Abstract
Carcinogenesis is a dynamic and stepwise process, which is accompanied by a variety of somatic and epigenetic alterations in response to a changing microenvironment. Hypoxic conditions will select for cells that have adjusted their metabolic profile and can maintain proliferation by successfully competing for scarce nutritional and oxygen resources. In the present study we have investigated the effects of energy depletion in the context of HPV (human papillomavirus)-induced pathogenesis. We show that cervical carcinoma cell lines are susceptible to undergoing either growth arrest or cell death under conditions of metabolic stress induced by AICAR (5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside), a known activator of the AMPK (AMP-activated protein kinase). Our results reveal that AICAR treatment leads to a reduced binding affinity of the transcription factor AP-1 (activator protein-1) and in turn to a selective suppression of HPV transcription. Moreover, the outcome of AICAR on proliferation and survival was dependent on p53 activation and the presence of LKB1, the major upstream kinase of AMPK. Using non-malignant LKB1-expressing somatic cell hybrids, which lose expression after tumorigenic segregation, as well as small interfering RNA LKB1 knockdown approaches, we could further demonstrate that expression of LKB1 protects cells from cytotoxicity induced by agents which modulate the ATP/AMP ratio. Since simulation of low energy status can selectively eradicate LKB1-negative cervical carcinoma cells, AICAR may represent a novel drug in the treatment of cervical cancer.
Keywords: activator protein-1 (AP-1), AMP-activated protein kinase (AMPK), human papillomavirus (HPV) transcription, p53
Abbreviations: ACC, acetyl-CoA-carboxylase; AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; AMPK, AMP-activated protein kinase; AP-1, activator protein-1; 2-DG, 2-deoxyglucose; EMSA, electrophoretic mobility-shift assay; 5-FU, fluorouracil; GSK3, glycogen synthase kinase-3; HPV, human papillomavirus; M2-PK, M2 pyruvate kinase; MAb, monoclonal antibody; PARP, poly (ADP-ribose) polymerase; RT, reverse transcriptase; siRNA, small interfering RNA; Sp1, stimulating protein 1; TBS, Tris-buffered saline; URR, upstream regulatory region
INTRODUCTION
Cervical cancer is a sexually transmitted disease with an apparent causal relationship between the infection with certain ‘high-risk’ HPV (human papillomavirus) types (mostly HPV-16, but also HPV-18, -33, -45 and some others) and the onset of tumour formation [1]. While the multi-step process of virus-induced carcinogenesis can be mimicked in part under tissue culture conditions [2], accumulation of malignant cells in a patient represents a more complicated situation, which is determined by the three-dimensional environment, depending on external parameters such as blood vessel formation, nutritional supplementation, growth hormones and oxygen [3]. To compensate for initial poor vascularization and low nutritional supply, transformed cells have to adapt to their new surroundings through a profound change in their energy metabolism [3]. Indeed many tumours, including cancer of the cervix uteri, are characterized by an increased anaerobic catabolism of glucose as the energy source [4]. Furthermore, glycolytic intermediates are used as precursors for synthetic processes debranching from glycolysis such as nucleic acid, phospholipid and amino acid synthesis [5,6].
Although the glycolytic phenotype seems to be disadvantageous, yielding 18 times less ATP than oxidative phosphorylation [5], its maintenance in primary and metastatic malignancies has a quite obvious selective benefit; cells which shift to anaerobic glycolysis not only survive the hypoxic microenvironment, but also select for a phenotype resistant to acid-induced toxicity, caused by secretion of lactic acid during incomplete glucose metabolism [3]. Likewise, chronic acidification can even promote invasion of formerly premalignant cells since adjacent normal populations, which are sensitive to acidosis, are destroyed [3]. Consequently, fully transformed cells have undergone genetic mutations to an extent that they have partly lost their flexibility to respond to changing conditions, such as deprivation of glucose, which makes them susceptible to drugs interfering with energy metabolism [7]. HPV-positive cells derived from highly invasive cancers depend mainly on glycolysis for energy production [8]. In several studies, including animal models, interference with glycolysis by particular inhibitors or glucose withdrawal disturbs the proliferation of tumour cells [9,10]. In our previous study [11] we have shown that the glucose availability as the energy source is not only advantageous for an increased metabolism, but seems to be even indispensable in maintaining HPV-18 oncogene transcription in cervical carcinoma cells. This was mainly deduced from the fact that the glucose analogue 2-DG (2-deoxyglucose) was able to suppress viral expression at the level of the initiation of transcription. Moreover, 2-DG restored the normal half-life of the tumour suppressor p53, in response to viral E6/E7 oncogene suppression [11].
Besides a general inhibitory effect on protein glycosylation [12], 2-DG also reduces the intracellular ATP level and in turn increases AMP. Alteration of the intracellular ATP/AMP ratio as a consequence of nutrient starvation or hypoxia usually activates the AMPK (AMP-activated protein kinase), a metabolic stress-sensing protein, which has attracted increasing attention in cancer research due to its central role in controlling energy consumption and cell proliferation [13]. AMPK represents a multicomponent enzyme complex which, once activated, switches off many ATP-utilizing processes in order to sustain energy homeostasis [13]. AMP binding allosterically activates AMPK, facilitating the binding of upstream kinases which enhance its activity.
AICAR (5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside), an adenosine analogue, has been shown to mimic AMP once phosphorylated to ZMP by adenosine kinase [14]. Its effect on the survival of cells depends strongly on the cell type and concentration used and varies from induction of growth arrest, prolonged survival and induction of senescence and apoptosis [15]. Moreover, AICAR can even induce apoptosis without AMPK activation [16], which is evidently causally linked to the absence of LKB1, a serine/threonine tumour suppressor kinase, which normally phosphorylates AMPK [17].
In the present study we describe the effects of cellular metabolic stress on HPV-positive cervical carcinoma cells. We show that both viral transcription and cellular growth are strongly affected by AICAR treatment. Notably, the final outcome of the biological response upon AICAR application was determined by the AMPK upstream kinase LKB1, whose expression protects non-malignant HPV-positive cells from apoptosis, whereas LKB1-negative cells were eliminated. This may provide new strategies in the treatment of cervical cancer, using LKB1 expression as a predictor for the therapeutic success.
MATERIALS AND METHODS
Cell lines and somatic cell hybrids
The cervical carcinoma cell lines HeLa, SiHa, CaSki, SW756, C33a, the non-tumorigenic somatic cell hybrid (referred to as ‘444’) of HeLa and primary human lung fibroblasts, its tumorigenic segregant (referred to as ‘CGL3’) [18], β-‘444’, the lung carcinoma cell line H1299 and the chronic myelogenous leukaemia cell line K562 were maintained in DMEM (Dulbecco's modified Eagle's medium; Sigma) supplemented with 10% fetal calf serum and 1% penicillin/streptomycin (Invitrogen). β-444 cells were generated by stable transfection of 444 cells with a β-actin-promoter-driven E6/E7 transcription cassette, representing a viral-cellular chimeric cDNA isolated from SW756 cells. AICAR, 2-DG and uridine (all from Sigma) were dissolved in sterile water. Usually, 2×104 cells per cm2 of the respective cell lines were seeded, and left for 24 h before treatment.
RNA extraction and Northern blot analysis
Cytoplasmic RNA was extracted according to the guanidinium-thiocyanate method or using an RNeasy RNA purification kit (Qiagen). Total cellular RNA (5 μg) was separated on 1% agarose gels in the presence of ethidium bromide under non-denaturating conditions and transferred to nylon membranes (GeneScreen, NEN Life Science). The membranes were subsequently hybridized under stringent conditions with specific 32P-labelled DNA probes [11].
DNA hybridization probes
pHPV-16/18 represent the unit-length genome of HPV-16/18 DNA cloned in pBR322. pHF-βA1 harbours an approximately full-length insert of the fibroblast β-actin gene. All probes were labelled by random-priming in the presence of [32P]dCTP [11].
