Abstract
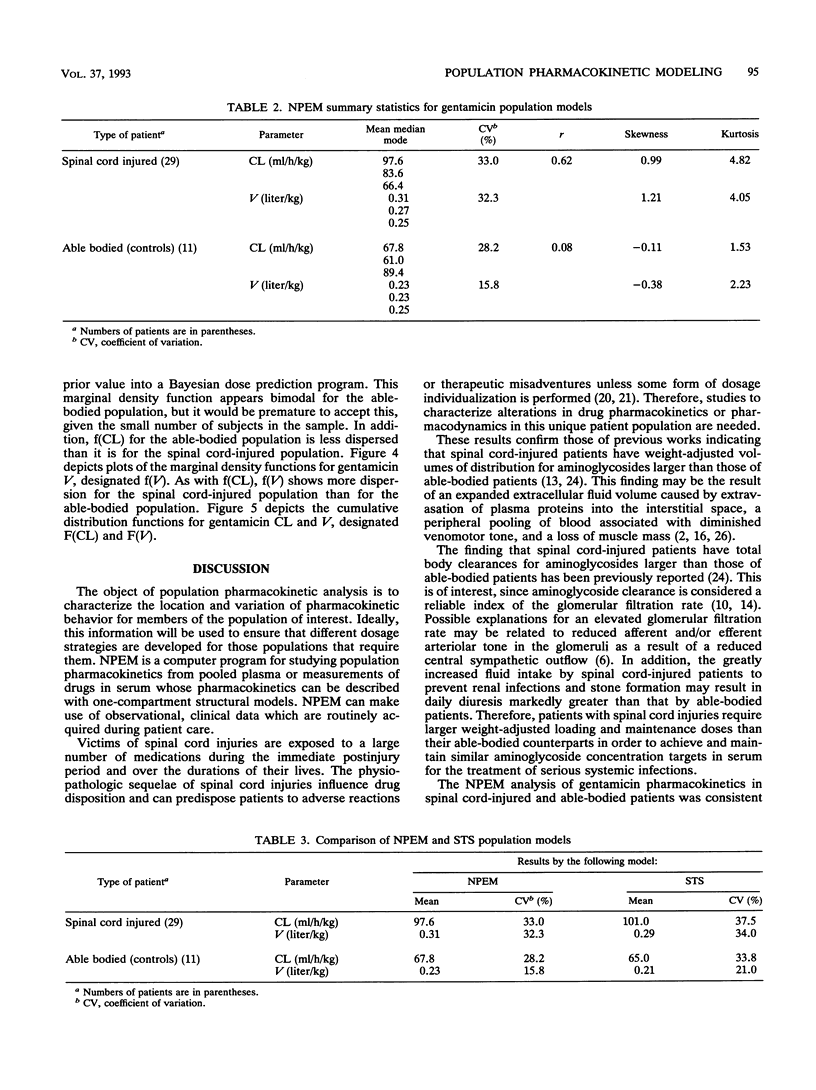
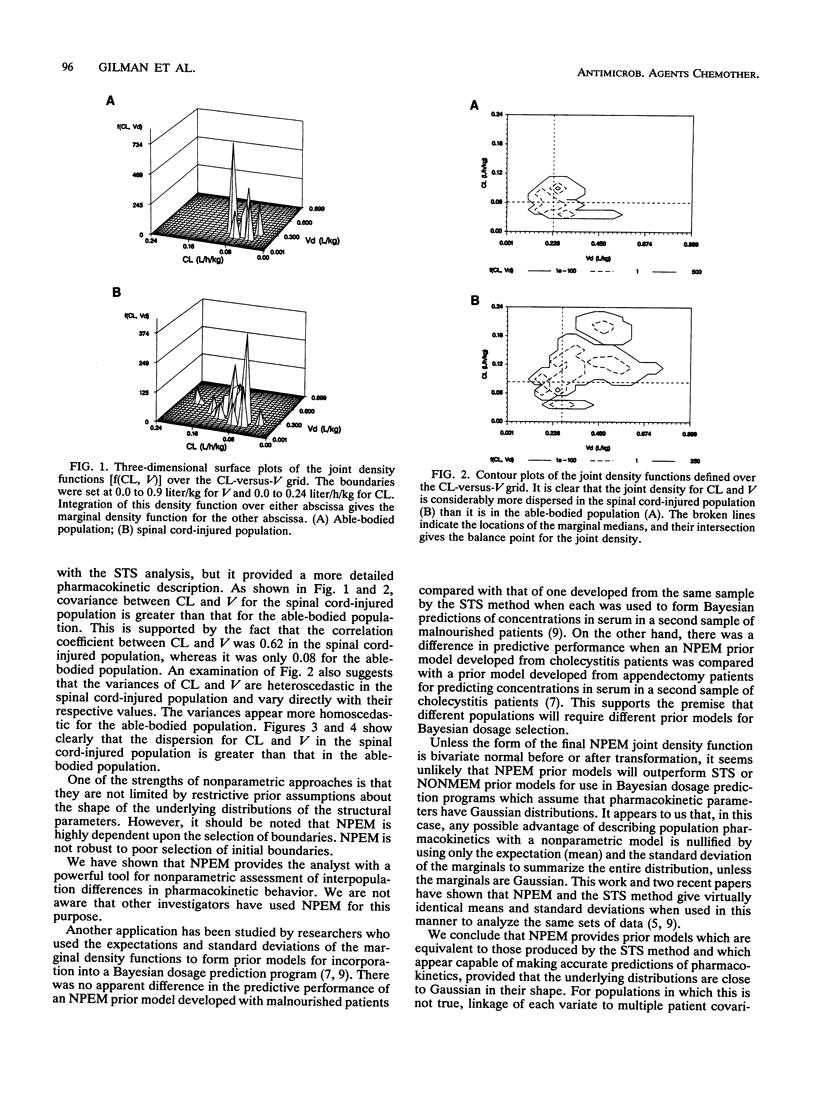
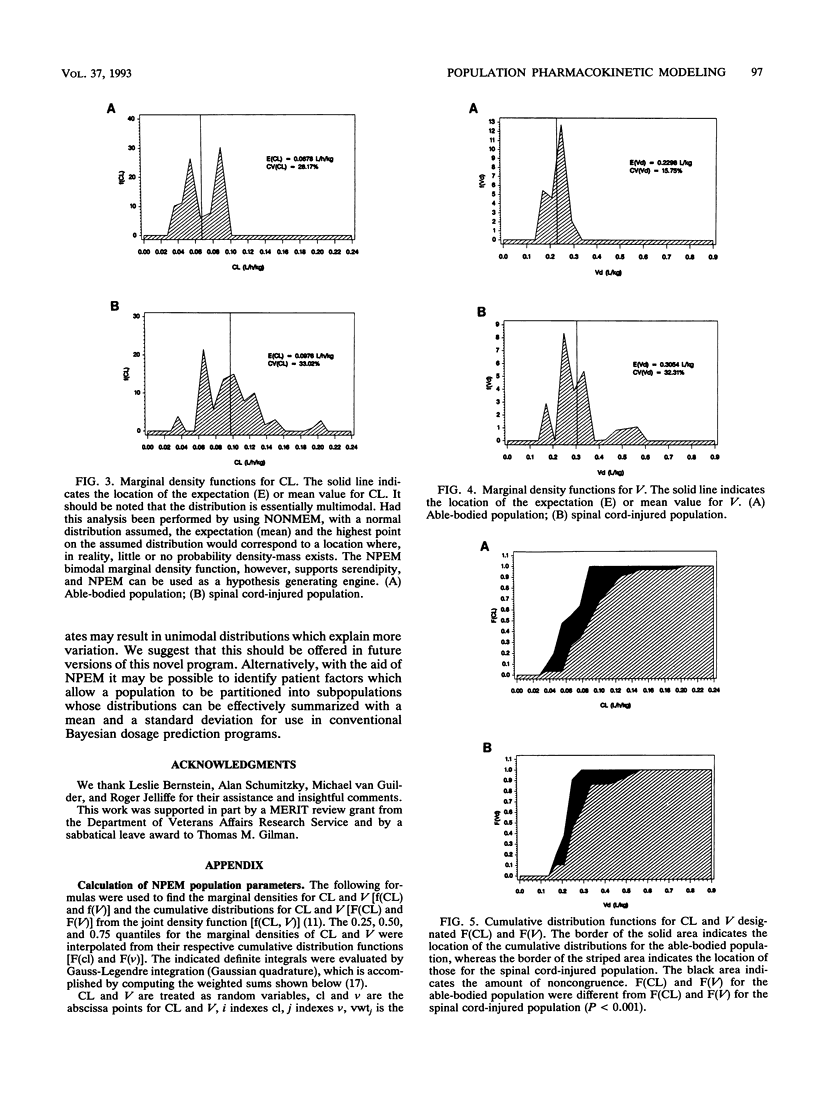
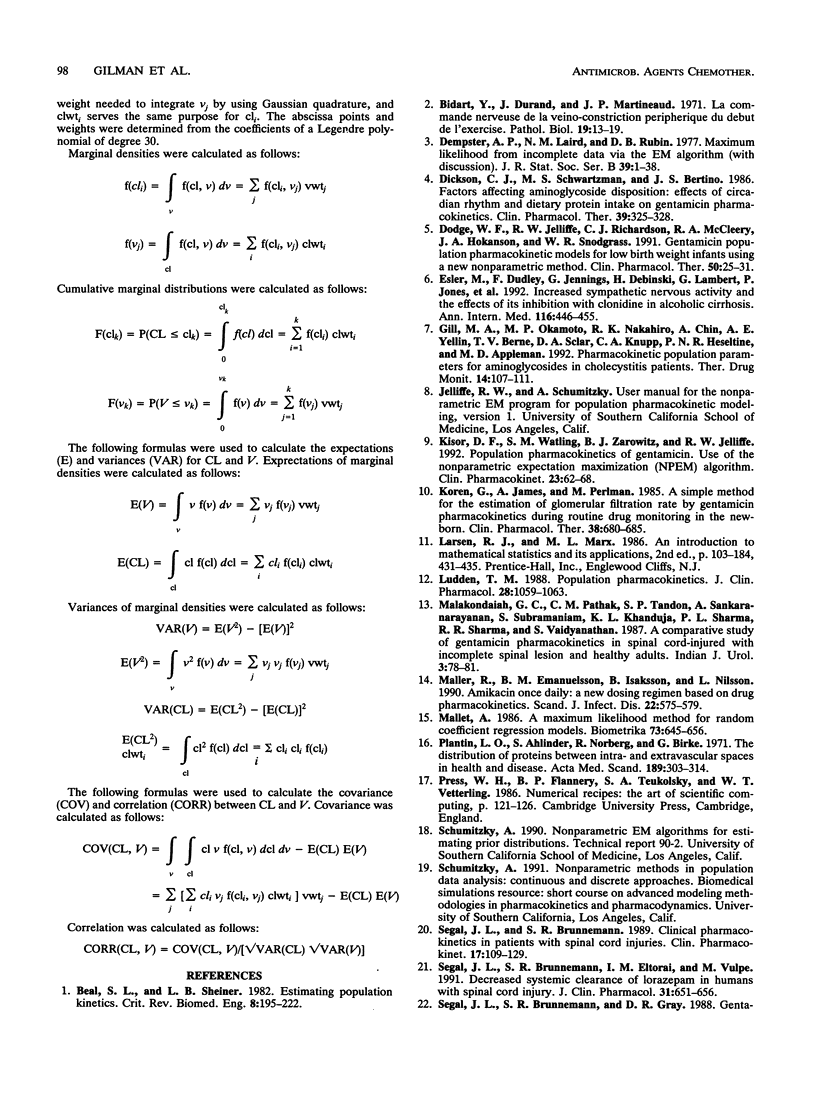
Population pharmacokinetic models for gentamicin were developed by using data obtained from 29 spinal cord-injured patients and 11 able-bodied control patients. With a one-compartment model, the population parameters were clearance (CL), volume of distribution (V), and their associated variances. Parameter estimates were found by using the computer program NPEM and by the standard two-stage (STS) method. NPEM uses a nonparametric approach incorporating the expectation maximization algorithm to evaluate a joint probability density function at 900 intersections over a bivariate grid. In contrast, the STS method requires conventional assumptions of normality for the underlying distributions. For NPEM, the mean CL was 97.6 ml/h/kg of body weight (coefficient of variation, 33.0% in the spinal cord-injured patients and 67.8 ml/h/kg +/- 28.2% in the able-bodied patients; the mean V was 0.31 liter/kg +/- 32.3% in the spinal cord-injured patients and 0.23 liter/kg +/- 15.8% in the able-bodied patients. For STS, the mean CL was 101.0 ml/h/kg +/- 37.5% in the spinal cord-injured patients and 65.0 ml/h/kg +/- 33.8% in the able-bodied patients; the mean V was 0.29 liter/kg +/- 34.0% in the