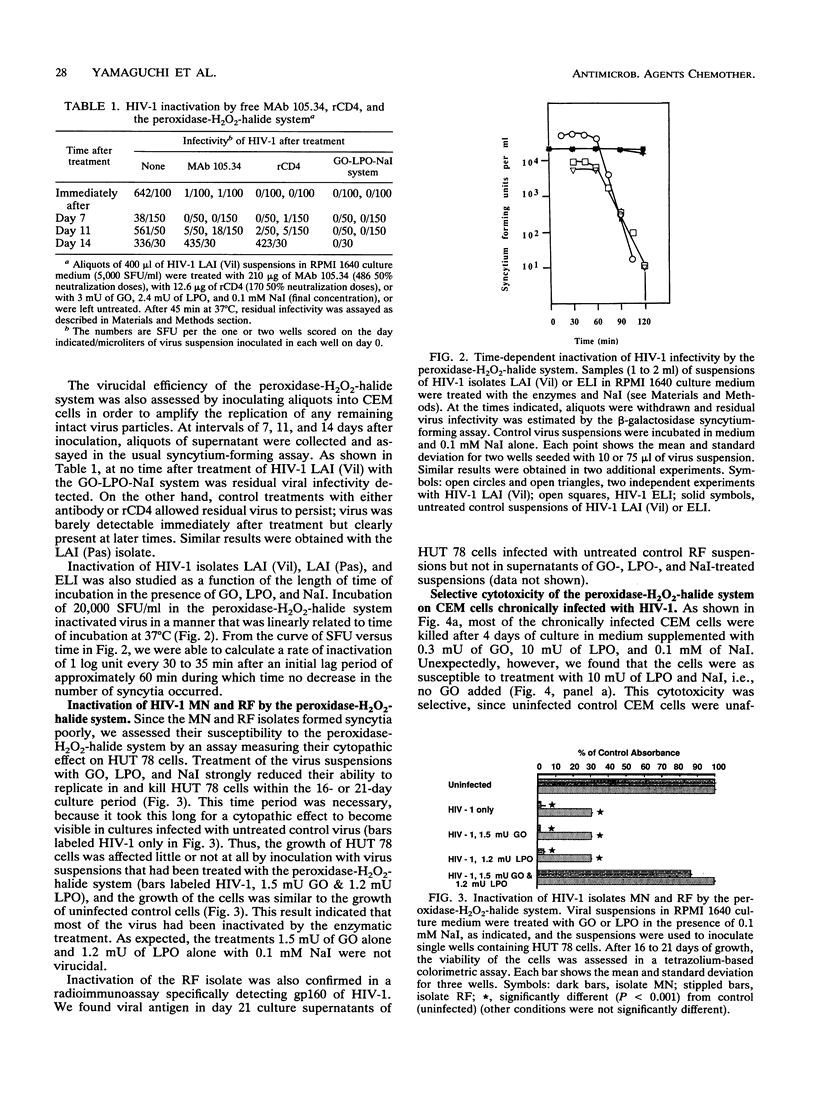
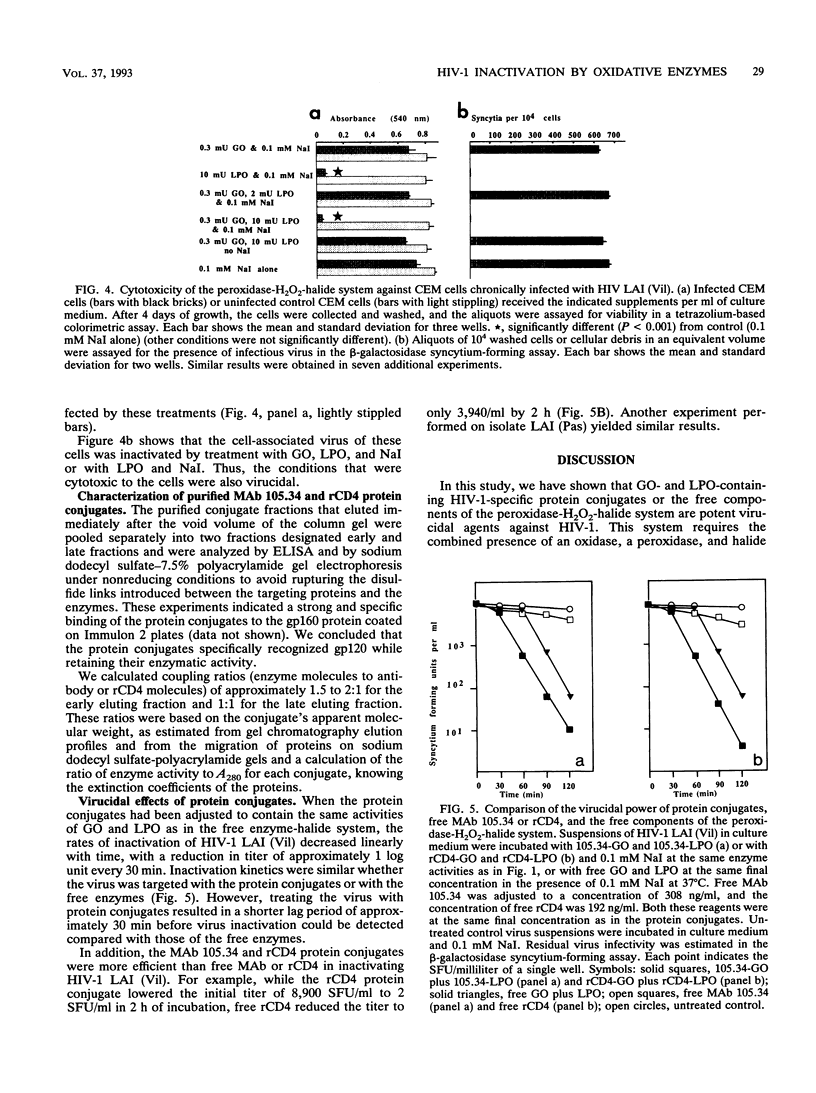
Glucose oxidase and peroxidase (lactoperoxidase or myeloperoxidase) are virucidal to human immunodeficiency virus type 1 (HIV-1) in the presence of sodium iodide, as assessed by the loss of viral replication in a syncytium-forming assay or by the inhibition of cytopathic effects on infected cells. In the presence of low concentrations of sodium iodide, five HIV-1 isolates were equally susceptible to this virucidal system at enzyme concentrations of a few milliunits. The loss of viral replication was linearly related to the time of incubation in the enzyme solutions, with an inactivation rate of 1 log unit every 30 min. These enzymes and this halide were also cytotoxic to chronically infected, but not to uninfected, cultured CEM cells. Protein conjugates were prepared by using the enzymes and murine antibody 105.34, which recognized the V3 loop of HIV-1 LAI isolate surface glycoprotein, or recombinant human CD4. The protein conjugates inactivated free virus at rates similar to those of the free enzymes and were more effective than antibody or recombinant CD4 alone. These in vitro findings demonstrate that the peroxidase-H2O2-halide system provides potent virucidal activity against HIV-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belding M. E., Klebanoff S. J., Ray C. G. Peroxidase-mediated virucidal systems. Science. 1970 Jan 9;167(3915):195–196. doi: 10.1126/science.167.3915.195. [DOI] [PubMed] [Google Scholar]
- Casentini-Borocz D., Bringman T. Enzyme immunoconjugates utilizing glucose oxidase and myeloperoxidase are cytotoxic to Candida tropicalis. Antimicrob Agents Chemother. 1990 May;34(5):875–880. doi: 10.1128/aac.34.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase M. J., Klebanoff S. J. Viricidal effect of stimulated human mononuclear phagocytes on human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5582–5585. doi: 10.1073/pnas.89.12.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Mizukami T., Fuerst T. R., FitzGerald D. J., Moss B., Pastan I., Berger E. A. Selective killing of HIV-infected cells by recombinant human CD4-Pseudomonas exotoxin hybrid protein. Nature. 1988 Sep 22;335(6188):369–372. doi: 10.1038/335369a0. [DOI] [PubMed] [Google Scholar]