Reverse transcription and PCR
For reverse transcription, 2 μg of DNase-purified RNA was mixed with 0.2 μg of random primers (Roche), heated at 70 °C for 10 min, and then chilled on ice. After annealing, the mixture was supplemented with reaction buffer [50 mM Tris/HCl (pH 8.3), 75 mM KCl and 3 mM MgCl2], 10 mM dithiothreitol, 500 μM deoxynucleoside triphosphate mixture (Invitrogen) and incubated at 25 °C for 10 min. RT (reverse transcriptase) SuperScript® II (100 units; Invitrogen) was added, and the reaction was incubated at 42 °C for 50 min, heated to 70 °C for 15 min, and finally stored at −20 °C until use. PCR reactions were performed in a solution containing 20 mM Tris/HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, 200 μM deoxynucleoside triphosphate mix (Invitrogen), 40 pM of upstream and downstream primers, 1 unit of Taq polymerase (Sigma) and 2 μl of reverse-transcribed product. The amplification was performed in an MJ Research PTC-200 thermal cycler in a total volume of 50 μl. The following primers and conditions were used: p21: 5′-ATGTCAGAACCGGCTGGGGATG-3′ and 5′-TTAGGGCTTCCTCTTGGAGAAG-3′ located in exon 3 and 4 (product length: 494 bp), 4 min at 94 °C, 28 cycles of 30 s at 94 °C, 45 s at 55 °C, and 30 s at 72 °C, final extension of 10 min at 72 °C; p53: 5′-CTGAGGTTGGCTCTGACTGTACCACCATCC-3′ and 5′-CTCATTCAGCTCTCGGAACATCTCGAAGCG-3′, located in exons 7 and 10 (product length: 371 bp), 4 min at 94 °C, 30 cycles of 30 s at 94 °C, 45 s at 57 °C, and 30 s at 72 °C, final extension of 10 min at 72 °C; LKB1: located in exons 9 and 10 (product length: 314 bp), 5′-ATGGAGTACTGCGTGTGTGG-3′ and 5′-CCAGATGTCCACCTTGAAGC-3′, 4 min at 94 °C, 35 cycles of 30 s at 94 °C, 45 s at 58 °C, and 1 min at 72 °C, final extension of 10 min at 72 °C; GAPDH (internal control): 5′-TGGATATTGTTGCCATCAATGACC-3′ and 5′-GATGGCATGGACTGTGGTCATG-3′, 4 min at 94 °C, 25 cycles of 30 s at 94 °C, 45 s at 65 °C, and 30 s at 72 °C, final extension of 10 min at 72 °C. The PCR products were analysed on 2% agarose gels containing ethidium bromide.
SDS/PAGE and Western blot analysis
Total cellular protein extracts were prepared using RIPA buffer [0.5% deoxycholate, 0.5% Nonidet P-40, 0.5% SDS, 50 mM Tris (pH 7.4) and 100 mM NaCl] supplemented with the serine protease inhibitor PMSF to a final concentration of 10 μg/ml. Cells were sonified using a Sonifier 250 (Branson Ultrasonics) and after removing the cell debris by centrifugation (16000 g for 5 min at 4 °C), the supernatant was immediately frozen and stored at −80 °C. Nuclear and cytosolic extracts were prepared according to the method of Schreiber et al. [19], adding the protease inhibitors N-N-(L-3-trans-carboxyoxirane-2-carbonyl)-L-leucyl-agmatine (E64) and 4-(2-aminoethyl)-benzenesulfonylfluoride (Pefabloc SC) in concentrations suggested by the manufacturer (Roche). Concentrations of RIPA buffer and nuclear extracts were determined using the Bio-Rad DC Protein Assay Kit. Cellular (20 μg) and total (50 μg) protein extracts were separated on SDS/PAGE (8–12% gels), electrotransferred to Immobilon-P membranes (Millipore) and probed with the following antibodies: human-specific murine MAb (monoclonal antibody) directed against p53 (DO1, Santa Cruz); p21 MAb (Anti Cip/Waf1, BD Transduction Laboratories); PARP [poly (ADP-ribose) polymerase] MAb (Sc-8007, Santa Cruz); actin MAb (clone 4, ICN Biomedicals); human-specific rabbit antibodies c-Jun (H-79, Santa Cruz) and phosphorylated acetyl-CoA carboxylase (ACC) MAb (p-ACC, Cell Signalling). The incubation was carried out overnight at 4 °C in TBS (Tris-buffered saline) supplemented with 5% non-fat skimmed milk powder (Sigma), 0.05% Tween 20 (Sigma) and the diluted antibody. The bands were visualized with anti-mouse IgG antibodies conjugated with horseradish peroxidase using the ECL® detection system (Amersham Biosciences). For reincubation with additional antibodies, the membranes were stripped with 200 mM NaOH for 5 min at room temperature (25 °C). Equal protein transfer and loading was controlled by reincubation with an actin-specific antibody.
EMSAs (electrophoretic mobility-shift assays)
Oligonucleotides for an HPV-18-specific AP-1 (activator protein-1)-binding site 5′-CGCACCTGGTATTAGTCATTTTCC-3′ within the enhancer region (position 7596–7620), the AP-1 consensus sequence 5′-CGCTTGATGACTCAGCCGGAA-3′ from the human collagenase promoter [20] and Oct-1 consensus sequences 5′-TGTCGAATGCAAATCACTAGAA-3′ derived from the immunoglobulin light chain enhancer [21] were generated in an Applied Biosystems synthesizer and purified by HPLC. The annealed oligonucleotides were labelled with 3000 Ci/mmol [γ-32P]ATP (Amersham Biosciences) and T4 polynucleotide kinase (New England Biolabs) and gel-purified. Binding reactions were performed in 20 μl containing 10% glycerol, 12 mM Hepes (pH 7.9), 4 mM Tris/HCl, 60 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 0.6 mg/ml BSA, 2 μg of poly(dI-dC) and 2 μg of nuclear extract. After 5 min, 10000 cpm of the probe was added and incubation was continued for 30 min at room temperature. For supershifts, 2 μg of either c-Jun (sc-822X), Fra-1 (sc-183X) or c-Fos (sc-52X) were added and incubated for 1 h (all antibodies for the supershift assays were from Santa Cruz). The complexes were resolved using 5.5% non-denaturing polyacrylamide gels (29:1 cross-linking ratio). The gels were dried and exposed to X-ray film (Amersham Biosciences).
Transfection of an HPV-18 URR (upstream regulatory region) reporter construct
To circumvent corrections for transfection efficiencies, a luciferase reporter construct under the control of the complete HPV-18 URR was transfected on 1×106 HeLa cells in a semi-stable fashion. In detail, cells were seeded in 6-cm tissue culture dishes for 24 h. The following day, cells were transfected using Effectene reagent (Qiagen) containing 2 μg of the HPV-18 URR-luciferase plasmid and 0.2 μg of a plasmid carrying a dominant selection marker for neomycin resistance. The cells were first incubated in the absence of the selective antibiotic G-418 (Geneticin). Post transfection (24 h), the medium was changed and supplemented with 1 mg/ml G-418. The outgrowing clones were pooled and used for the luciferase assay. H1299 cells semi-stably transfected with the HPV-18 URR-luciferase construct were kindly provided by Daniela Holland (DKFZ, Heidelberg, Germany) and served as a control for HPV-negative cells. All experiments were performed in triplicate. For the luciferase assay, six-well plates were seeded with 5×105 cells. The next day, three wells were supplemented with 4 mM AICAR without changing the medium and the remaining three wells were used as controls. Following 24 h treatment, cells were collected and lysed in passive lysis buffer (Promega) and the luciferase activity was evaluated in a luminometer (Berthold) using Luciferase Assay Reagent II (Promega).
siRNA (small interfering RNA) transfection
For knockdown experiments, 75 nM SMARTpool siRNA against LKB1 (Dharmacon) was transfected using HiPerFect transfection reagent (Qiagen) according to the manufacturer's instructions. siRNA directed against the luciferase gene served as a negative control. Cells (2×106) were transfected and seeded in 10-cm plates. The following day, cells were split 1:4 and cultured for an additional 24 h. To control the efficiency of the knockdown, cells were collected for RNA preparation using the RNeasy kit (Qiagen) according to the manufacturer's instructions. RT-PCR was performed to monitor LKB1 expression.