spinal cord-injured patients and 0.21 liter/kg +/- 21.0% in the able-bodied patients. Although the means and variances found by NPEM and the STS method were similar, the NPEM analysis revealed that the distributions of CL and V, even after they were linked to weight, were positively skewed and kurtotic. The cumulative distribution functions for CL (P < 0.001) and V (P < 0.001) in spinal cord-injured patients were different from those in able-bodied patients. Unique population models are required for the initial dosage selection for spinal cord-injured patients. Future approaches for developing population models should allow the linkage of structural parameters to multiple patient covariates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beal S. L., Sheiner L. B. Estimating population kinetics. Crit Rev Biomed Eng. 1982;8(3):195–222. [PubMed] [Google Scholar]
- Bidart Y., Durand J., Martineaud J. P. La commande nerveuse de la veino-constriction périphérique du début de l'exercice. Etude comparative chez l'homme normal et chez le paraplégique par section de la moelle dorsale. Pathol Biol (Paris) 1971 Jan;19(1):13–19. [PubMed] [Google Scholar]
- Dickson C. J., Schwartzman M. S., Bertino J. S., Jr Factors affecting aminoglycoside disposition: effects of circadian rhythm and dietary protein intake on gentamicin pharmacokinetics. Clin Pharmacol Ther. 1986 Mar;39(3):325–328. doi: 10.1038/clpt.1986.47. [DOI] [PubMed] [Google Scholar]