- Chouchane L., Bringman T., Barbier S., Traincard F., Strosberg A. D. Targeted killing of yeast expressing a HIV-1 peptide by antibody-conjugated glucose oxidase and horseradish peroxidase. Immunol Lett. 1990 Sep;25(4):359–365. doi: 10.1016/0165-2478(90)90208-8. [DOI] [PubMed] [Google Scholar]
- Demignot S., Domurado D. Effect of prosthetic sugar groups on the pharmacokinetics of glucose-oxidase. Drug Des Deliv. 1987 May;1(4):333–348. [PubMed] [Google Scholar]
- Dionysius D. A., Grieve P. A., Vos A. C. Studies on the lactoperoxidase system: reaction kinetics and antibacterial activity using two methods for hydrogen peroxide generation. J Appl Bacteriol. 1992 Feb;72(2):146–153. doi: 10.1111/j.1365-2672.1992.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Eck H. P., Gmünder H., Hartmann M., Petzoldt D., Daniel V., Dröge W. Low concentrations of acid-soluble thiol (cysteine) in the blood plasma of HIV-1-infected patients. Biol Chem Hoppe Seyler. 1989 Feb;370(2):101–108. doi: 10.1515/bchm3.1989.370.1.101. [DOI] [PubMed] [Google Scholar]
- Fultz P. N. Components of saliva inactivate human immunodeficiency virus. Lancet. 1986 Nov 22;2(8517):1215–1215. doi: 10.1016/s0140-6736(86)92218-x. [DOI] [PubMed] [Google Scholar]
- Ito H., Morizet J., Coulombel L., Stanislawski M. T cell depletion of human bone marrow using an oxidase-peroxidase enzyme immunotoxin. Bone Marrow Transplant. 1990 Dec;6(6):395–398. [PubMed] [Google Scholar]
- Kim Y. W., Fung M. S., Sun N. C., Sun C. R., Chang N. T., Chang T. W. Immunoconjugates that neutralize HIV virions kill T cells infected with diverse strains of HIV-1. J Immunol. 1990 Feb 15;144(4):1257–1262. [PubMed] [Google Scholar]
- Klebanoff S. J., Coombs R. W. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role of the myeloperoxidase system. J Clin Invest. 1992 Jun;89(6):2014–2017. doi: 10.1172/JCI115810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klebanoff S. J. Iodination of bacteria: a bactericidal mechanism. J Exp Med. 1967 Dec 1;126(6):1063–1078. doi: 10.1084/jem.126.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K., Watanabe T. Distribution of oncodevelopmental markers in neoplastic cells: therapeutic implications. J Histochem Cytochem. 1984 Aug;32(8):894–898. doi: 10.1177/32.8.6747276. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Hatch W. C., Dunlop N. M., Robey W. G., Arthur L. O., Gonda M. A., Fischinger P. J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987 Fall;3(3):283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- Pincus S. H., Wehrly K., Chesebro B. Treatment of HIV tissue culture infection with monoclonal antibody-ricin A chain conjugates. J Immunol. 1989 May 1;142(9):3070–3075. [PubMed] [Google Scholar]