DNA staining, flow cytometry and quantification of apoptosis
Cells, including supernatants, were harvested by trypsination, washed twice with PBS and fixed overnight with 70% ethanol. After fixation and centrifugation, the cell pellets were resuspended in DNA staining solution containing 5 μM DAPI for DNA and 5 μM SR 101 as a protein counter-stain following the protocol published by Stoehr et al. [22]. Processing and cell cycle analysis were performed according to Dean and Jett [23] with a cytofluorograph 30-L (Ortho). Cells were additionally analysed by fluorescence microscopy (Leica DMRD).
RESULTS
Selective down-regulation of HPV transcription in cervical carcinoma cell lines
2-DG treatment has been reported to selectively reduce the transcription of viral mRNA encoding HPV-18-specific oncogenes E6/E7 in HeLa cells concomitant with a decrease in intracellular ATP levels [11]. It was subsequently shown that other conditions of energy deprivation, such as hypoxia and low glucose levels, have the same effect on HPV gene expression [12]. However, 2-DG is only effective as an ATP-depleting agent at high doses, since glucose and 2-DG compete for uptake [8]. To mimic a decreased ATP/AMP ratio at a constant ATP level, cells were treated with different concentrations of AICAR. AICAR is a cell-permeable drug and its phosphorylated form, ZMP, structurally resembles AMP, a known activator of AMPK [13].
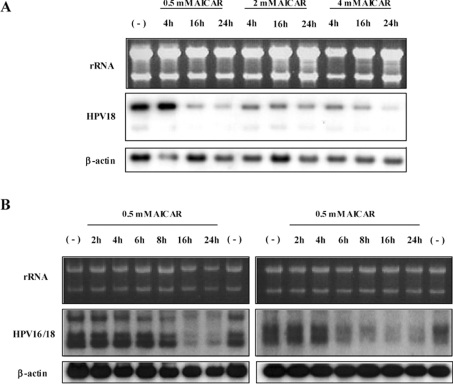
As depicted in Figure 1(A), 0.5 mM AICAR was sufficient to down-regulate E6/E7 viral transcription in HPV-18-positive cervical carcinoma HeLa cells in a time-dependent manner. The strongest reduction was detected with 4 mM AICAR after 24 h treatment. HPV-18 suppression was selective since other reference genes such as the housekeeping gene β-actin were not affected. To demonstrate that the effect of AICAR was not merely a cell-line-specific phenomenon, additional HPV-16/18-positive cells were examined. CaSki cells contain approx. 500 both full-length and truncated copies of HPV-16 integrated at different chromosomal locations, SW756 cells harbour multiple truncated copies of HPV-18 located at a single integration site [24]. As demonstrated in Figure 1(B), both cell lines also showed a strong decrease in HPV expression, clearly indicating that AICAR treatment negatively regulates viral transcription independently of the HPV type, the copy number or the viral integration locus (Figure 1B).
Figure 1. Selective down-regulation of HPV transcription in cervical carcinoma cells after treatment with AICAR.
Northern blots of different cervical cancer cell lines treated with AICAR. (A) HPV-18-positive HeLa cells were incubated for the times indicated with different concentrations of AICAR. (B) RNA obtained from HPV-16-positive CaSki cells (left-hand panels) and HPV-18-positive SW756 cells (right-hand panels) after treatment with 0.5 mM AICAR for different periods of time. For Northern blot analysis, 5 μg/lane of total RNA was separated under non-denaturing conditions in 1% agarose gels. After transfer, the membranes were consecutively hybridized with HPV-16/18- and β-actin-specific DNA probes. (−), Untreated control.
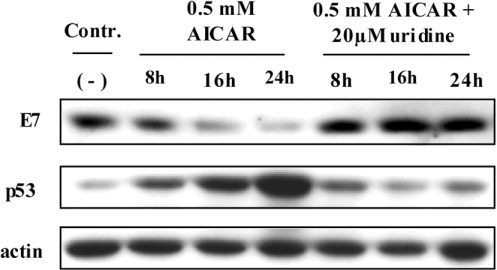
Consistent with the down-regulation of HPV-18 RNA (Figure 1A), 0.5 mM AICAR treatment also led to a selective and time-dependent reduction of the E7 oncoprotein (Figure 2). Due to the unavailability of appropriate antibodies, we could only indirectly measure the fate of the E6 oncoprotein. Figure 2 shows that in the same way as E7 and viral transcription was decreased (Figure 1A), p53 was stabilized compared with untreated control cells. Cells cultured in the presence of AICAR showed a strong decrease in UTP and CTP levels as a result of PRPP (5-phospho-D-ribosyl-1-pyrophosphate) depletion, an important precursor for pyrimidine nucleotide synthesis [25]. To overcome pyrimidine depletion and to demonstrate that the metabolism of the treated cells was not irreversibly disturbed, HeLa cells were cultured with 0.5 mM AICAR in the presence of 20 μM uridine. Under these conditions, the inhibitory effect on oncogene expression and stabilization of p53 could be completely prevented (Figure 2). Conversely, at higher AICAR concentrations uridine failed to inhibit the transcriptional attenuation of HPV expression even when uridine was supplemented in concentrations up to 1 mM (results not shown).
Figure 2. Reciprocal regulation of E7 and p53 after AICAR treatment: abrogation of the AICAR effect by uridine.
HeLa cells were treated with 0.5 mM AICAR for different periods of time with and without supplementation of 20 μM uridine. Immunoblots were performed using antibodies raised against HPV-18 E7 and p53. Equal loading and protein transfer were confirmed by incubating with an anti-actin-specific antibody. (−), Untreated control.
The viral control region determines down-regulation of HPV transcription
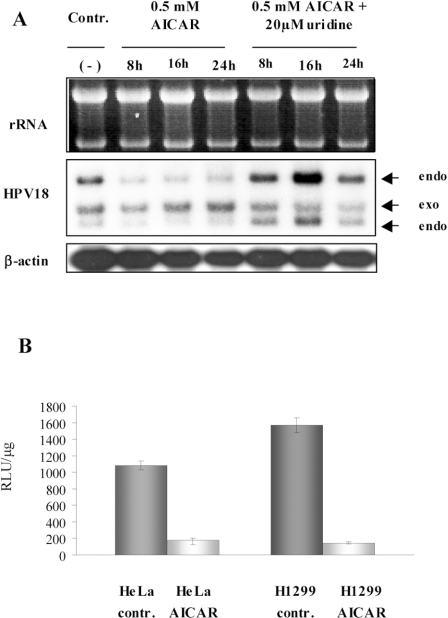
A decrease in oncogene mRNA levels can be either due to a labilization and shortening of the cytoplasmic mRNA half-life or due to direct suppression of viral URR-directed transcriptional activity. To investigate the effect of AICAR treatment on the HPV-URR, we established a clonal sub-line derived from HeLa cells fused with normal human fibroblasts, which expresses an additional E6/E7 cDNA transcription cassette under the control of the β-actin promoter [26]. As depicted in Figure 3(A), incubation with 0.5 mM AICAR only resulted in a suppression of the URR-directed E6/E7 transcription (referred to as ‘endo’ for endogenous HPV-18 expression), while the β-actin-driven viral cDNA (referred to as ‘exo’) was not affected. Uridine supplementation was able to abrogate the AICAR effect indicating that down-regulation of viral gene expression was regulated at the level of initiation of transcription rather than by post-transcriptional mechanisms. Additionally, to exclude any positional effect due to integration and to unequivocally demonstrate that the URR was indeed a target of the negative regulation upon AICAR treatment, HeLa cells were transfected with an HPV-18-URR luciferase reporter plasmid. H1299 cells, which contain the same reporter construct, were used to monitor the effect of AICAR on the HPV-18-URR-directed transcription in HPV-negative cells. As shown in Figure 3(B), in both cell lines, 4 mM AICAR treatment led to a 6–10 times reduced luciferase activity, clearly indicating that the HPV-18-URR was a target of the negative regulatory process induced by AICAR.