- Dodge W. F., Jelliffe R. W., Richardson C. J., McCleery R. A., Hokanson J. A., Snodgrass W. R. Gentamicin population pharmacokinetic models for low birth weight infants using a new nonparametric method. Clin Pharmacol Ther. 1991 Jul;50(1):25–31. doi: 10.1038/clpt.1991.100. [DOI] [PubMed] [Google Scholar]
- Esler M., Dudley F., Jennings G., Debinski H., Lambert G., Jones P., Crotty B., Colman J., Willett I. Increased sympathetic nervous activity and the effects of its inhibition with clonidine in alcoholic cirrhosis. Ann Intern Med. 1992 Mar 15;116(6):446–455. doi: 10.7326/0003-4819-116-6-446. [DOI] [PubMed] [Google Scholar]
- Gill M. A., Okamoto M. P., Nakahiro R. K., Chin A., Inagaki K., Sclar D. A. Pharmacokinetic population parameters for aminoglycosides in cholecystitis patients. Ther Drug Monit. 1992 Apr;14(2):107–111. doi: 10.1097/00007691-199204000-00005. [DOI] [PubMed] [Google Scholar]
- Kisor D. F., Watling S. M., Zarowitz B. J., Jelliffe R. W. Population pharmacokinetics of gentamicin. Use of the nonparametric expectation maximisation (NPEM) algorithm. Clin Pharmacokinet. 1992 Jul;23(1):62–68. doi: 10.2165/00003088-199223010-00005. [DOI] [PubMed] [Google Scholar]