- Pourtois M., Binet C., Van Tieghem N., Courtois P. R., Vandenabbeele A., Thirty L. Saliva can contribute in quick inhibition of HIV infectivity. AIDS. 1991 May;5(5):598–600. [PubMed] [Google Scholar]
- Rocancourt D., Bonnerot C., Jouin H., Emerman M., Nicolas J. F. Activation of a beta-galactosidase recombinant provirus: application to titration of human immunodeficiency virus (HIV) and HIV-infected cells. J Virol. 1990 Jun;64(6):2660–2668. doi: 10.1128/jvi.64.6.2660-2668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roederer M., Staal F. J., Osada H., Herzenberg L. A., Herzenberg L. A. CD4 and CD8 T cells with high intracellular glutathione levels are selectively lost as the HIV infection progresses. Int Immunol. 1991 Sep;3(9):933–937. doi: 10.1093/intimm/3.9.933. [DOI] [PubMed] [Google Scholar]
- Stanislawski M., Rousseau V., Goavec M., Ito H. Immunotoxins containing glucose oxidase and lactoperoxidase with tumoricidal properties: in vitro killing effectiveness in a mouse plasmacytoma cell model. Cancer Res. 1989 Oct 15;49(20):5497–5504. [PubMed] [Google Scholar]
- Till M. A., Ghetie V., Gregory T., Patzer E. J., Porter J. P., Uhr J. W., Capon D. J., Vitetta E. S. HIV-infected cells are killed by rCD4-ricin A chain. Science. 1988 Nov 25;242(4882):1166–1168. doi: 10.1126/science.2847316. [DOI] [PubMed] [Google Scholar]
- Wain-Hobson S., Vartanian J. P., Henry M., Chenciner N., Cheynier R., Delassus S., Martins L. P., Sala M., Nugeyre M. T., Guétard D. LAV revisited: origins of the early HIV-1 isolates from Institut Pasteur. Science. 1991 May 17;252(5008):961–965. doi: 10.1126/science.2035026. [DOI] [PubMed] [Google Scholar]
- Zarling J. M., Moran P. A., Haffar O., Sias J., Richman D. D., Spina C. A., Myers D. E., Kuebelbeck V., Ledbetter J. A., Uckun F. M. Inhibition of HIV replication by pokeweed antiviral protein targeted to CD4+ cells by monoclonal antibodies. Nature. 1990 Sep 6;347(6288):92–95. doi: 10.1038/347092a0. [DOI] [PubMed] [Google Scholar]