Figure 3. URR-mediated down-regulation of viral transcription following AICAR treatment.
(A) 444 cells expressing an additional E6/E7 transcription cassette under the control of the β-actin promoter were treated with 0.5 mM AICAR with and without supplementation of 20 μM uridine for the times indicated. Total RNA (5 μg/lane) was separated under non-denaturing conditions on a 1% agarose gel and the same membranes were consecutively hybridized with HPV-18- and β-actin-specific DNA probes. (−), Untreated control. (B) Luciferase reporter assay with HeLa and H1299 cells carrying the luciferase reporter construct under the control of the HPV-18 URR. Semi-stably transfected cells were treated with 4 mM AICAR for 24 h and the luciferase activity was compared with untreated controls. RLU/μg, renilla luciferase units per μg of total cellular protein.
Selective reduction of AP-1 after AICAR treatment
HPV transcriptional activity is strongly determined by a certain set of host-specific transcription factors, which bind to their cognate cis-regulatory recognition sites within the URR [27]. In the case of 2-DG it was shown that the transactivating potential of the transcription-factor Sp1 (stimulating protein-1) was diminished by an increase in O-linked glycosylation [12]. Since the β-actin promoter contains numerous Sp1-like sequences [28], but was not affected upon AICAR incubation (Figure 3), we focused our attention on the transcription factor AP-1, which is known to play a central role in the transcriptional regulation of almost all HPV types investigated so far [29].
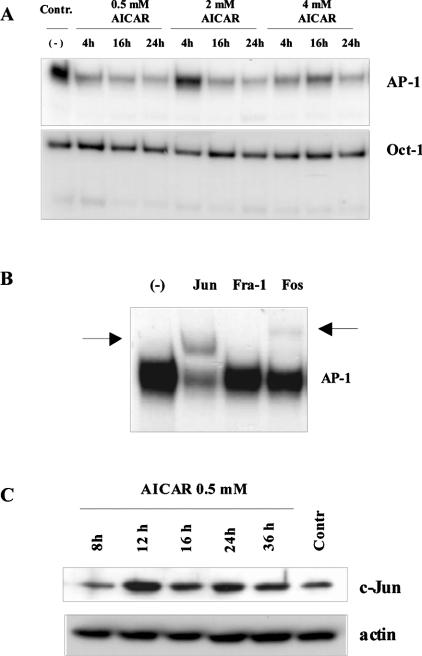
To test this notion, we examined the AP-1 binding using EMSAs. Incubation of nuclear extracts obtained from cells treated with different concentrations of AICAR with 32P-labelled AP-1 oligonucleotides resulted in a strong reduction of AP-1 binding (Figure 4A). Supershift analysis confirmed that c-Jun is the main dimerization partner of the AP-1 complex in HeLa cells (Figure 4B). However, monitoring the c-Jun steady-state levels by Western blot, no quantitative reduction was detectable under conditions of low AP-1 binding (Figure 4C). These data suggest that decreased AP-1 affinity in the EMSA might be the result of a post-translational modification of c-Jun, probably interfering with its affinity for DNA. To demonstrate that suppression of AP-1 binding was a selective process, EMSAs with 32P-labelled Oct-1- specific oligonucleotides were performed. In fact, under conditions where AP-1 binding was reduced (Figure 4A), both Oct-1 and Sp1 binding (results not shown) were not affected. These data clearly demonstrate that an unbalanced energy metabolism triggers a decrease of AP-1, but apparently did not impair the binding of transcription factors in general.
Figure 4. Selective reduction of AP-1 binding after AICAR treatment.
(A) EMSA of nuclear extracts obtained from treated and non-treated cells were incubated with 32P-labelled oligonucleotides specific for AP-1 or Oct-1. The incubation time and the AICAR concentrations are indicated. (B) Supershifts showing the main components in the AP-1 complex of HeLa cells. The shifted bands are indicated by arrows. (C) Nuclear protein extracts were used for immunoblots with a c-Jun-specific antibody. Equal loading and protein transfer were confirmed by incubating the same membrane with an anti-actin-specific antibody.
The effect of AICAR on the cell cycle and p53/p21CIP1 expression
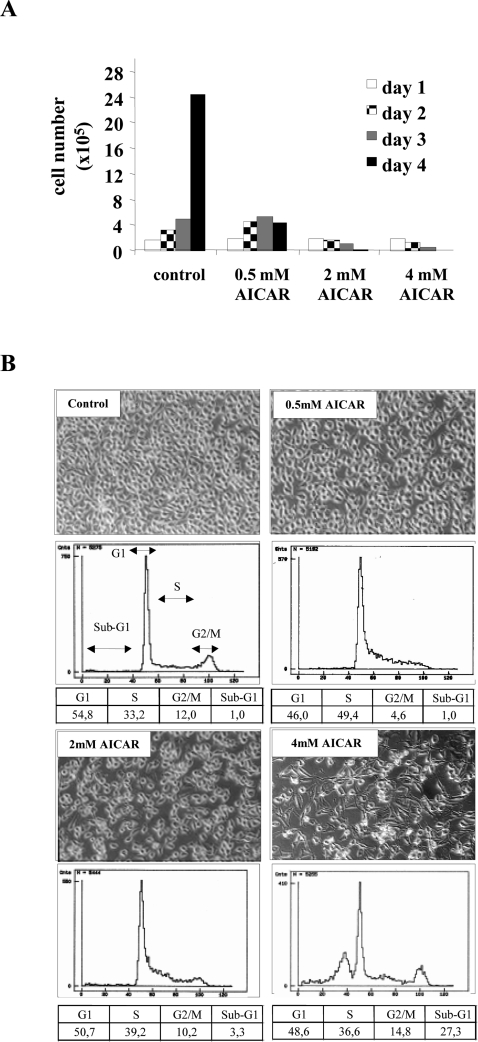
Since viral expression is necessary to maintain a proliferative phenotype of cervical carcinoma cells [30], we examined the fate of HeLa cells at different time points after seeding in the presence of increasing concentrations of AICAR. As shown in Figure 5(A), 0.5 mM AICAR completely blocked cellular growth, while at higher amounts of AICAR the number of cells were actually diminished. In fact, as revealed by phase-contrast microscopy (Figure 5B), addition of 4 mM AICAR for 24 h led to the appearance of typical apoptotic features indicated by condensation of the cytoplasm and nuclear shrinkage (karyorhexis). In order to analyse at which phase of the cell cycle the cells became growth arrested and to quantify the extent of apoptosis, flow-cytometric analyses were performed. As depicted in Figure 5(B), lower concentrations of AICAR blocked cells within S-phase, which in turn resulted in a reduced number of cells in G2/M. At 4 mM AICAR, a substantial fraction of cells (approx. 27%) accumulated in a sub-G1 position, confirming that apoptosis was taking place. At 2 mM AICAR, S-phase arrest was partially released and cell morphology changed in a similar manner to cells treated with 4 mM but without the appearance of apoptotic features. In contrast, the HPV-negative cervical carcinoma cell line C33a did not undergo apoptosis, but a reduced growth rate was observed (see Supplementary Figure at http://www.BiochemJ.org/bj/403/bj4030501add.htm).
Figure 5. Growth curves and cell cycle analysis after AICAR treatment.
(A) Follow-up of the cellular growth of 1×104 HeLa cells for 1–4 days in the presence of AICAR. (B) Representative flow cytometric profiles and the corresponding morphology of HeLa cells after incubation with AICAR. The percentage of cells present in the different phases of the cell cycle (G1, S, G2/M) as well as the percentage of apoptotic cells (reflected by the sub-G1 peak) are given in the table below the profiles and represent the mean of three different experiments. The magnification of the phase-contrast micrographs is ×200.
