Abstract
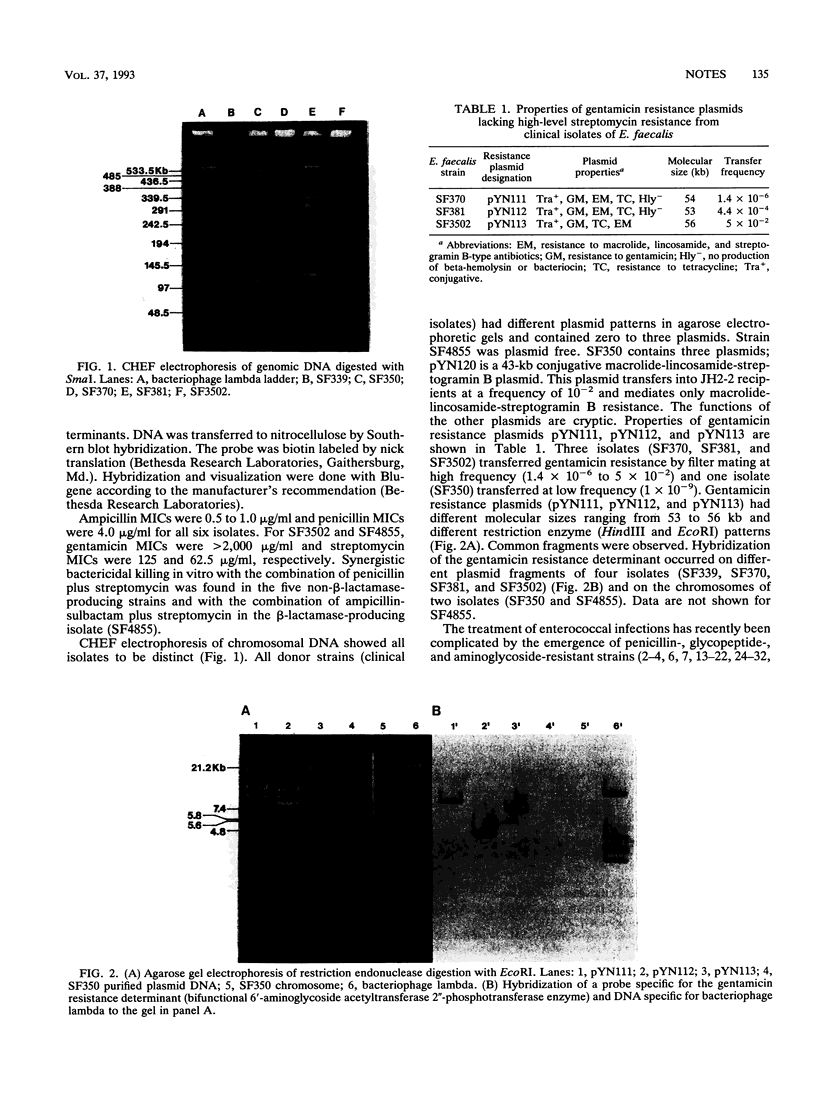
Antimicrobial susceptibilities and DNA contents were analyzed for six clinical isolates of Enterococcus faecalis that had high-level resistance to gentamicin (MIC > 2,000 micrograms/ml) but not streptomycin and were obtained from patients in diverse geographic areas. Contour-clamped homogeneous electric field electrophoresis of genomic DNA showed all isolates to be different strains. Gentamicin resistance was transferred from four isolates to plasmid-free enterococcal recipients in filter matings. Restriction enzyme analysis of transconjugants showed distinct gentamicin resistance plasmids. A probe specific for the gentamicin resistance determinant hybridized to the plasmids of four isolates and to the chromosomes of two isolates. These findings suggest that clonal dissemination is not responsible for the spread of these resistant strains, that resistance determinants occur on different plasmids as well as on the chromosome of E. faecalis, and that the genetic determinants of resistance are related.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush L. M., Calmon J., Cherney C. L., Wendeler M., Pitsakis P., Poupard J., Levison M. E., Johnson C. C. High-level penicillin resistance among isolates of enterococci. Implications for treatment of enterococcal infections. Ann Intern Med. 1989 Apr 1;110(7):515–520. doi: 10.7326/0003-4819-110-7-515. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Movable genetic elements and antibiotic resistance in enterococci. Eur J Clin Microbiol Infect Dis. 1990 Feb;9(2):90–102. doi: 10.1007/BF01963632. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981 Sep;45(3):409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Dunny G. M., Schultz S. K. Characterization of three plasmid deoxyribonucleic acid molecules in a strain of Streptococcus faecalis: identification of a plasmid determining erythromycin resistance. J Bacteriol. 1974 Jan;117(1):283–289. doi: 10.1128/jb.117.1.283-289.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Zighelboim-Daum S., Reiszner E., Goldmann D., Moellering R. C., Jr High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob Agents Chemother. 1988 Oct;32(10):1528–1532. doi: 10.1128/aac.32.10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam R. R., Collins M. D. Identification of Enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989 Apr;27(4):731–734. doi: 10.1128/jcm.27.4.731-734.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes B. A., Schaberg D. R. Transfer of resistance plasmids from Staphylococcus epidermidis to Staphylococcus aureus: evidence for conjugative exchange of resistance. J Bacteriol. 1983 Feb;153(2):627–634. doi: 10.1128/jb.153.2.627-634.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob Agents Chemother. 1991 Jun;35(6):1147–1152. doi: 10.1128/aac.35.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A. H., Gilligan P. H., Facklam R. R. Recovery of resistant enterococci during vancomycin prophylaxis. J Clin Microbiol. 1988 Jun;26(6):1216–1218. doi: 10.1128/jcm.26.6.1216-1218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinberg A. N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971 May;77(5):821–828. [PubMed] [Google Scholar]