- Koren G., James A., Perlman M. A simple method for the estimation of glomerular filtration rate by gentamicin pharmacokinetics during routine drug monitoring in the newborn. Clin Pharmacol Ther. 1985 Dec;38(6):680–685. doi: 10.1038/clpt.1985.245. [DOI] [PubMed] [Google Scholar]
- Ludden T. M. Population pharmacokinetics. J Clin Pharmacol. 1988 Dec;28(12):1059–1063. doi: 10.1002/j.1552-4604.1988.tb05714.x. [DOI] [PubMed] [Google Scholar]
- Maller R., Emanuelsson B. M., Isaksson B., Nilsson L. Amikacin once daily: a new dosing regimen based on drug pharmacokinetics. Scand J Infect Dis. 1990;22(5):575–579. doi: 10.3109/00365549009027099. [DOI] [PubMed] [Google Scholar]
- Plantin L. O., Ahlinder S., Norberg R., Birke G. The distribution of proteins between intra- and extravascular spaces in health and disease. Acta Med Scand. 1971 Apr;189(4):309–314. doi: 10.1111/j.0954-6820.1971.tb04381.x. [DOI] [PubMed] [Google Scholar]
- Segal J. L., Brunnemann S. R. Clinical pharmacokinetics in patients with spinal cord injuries. Clin Pharmacokinet. 1989 Aug;17(2):109–129. doi: 10.2165/00003088-198917020-00004. [DOI] [PubMed] [Google Scholar]
- Segal J. L., Brunnemann S. R., Eltorai I. M., Vulpe M. Decreased systemic clearance of lorazepam in humans with spinal cord injury. J Clin Pharmacol. 1991 Jul;31(7):651–656. doi: 10.1002/j.1552-4604.1991.tb03751.x. [DOI] [PubMed] [Google Scholar]