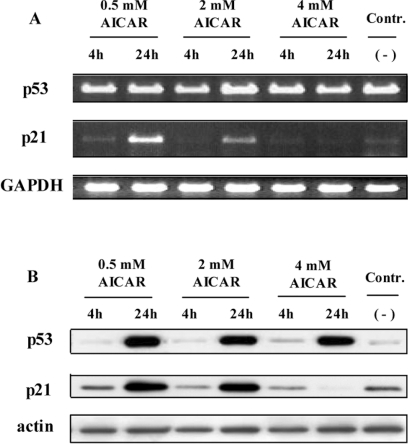
To get an insight into the dosage effect, we monitored the expression of p53 and p21CIP1 at different AICAR concentrations. As shown in Figure 2, treatment with AICAR resulted in a strong increase of the p53 protein. Using RT-PCR, no differences at the p53 mRNA level could be observed (Figure 6A, upper panel), indicating that the quantitative increase of p53 was the consequence of its reconstituted half-life due to viral oncogene suppression (Figure 1) rather than by a transcriptional effect. One major target gene involved in cell cycle control is p21CIP1, an inhibitor of cyclin-dependent kinases [31]. Interestingly, only when cellular growth arrest was accomplished was p21CIP1 up-regulated, both at the RNA and protein level (Figure 6). In contrast, under conditions of apoptosis, p21CIP1 was not induced transcriptionally despite increased p53 protein levels. Moreover, Western blot analysis revealed that p21CIP1 was not even expressed, indicating that under these conditions p21CIP1 induction was not necessary (Figure 6B). These findings suggest that growth of HeLa cells can be efficiently inhibited by lower concentrations of AICAR, whereas increasing concentrations of AICAR trigger apoptosis.
Figure 6. Dosage effect of AICAR on p53 and p21CIP1 expression.
(A) RT-PCR analysis of p53, p21CIP1 and GAPDH in AICAR-treated cells after separation on a 1% agarose gel. Contr., untreated cells. (B) Immunoblot with antibodies against p53 and p21CIP1 of total protein extracts. Equal loading and protein transfer were confirmed by incubating the same membrane with an anti-actin-specific antibody.
Expression of LKB1 and its role in induction of apoptosis
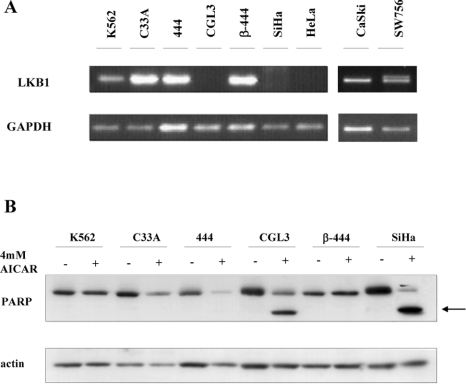
As already shown for cells without any HPV aetiology, AICAR also induces p53 and p21 accumulation [31]. Apparently, p53 is a direct target of activated AMPK [32], providing a novel link between cell cycle control and metabolic regulation. AMPK in turn is activated by AMPK upstream kinases, such as LKB1 [33]. To examine the expression of LKB1 in the context of HPV-induced carcinogenesis, we analysed the presence of the corresponding mRNA in different cervical carcinoma cells (Figure 7A). Consistent with previous results [17], HeLa cells lacked detectable LKB1 expression, whereas other cervical carcinoma cell lines, such as CaSki, SW756 and C33a respectively, were found to be positive for LKB1 mRNA. Exceptions were the HPV-16-positive cervical carcinoma cell line SiHa and the tumorigenic segregant (CGL3) of the former non-tumorigenic somatic cell hybrid made of HeLa and primary human fibroblasts (444), where the conversion to tumorigenicity was obviously accompanied by a loss of LKB1 expression. The chronic myelogenous leukaemia cell line K562, known to be LKB1 positive [34], was used as a positive control for the RT-PCR reaction (Figure 7A).
Figure 7. LKB1 expression and apoptosis.
(A) RNAs from different cervical carcinoma cell lines and derived somatic cell hybrids were reverse transcribed and the RT-PCR products of LKB1 and GAPDH were separated on a 1% agarose gel. (B) The indicated cell lines were treated for 24 h with 4 mM AICAR. For immunoblot analysis, total protein extracts were incubated with antibodies against PARP and actin. The arrow indicates the PARP cleavage product.
As suggested previously [17], the absence of LKB1 and therefore the lack of AMPK activation commit cells to undergo apoptosis when they are challenged with drugs signalling low cellular energy conditions [17]. To confirm this notion, various cell lines were treated with 4 mM AICAR and programmed cell death was monitored. In fact, as depicted in Figure 7B, all cells which lacked detectable LKB1 expression showed the appearance of PARP cleavage following AICAR treatment, a characteristic sign of ongoing apoptosis (see also Figure 8A for HeLa cells). This suggests that LKB1-deficient cervical carcinoma cells undergo apoptosis under conditions that mimic an elevated AMP/ATP ratio.
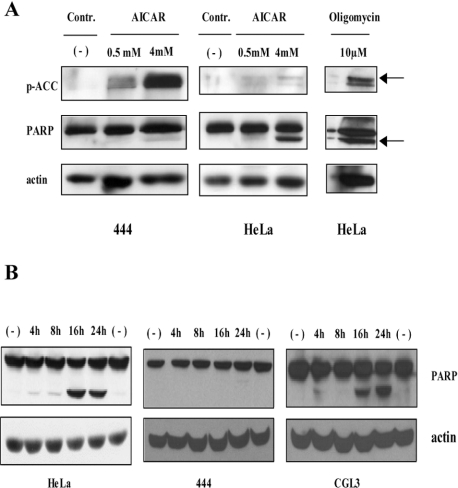
Figure 8. Absence of LKB1 activity in HeLa cells, but not in non-malignant somatic cell hybrids: selective induction of apoptosis by 2-DG in LKB-1-negative cells.
(A) HeLa and 444 cells were treated for 24 h with 0.5 and 4 mM AICAR or 10 μM oligomycin respectively. Total protein extracts were used for Western blot analysis to monitor the phosphorylation of the AMPK downstream substrate ACC (p-ACC) and cleavage of PARP. Actin was used as a loading and transfer control. (B) Indicated cell lines were treated with 45 mM 2-DG for different periods of time and total protein extracts were used for immunoblots. Membranes were incubated with antibodies against PARP and actin, the latter acting as a loading control.
AMPK functionality and apoptosis
In order to elucidate the activity of AMPK, phosphorylation of the downstream target ACC, an enzyme involved in the fatty acid synthesis pathway, was monitored [15]. For this purpose, we chose LKB1-positive 444 cells and LKB1-negative parental HeLa cells for comparison. As demonstrated in Figure 8(A), upon 4 mM AICAR treatment only 444 cells showed increased ACC phosphorylation, which is consistent with the presence of LKB1 expression and an activation of AMPK. In contrast, HeLa cells were completely refractory under the same experimental conditions, although apoptosis, as indicated by a strong PARP cleavage, was induced. To demonstrate that there still exists a redundant non-LKB1-dependent pathway activating AMPK, HeLa cells were treated with 10 μM oligomycin, an inhibitor of mitochondrial respiration that, like 2-DG, depletes the intracellular ATP levels [35]. Indeed, oligomycin activated AMPK and also led to a cleavage of PARP, whereas AICAR-mediated apoptosis seemed to be entirely independent of AMPK activity. Since LKB1 signalling is involved in the cellular response to low energy, we hypothesized that the presence of LKB1 should protect cells from apoptosis induced by drugs that further elevate intracellular AMP.
Taking advantage of our somatic cell hybrid model system metabolic stress, such as the application of 2-DG, can be investigated both in non-malignant cells expressing LKB1 and in tumorigenic segregants derived from the very same hybrid (CGL3), but lacking LKB1 (see Figure 7A). As predicted, 2-DG only induced apoptosis in LKB1-negative cells (HeLa and CGL3), while 444 cells were resistant (Figure 8B).