- Murray B. E., Lopardo H. A., Rubeglio E. A., Frosolono M., Singh K. V. Intrahospital spread of a single gentamicin-resistant, beta-lactamase-producing strain of Enterococcus faecalis in Argentina. Antimicrob Agents Chemother. 1992 Jan;36(1):230–232. doi: 10.1128/aac.36.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Markowitz S. M., Lopardo H. A., Patterson J. E., Zervos M. J., Rubeglio E., Eliopoulos G. M., Rice L. B., Goldstein F. W. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J Infect Dis. 1991 Apr;163(4):780–785. doi: 10.1093/infdis/163.4.780. [DOI] [PubMed] [Google Scholar]
- Nachamkin I., Axelrod P., Talbot G. H., Fischer S. H., Wennersten C. B., Moellering R. C., Jr, MacGregor R. R. Multiply high-level-aminoglycoside-resistant enterococci isolated from patients in a university hospital. J Clin Microbiol. 1988 Jul;26(7):1287–1291. doi: 10.1128/jcm.26.7.1287-1291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noskin G. A., Till M., Patterson B. K., Clarke J. T., Warren J. R. High-level gentamicin resistance in Enterococcus faecalis bacteremia. J Infect Dis. 1991 Dec;164(6):1212–1215. doi: 10.1093/infdis/164.6.1212. [DOI] [PubMed] [Google Scholar]
- Oster S. E., Chirurgi V. A., Goldberg A. A., Aiken S., McCabe R. E. Ampicillin-resistant enterococcal species in an acute-care hospital. Antimicrob Agents Chemother. 1990 Sep;34(9):1821–1823. doi: 10.1128/aac.34.9.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Masecar B. L., Kauffman C. A., Schaberg D. R., Hierholzer W. J., Jr, Zervos M. J. Gentamicin resistance plasmids of enterococci from diverse geographic areas are heterogeneous. J Infect Dis. 1988 Jul;158(1):212–216. doi: 10.1093/infdis/158.1.212. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Masecar B. L., Zervos M. J. Characterization and comparison of two penicillinase-producing strains of Streptococcus (Enterococcus) faecalis. Antimicrob Agents Chemother. 1988 Jan;32(1):122–124. doi: 10.1128/aac.32.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Wanger A., Zscheck K. K., Zervos M. J., Murray B. E. Molecular epidemiology of beta-lactamase-producing enterococci. Antimicrob Agents Chemother. 1990 Feb;34(2):302–305. doi: 10.1128/aac.34.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Zervos M. J. High-level gentamicin resistance in Enterococcus: microbiology, genetic basis, and epidemiology. Rev Infect Dis. 1990 Jul-Aug;12(4):644–652. doi: 10.1093/clinids/12.4.644. [DOI] [PubMed] [Google Scholar]
- Rhinehart E., Smith N. E., Wennersten C., Gorss E., Freeman J., Eliopoulos G. M., Moellering R. C., Jr, Goldmann D. A. Rapid dissemination of beta-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant-toddler surgical ward. N Engl J Med. 1990 Dec 27;323(26):1814–1818. doi: 10.1056/NEJM199012273232606. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Zervos M. Plasmid analysis in the study of the epidemiology of nosocomial gram-positive cocci. Rev Infect Dis. 1986 Sep-Oct;8(5):705–712. doi: 10.1093/clinids/8.5.705. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Wurtz R., Sahm D., Flaherty J. Gentamicin-resistant, streptomycin-susceptible Enterococcus (Streptococcus) faecalis bacteremia. J Infect Dis. 1991 Jun;163(6):1393–1394. doi: 10.1093/infdis/163.6.1393. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Mikesell T. S., Schaberg D. R. Heterogeneity of plasmids determining high-level resistance to gentamicin in clinical isolates of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Jul;30(1):78–81. doi: 10.1128/aac.30.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]