- Segal J. L., Brunnemann S. R., Gray D. R. Gentamicin bioavailability and single-dose pharmacokinetics in spinal cord injury. Drug Intell Clin Pharm. 1988 Jun;22(6):461–465. doi: 10.1177/106002808802200603. [DOI] [PubMed] [Google Scholar]
- Segal J. L., Gray D. R., Gordon S. K., Eltorai I. M., Khonsari F., Patel J. Gentamicin disposition kinetics in humans with spinal cord injury. Paraplegia. 1985 Feb;23(1):47–55. doi: 10.1038/sc.1985.8. [DOI] [PubMed] [Google Scholar]
- Sheiner L. B. The population approach to pharmacokinetic data analysis: rationale and standard data analysis methods. Drug Metab Rev. 1984;15(1-2):153–171. doi: 10.3109/03602538409015063. [DOI] [PubMed] [Google Scholar]
- Shizgal H. M., Roza A., Leduc B., Drouin G., Villemure J. G., Yaffe C. Body composition in quadriplegic patients. JPEN J Parenter Enteral Nutr. 1986 Jul-Aug;10(4):364–368. doi: 10.1177/0148607186010004364. [DOI] [PubMed] [Google Scholar]
- Steimer J. L., Mallet A., Golmard J. L., Boisvieux J. F. Alternative approaches to estimation of population pharmacokinetic parameters: comparison with the nonlinear mixed-effect model. Drug Metab Rev. 1984;15(1-2):265–292. doi: 10.3109/03602538409015066. [DOI] [PubMed] [Google Scholar]
- Whiting B., Kelman A. W., Grevel J. Population pharmacokinetics. Theory and clinical application. Clin Pharmacokinet. 1986 Sep-Oct;11(5):387–401. doi: 10.2165/00003088-198611050-00004. [DOI] [PubMed] [Google Scholar]