Knockdown of LKB1 transcription by siRNA
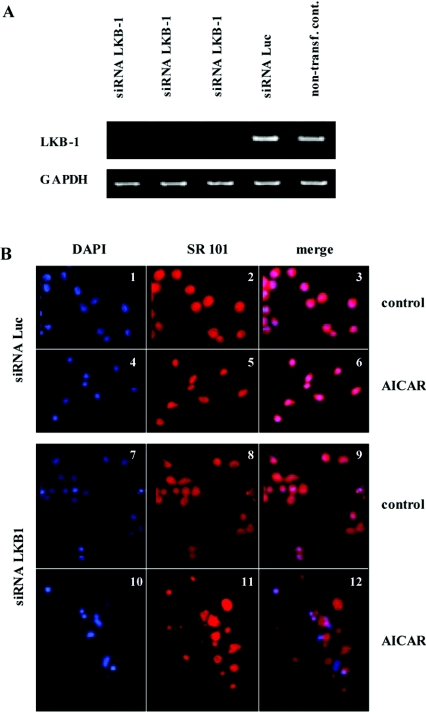
To study the fate of AICAR-treated cells after knocking down LKB1 expression, both C33a (results not shown) and 444 hybrids were transfected with LKB1 siRNA in comparison with an siRNA directed against the luciferase gene (siLuc) as a non-specific control. As shown in Figure 9(A), LKB1 siRNA delivery resulted in an almost complete reduction of the corresponding mRNA, whereas siLuc had no effect. When cells were incubated with 4 mM AICAR for 24 h, 100% of LKB1 knockdown cells were eradicated in contrast with siLuc-transfected controls. To monitor the mode of cell death, incubation with AICAR was reduced to 8 h, cells were stained with DAPI and SR 101 as a protein counter- stain and analysed by fluorescence microscopy. As depicted in Figure 9(B), LKB1 knockdown cells underwent extensive cell death (panels 10–12) in contrast with the siLuc controls, which showed no morphological changes (panels 4–6). Panel 12 revealed the presence of necrotic (loss of plasma integrity without morphological changes of the nuclei) rather than apoptotic cells, indicated by loss of cell integrity. This suggests that AICAR or ATP-depleting agents may represent potential therapeutic drugs that selectively eradicate LKB1-deficient cervical carcinoma cells via apoptosis or necrosis.
Figure 9. Knockdown of LKB1 transcription by siRNA.
444 Cells were transfected in triplicate with siRNA against LKB1 and against luciferase as a negative control. After 24 h cells were split 1:4 and 60 h post-transfection RNA from one of the plates was extracted. (A) For RT-PCR analysis, LKB1-specific primers and GAPDH primers (as an internal control) were used. AICAR treatment was started 60 h post transfection. (B) After 8 h treatment, cells were harvested, prepared and stained for fluorescence microscopy. 444 Cells transfected with siRNA against luciferase under control conditions (panels 1–3) and after 8 h 4 mM AICAR treatment (panels 4–6). Untreated LKB1 knockdown cells (panels 7–9) and after 8 h 4 mM AICAR treatment (panels 10–12). Magnification: ×200.
DISCUSSION
A hallmark of many highly invasive tumours, including cervical cancer, is their high glycolytic rate, strongly supporting Warburg's dictum of a relationship between a glycolytic shift and malignant progression. Furthermore, a glycolytic phenotype, which guarantees a constant energy supply even when oxygen levels are decreased is evidently also an essential prerequisite to maintain efficient transcriptional activity of the viral oncoproteins E6 and E7 [12]. In the present study, not only 2-DG and limited glucose availability selectively reduce HPV expression in cervical carcinoma cells [11,12,36], but also AICAR (Figure 1), a known activator of AMPK, which simulates low energy conditions without ATP depletion. The suppressive effect was not limited to HPV-18- positive HeLa cells, but could also be visualized in other cell lines (Figure 1), differing in their HPV type, their viral copy number and viral chromosomal localization (Figure 1B). Since both E6 and E7 transcription was down-regulated by AICAR treatment, p53 stabilization can be explained by reduced expression of E6 (Figure 2).
Remarkably, there seems to be an evolutionary well-designed circuit between sustained HPV transcription and oncoprotein functionality, since E7 itself is capable of reprogramming the metabolism of its host cell. E7 physically interacts with the M2-PK (M2 pyruvate kinase) [37] and the acid α-glucosidase [38], both key regulatory enzymes involved in the intracellular accumulation of glycolytic phosphometabolites and glycogen breakdown [11]. Binding of E7 to M2-PK, for instance, changes its catalytic properties, which finally leads to an increase of nucleotide precursors required for DNA synthesis and cell growth [37]. Accordingly, for a successful virus–host interaction it is reasonable to assume that viral transcription, which continuously needs a high metabolic state for keeping up cell proliferation, is down-regulated by a negative feedback loop when the host cell is sensing low energy conditions.
As depicted in Figure 3(A), we showed that AICAR exclusively affected URR-directed gene expression, while the transcriptional activity of a β-actin-driven E6/E7 transcription cassette, which contains a 3′-cellular poly-adenylation site from SW756 cells remained unchanged. The same was true when HPV-18-URR- driven luciferase reporter constructs were transfected (Figure 3B), clearly supporting the notion that HPV is suppressed at the level of the initiation of transcription rather than post-transcriptionally. Recently, it has been demonstrated that 2-DG, hypoglycaemia and hypoxia can affect the binding affinity of the transcription factor Sp1 by changing its glycosylation profile, which is added in a post-translational manner [12]. In our experiments however, AICAR treatment had no effect on Sp1 (results not shown), but a decreased binding affinity of the transcription factor AP-1 could be discerned (Figure 4A). In agreement with our findings, evidence has been provided that AICAR also interferes with AP-1 binding in other systems [39], suggesting a general functional relationship between AP-1 activity and the metabolic state of the cell.
Actually AP-1 is not only involved in various cellular regulatory processes [40], but also in the maintenance of HPV expression [29]. This notion has been confirmed by site-directed mutagenesis of the AP-1 sites within the viral enhancer/promoter region, where in contrast with other cis-regulatory sequences, URR-driven transcription was almost completely abolished [27]. The finding that Oct-1 binding was not reduced under these conditions clearly demonstrated the selectivity of this process. Decreased AP-1 binding in the EMSA, however, could not be attributed to a reduction of c-Jun, the major dimerization partner of the Jun-family members in cervical carcinoma cells (Figure 4B) [41], since Western blot analysis of nuclear extracts revealed that similar expression levels were preserved (Figure 4C). In respect of functionality it should be stressed that AICAR also causes activation of the GSK3 (glycogen synthase kinase-3) [42], which phosphorylates c-Jun at its C-terminal DNA-binding domain thereby preventing DNA binding [43]. This may explain the reduced AP-1 signals observed in the EMSA (Figure 4A) despite unchanged amounts of c-Jun, even after longer AICAR treatment (Figure 4C). Note that an inhibitor of GSK3, lithium chloride [44] does not relieve the transcriptional block on HPV-18 transcription (results not shown). How AICAR is definitively diminishing AP-1 binding still remains to be elucidated.
Although all AICAR concentrations tested reduced HPV-18 transcription in HeLa cells to a similar extent (Figure 1), the effects on proliferation and survival differed significantly (Figure 5A). While 0.5 mM AICAR caused accumulation of cells in S-phase with a depletion of the G2/M fraction, addition of 4 mM AICAR for 24 h led to the induction of apoptosis, which was indicated by the appearance of the characteristic sub-G1 peak after flow cytometric measurement (Figure 5B). Growth arrest at lower and moderate AICAR concentrations was in line with a post-translational increase of p53 and the induction of its major target gene p21CIP1, both at RNA and protein levels (Figure 6). Elevation of p53 and inhibition of oncogene expression could be completely prevented when 0.5 mM AICAR was applied in combination with 20 μM uridine, pointing to an inhibition of phospho-ribosyl-pyrophosphate-synthetase and nucleic acid synthesis as a further target of AICAR (Figure 2) [25]. p53 is a central protein in cell cycle control that signals either cessation of growth or induction of apoptosis, depending on the severity of cellular damage [32]. Moreover, its activity is also regulated by AMPK, demonstrating that p53 is part of a metabolic checkpoint, which determines the outcome of the biological response after energy depletion [32]. In HepG2 cells, induction of p53 and p21CIP1 after AICAR treatment has also been described [31]. However, p53 was induced at the transcriptional level, whereas in the present study p53 was stabilized as a consequence of viral oncogene suppression (Figures 1 and 2). Note that under conditions when cells underwent apoptosis, p21CIP1 mRNA was not induced and the corresponding protein was not detectable (Figure 6A). This is consistent with previous reports, suggesting that p21CIP1 has anti-apoptotic activity [45] and can be cleaved by a caspase-like mechanism [46].
Despite the fact that AICAR is considered to be a specific inducer of AMPK [13], no phosphorylation of its downstream target ACC [15] could be detected in HeLa cells (Figure 8A). This correlates with the absence of the AMPK upstream kinase LKB1 [42] (see also Figure 7), a putative tumour suppressor gene first described to be mutated in the rare inherited disease Peutz–Jeghers syndrome [47]. Monitoring the presence of LKB1 mRNA in other cervical carcinoma cells, the picture was not uniform (Figure 7A), suggesting that loss of LKB1 expression is not a general feature of HPV-induced carcinogenesis. There is strong evidence that it is not AMPK itself but one of the upstream kinases (either LKB1 or the calmodulin-dependent kinase) that determines the biological outcome following AICAR treatment [17,35]. LKB1-deficient mouse embryonic fibroblasts, for example, but not wild-type cells or their heterozygous controls, undergo extensive apoptosis [17]. In HeLa cells, LKB1 seems to be not transcribed due to promotor de novo methylation [17], but is reconstituted upon somatic cell hybridization with normal human fibroblasts (444). Tumorigenic segregants derived from the same hybrids (CGL3) have lost LKB1 expression, suggesting that progression to malignancy is paralleled by LKB1 down-regulation. Remarkably, similar to the aforementioned situation, only cells lacking LKB1 underwent cell death, while others were found to be resistant (Figures 7B and 8A). According to the model presented by Shaw et al. [17], lack of LKB1 expression should also sensitize cells to apoptosis when the ATP/AMP ratio is altered. In fact, 2-DG, which activates AMPK via the calmodulin-dependent kinase [35] only induced apoptosis in LKB1-negative HeLa cells and CGL3 hybrids, but not in their non-malignant, LKB1-positive counterparts (Figure 8B), clearly confirming the relationship between AICAR-induced cytotoxicity and the absence of LKB1 expression.
However, apoptosis (Figures 7 and 8) is apparently not the only way to eliminate LKB1-negative cells. Knocking down LKB1 via transient delivery of siRNA resulted in a complete eradication 24 h after AICAR treatment. Morphological changes typical for apoptosis were absent and cells seemed to be eliminated via disruption of the cellular membrane and release of DNA (Figure 9, panel 12). In comparison with cells which had already acquired a loss of LKB1 expression during the carcinogenic process (e.g. HeLa and CGL3), a short-term knockdown of LKB1 followed by AICAR treatment apparently led to a different biological outcome. LKB1-deficient cells underwent apoptosis (Figures 5B and 8B), whereas siRNA-mediated knockdown led to necrotic cell death. Since the activation of AMPK by AICAR in 444 cells (Figure 8A) did correlate with survival, it can be concluded that by short-term LKB1 suppression the cells cannot counteract the AICAR-induced metabolic stress, resulting in extensive cytotoxicity (Figure 9B).
The susceptibility of cells lacking LKB1 to undergo cell death raises the question as to whether interference with energy metabolism might be an additional option to eliminate chemoresistant cancer cells by a combinatorial therapy. Several strategies combining ATP depletion and anticancer agents have been tested in vitro and in vivo. Inhibition of glycolysis by either glucose withdrawal or 2-DG application enhanced radiosensitization [48], but also death ligand-induced apoptosis [46]. Furthermore, the combination of the widely used 5-FU (fluorouracil) with AICAR induced cell death in 5-FU-resistant cell lines [49]. Preliminary results from our laboratory are in agreement with these results showing a synergistic effect of AICAR treatment and CD95-ligand addition in the induction of apoptosis. This may open new strategies in the treatment of cervical cancer, using LKB1 expression as a predictor for therapeutic success.
Online data
Acknowledgments
We thank Daniela Holland for the H1299-transfected cells, Karin Butz for the luciferase reporter plasmid (both Department of Molecular Therapy of Virus-Associated Cancer, DKFZ, Heidelberg, Germany), Eric Stanbridge (Department of Microbiology and Molecular Genetics, University of Irvine, Irvine, CA, U.S.A.) for the hybrid cell lines, Monika Frank-Stoehr for cell cycle analysis, Rainer Schmidt (both Department of Viral Transformation Mechanisms, DKFZ, Heidelberg, Germany) and Thomas Hofmann (Division of Cellular Senescence, DKFZ, Heidelberg, Germany) for helpful advice and discussions.
References
- 1.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer. 2002;2:342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 2.Münger K., Howley P. M. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–228. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 3.Gatenby R. A., Gillies R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 4.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 5.Eigenbrodt E., Glossmann H. Glycolysis: one of the keys to cancer? Trends Pharmacol. Sci. 1980;1:240–245. [Google Scholar]
- 6.Mazurek S., Boschek C. B., Hugo F., Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin. Cancer Biol. 2005;15:300–308. doi: 10.1016/j.semcancer.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Seyfried T. N., Mukherjee P. Targeting energy metabolism in brain cancer: review and hypothesis. Nutr. Metab. 2005;2:30. doi: 10.1186/1743-7075-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vrbacky M., Krijt J., Drahota Z., Melkova Z. Inhibitory effects of Bcl-2 on mitochondrial respiration. Physiol. Res. 2003;52:545–554. [PubMed] [Google Scholar]
- 9.Rattan R., Giri S., Singh A. K., Singh I. 5-Aminoimidazole-4-carboxamide-1-β-D-ribofuranoside inhibits cancer cell proliferation in vitro and in vivo via AMP-activated protein kinase. J. Biol. Chem. 2005;280:39582–39593. doi: 10.1074/jbc.M507443200. [DOI] [PubMed] [Google Scholar]
- 10.Swinnen J. V., Beckers A., Brusselmans K., Organe S., Segers J., Timmermans L., Vanderhoydonc F., Deboel L., Derua R., Waelkens E., et al. Mimicry of a cellular low energy status blocks tumor cell anabolism and suppresses the malignant phenotype. Cancer Res. 2005;65:2441–2448. doi: 10.1158/0008-5472.CAN-04-3025. [DOI] [PubMed] [Google Scholar]
- 11.Maehama T., Patzelt A., Lengert M., Hutter K. J., Kanazawa K., Hausen H., Rösl F. Selective down-regulation of human papillomavirus transcription by 2-deoxyglucose. Int. J. Cancer. 1998;76:639–646. doi: 10.1002/(sici)1097-0215(19980529)76:5<639::aid-ijc5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 12.Kang H. T., Ju J. W., Cho J. W., Hwang E. S. Down-regulation of Sp1 activity through modulation of O-glycosylation by treatment with a low glucose mimetic, 2-deoxyglucose. J. Biol. Chem. 2003;278:51223–51231. doi: 10.1074/jbc.M307332200. [DOI] [PubMed] [Google Scholar]
- 13.Hardie D. G., Hawley S. A. AMP-activated protein kinase: the energy charge hypothesis revisited. BioEssays. 2001;23:1112–1119. doi: 10.1002/bies.10009. [DOI] [PubMed] [Google Scholar]
- 14.Sabina R. L., Patterson D., Holmes E. W. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J. Biol. Chem. 1985;260:6107–6114. [PubMed] [Google Scholar]
- 15.Luo Z., Saha A. K., Xiang X., Ruderman N. B. AMPK, the metabolic syndrome and cancer. Trends Pharmacol. Sci. 2005;26:69–76. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Lopez J. M., Santidrian A. F., Campas C., Gil J. 5-Aminoimidazole-4-carboxamide riboside induces apoptosis in Jurkat cells, but the AMP-activated protein kinase is not involved. Biochem. J. 2003;370:1027–1032. doi: 10.1042/BJ20021053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shaw R. J., Kosmatka M., Bardeesy N., Hurley R. L., Witters L. A., DePinho R. A., Cantley L. C. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanbridge E. J. Genetic analysis of human malignancy using somatic cell hybrids and monochromosome transfer. Cancer Surv. 1988;7:317–324. [PubMed] [Google Scholar]
- 19.Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee W., Mitchell P., Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- 21.Scheidereit C., Cromlish J. A., Gerster T., Kawakami K., Balmaceda C. G., Currie R. A., Roeder R. G. A human lymphoid-specific transcription factor that activates immunoglobulin genes is a homoeobox protein. Nature. 1988;336:551–557. doi: 10.1038/336551a0. [DOI] [PubMed] [Google Scholar]
- 22.Stoehr M., Vogt-Schaden M., Knobloch M., Vogel R., Futterman G. Evaluation of eight fluorochrome combinations for simultaneous DNA–protein flow analyses. Stain Technol. 1978;53:205–215. doi: 10.3109/10520297809111467. [DOI] [PubMed] [Google Scholar]
- 23.Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J. Cell Biol. 1974;60:523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med. Microbiol. Immunol. 1987;176:245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C. B., Meade J. C., Holmes E. W. Aminoimidazole carboxamide ribonucleoside toxicity: a model for study of pyrimidine starvation. J. Cell Physiol. 1981;107:335–344. doi: 10.1002/jcp.1041070305. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann A., Hanke B., Zawatzky R., Soto U., van Riggelen J., zur Hausen H., Rösl F. Disturbance of tumor necrosis factor α-mediated β-interferon signaling in cervical carcinoma cells. J. Virol. 2002;76:280–291. doi: 10.1128/JVI.76.1.280-291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butz K., Hoppe-Seyler F. Transcriptional control of human papillomavirus (HPV) oncogene expression: composition of the HPV type 18 upstream regulatory region. J. Virol. 1993;67:6476–6486. doi: 10.1128/jvi.67.11.6476-6486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quitschke W. W., Lin Z. Y., DePonti-Zilli L., Paterson B. M. The β-actin promoter. High levels of transcription depend upon a CCAAT binding factor. J. Biol. Chem. 1989;264:9539–9546. [PubMed] [Google Scholar]
- 29.Rösl F., Schwarz E. Regulation of E6 and E7 oncogene transcription. In: Tommasino M., editor. Papillomaviruses in Human Cancer. Heidelberg: Springer Verlag; 1997. pp. 25–70. [Google Scholar]
- 30.von Knebel-Döberitz M., Oltersdorf T., Schwarz E., Gissmann L. Correlation of modified human papillomavirus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res. 1988;48:3780–3786. [PubMed] [Google Scholar]
- 31.Imamura K., Ogura T., Kishimoto A., Kaminishi M., Esumi H. Cell cycle regulation via p53 phosphorylation by a 5′-AMP activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, in a human hepatocellular carcinoma cell line. Biochem. Biophys. Res. Commun. 2001;287:562–567. doi: 10.1006/bbrc.2001.5627. [DOI] [PubMed] [Google Scholar]
- 32.Jones R. G., Plas D. R., Kubek S., Buzzai M., Mu J., Xu Y., Birnbaum M. J., Thompson C. B. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Kyriakis J. M. At the crossroads: AMP-activated kinase and the LKB1 tumor suppressor link cell proliferation to metabolic regulation. J. Biol. 2003;2:26. doi: 10.1186/1475-4924-2-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiainen M., Ylikorkala A., Makela T. P. Growth suppression by LKB1 is mediated by a G(1) cell cycle arrest. Proc. Natl. Acad. Sci. U.S.A. 1999;66:9248–9251. doi: 10.1073/pnas.96.16.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurley R. L., Anderson K. A., Franzone J. M., Kemp B. E., Means A. R., Witters L. A. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J. Biol. Chem. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 36.Kang H. T., Hwang E. S. 2-Deoxyglucose: an anticancer and antiviral therapeutic, but not any more a low glucose mimetic. Life Sci. 2006;78:1392–1399. doi: 10.1016/j.lfs.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Zwerschke W., Mazurek S., Massimi P., Banks L., Eigenbrodt E., Jansen-Dürr P. Modulation of type M2 pyruvate kinase activity by the human papillomavirus type 16 E7 oncoprotein. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1291–1296. doi: 10.1073/pnas.96.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwerschke W., Mannhardt B., Massimi P., Nauenburg S., Pim D., Nickel W., Banks L., Reuser A. J., Jansen-Dürr P. Allosteric activation of acid α-glucosidase by the human papillomavirus E7 protein. J. Biol. Chem. 2000;275:9534–9541. doi: 10.1074/jbc.275.13.9534. [DOI] [PubMed] [Google Scholar]
- 39.Weigert C., Sauer U., Brodbeck K., Pfeiffer A., Haring H. U., Schleicher E. D. AP-1 proteins mediate hyperglycemia-induced activation of the human TGF-β1 promoter in mesangial cells. J. Am. Soc. Nephrol. 2000;11:2007–2016. doi: 10.1681/ASN.V11112007. [DOI] [PubMed] [Google Scholar]
- 40.Eferl R., Wagner E. F. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 41.Soto U., Das B. C., Lengert M., Finzer P., zur Hausen H., Rösl F. Conversion of HPV 18 positive non-tumourigenic HeLa-fibroblast hybrids to invasive growth involves loss of TNF-α mediated repression of viral transcription and modification of the AP-1 transcription complex. Oncogene. 1999;18:3187–3198. doi: 10.1038/sj.onc.1202765. [DOI] [PubMed] [Google Scholar]
- 42.King T. D., Song L., Jope R. S. AMP-activated protein kinase (AMPK) activating agents cause dephosphorylation of Akt and glycogen synthase kinase-3. Biochem. Pharmacol. 2006;71:1637–1647. doi: 10.1016/j.bcp.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 44.Stambolic V., Ruel L., Woodgett J. R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 45.Chan T. A., Hwang P. M., Hermeking H., Kinzler K. W., Vogelstein B. Cooperative effects of genes controlling the G(2)/M checkpoint. Genes Dev. 2000;14:1584–1588. [PMC free article] [PubMed] [Google Scholar]
- 46.Munoz-Pinedo C., Ruiz-Ruiz C., Ruiz de Almodovar C., Palacios C., Lopez-Rivas A. Inhibition of glucose metabolism sensitizes tumor cells to death receptor-triggered apoptosis through enhancement of death-inducing signaling complex formation and apical procaspase-8 processing. J. Biol. Chem. 2003;278:12759–12768. doi: 10.1074/jbc.M212392200. [DOI] [PubMed] [Google Scholar]
- 47.Alessi D. R., Sakamoto K., Bayascas J. R. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 2006;75:137–163. doi: 10.1146/annurev.biochem.75.103004.142702. [DOI] [PubMed] [Google Scholar]
- 48.Lin X., Zhang F., Bradbury C. M., Kaushal A., Li L., Spitz D. R., Aft R. L., Gius D. 2-Deoxy-D-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Res. 2003;63:3413–3417. [PubMed] [Google Scholar]
- 49.Hwang J. T., Ha J., Park O. J. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun. 2005;332:433–440. doi: 10.1016/j.bbrc.2005.04.143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.