Abstract
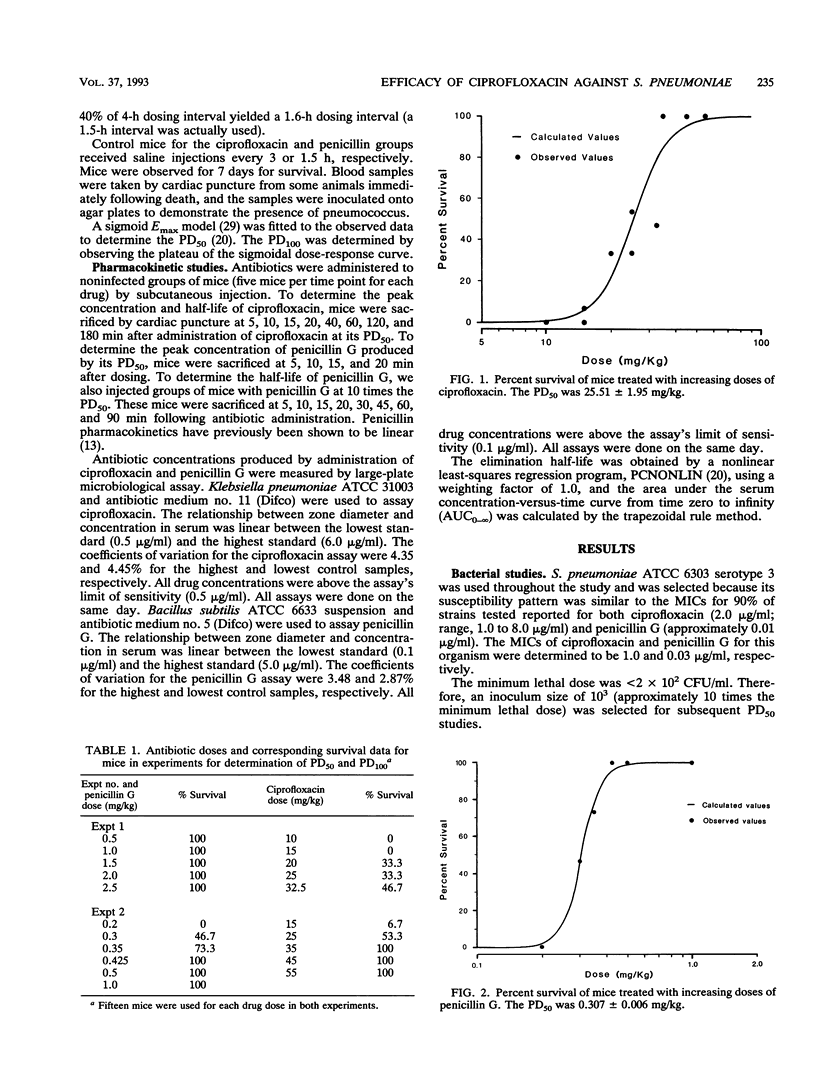
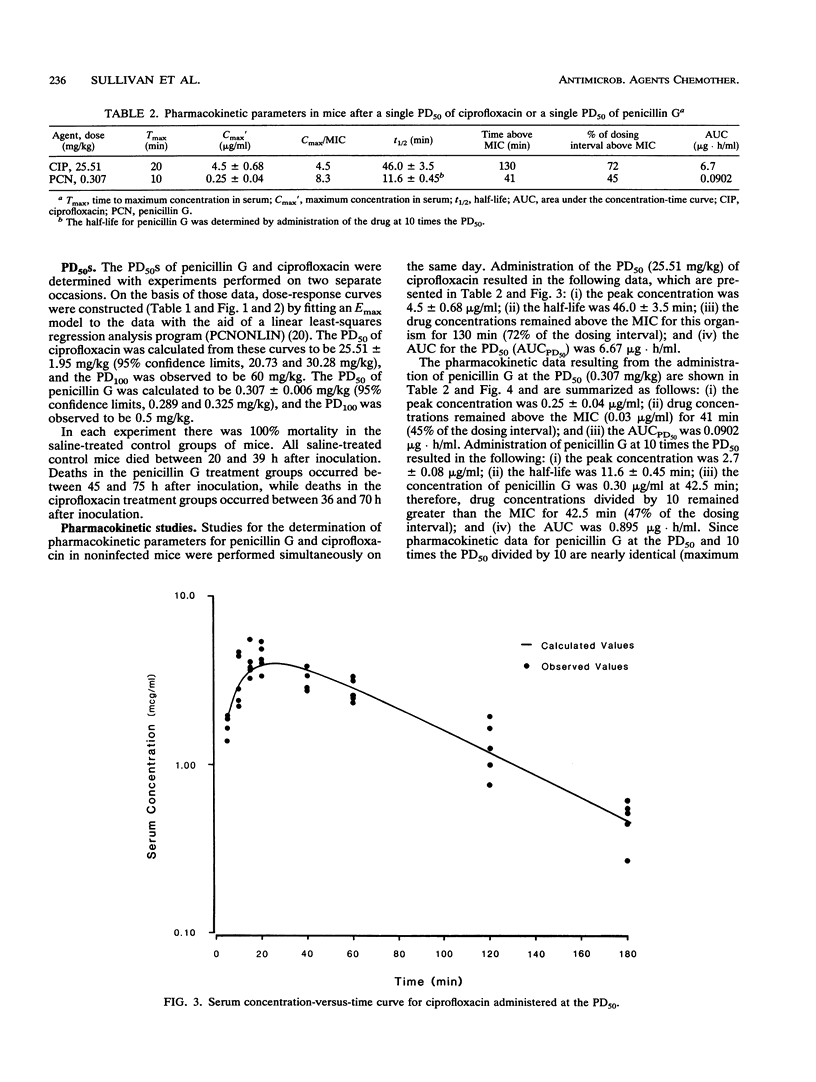
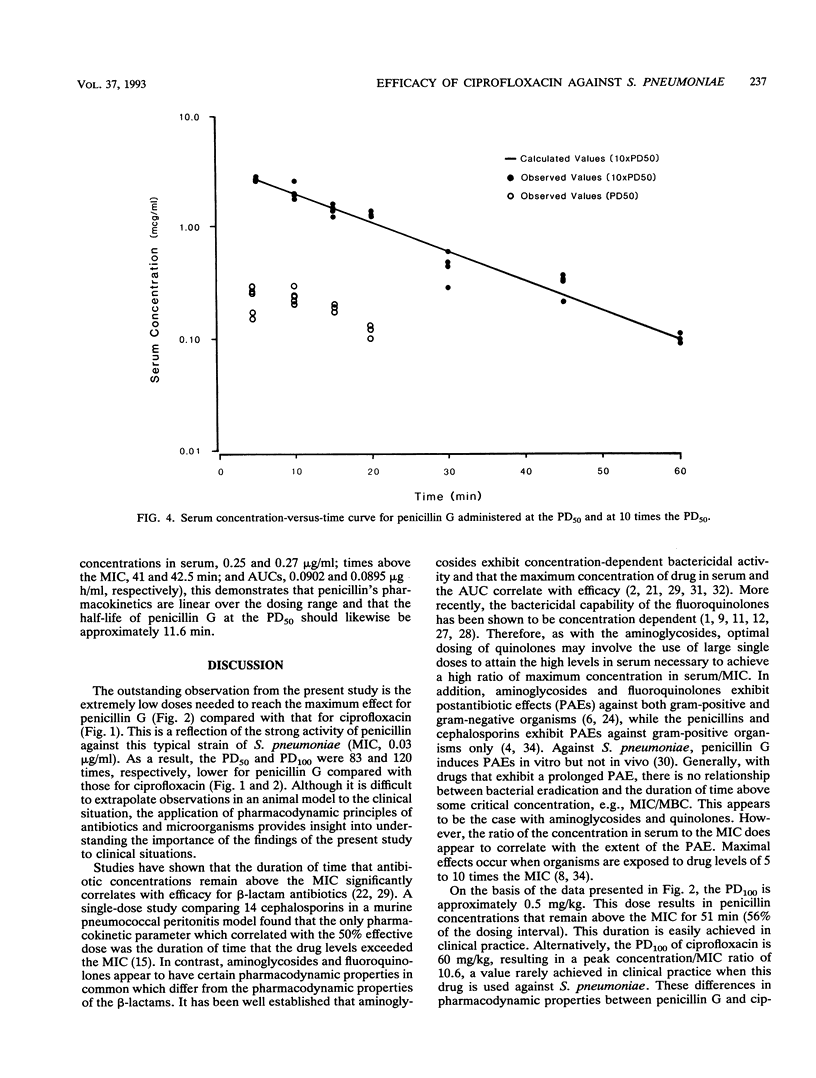
A mouse protection model was used to investigate the association of the pharmacokinetics and pharmacodynamics with the in vivo efficacy of ciprofloxacin compared with that of penicillin G in the treatment of mice infected with Streptococcus pneumoniae ATCC 6303. Mice were inoculated intraperitoneally with 10 times the minimum lethal dose of S. pneumoniae. For determination of the 50% protective dose, subcutaneous antibiotics were begun 1 h after infection and were continued for 24 h. The 50% protective doses of ciprofloxacin and penicillin G were 25.52 +/- 1.95 and 0.307 +/- 0.006 mg/kg of body weight, respectively, an 83-fold difference in efficacy. For 100% protection with penicillin G, the time that the drug concentration needed to remain above the MIC was 51 min, a value easily achieved in most clinical situations. For 100% protection with ciprofloxacin, the peak concentration/MIC ratio must reach a value of 10.6. This ratio is rarely achieved with this drug against S. pneumoniae in clinical practice. These pharmacodynamic differences probably contribute to the reported differences in clinical success between these agents.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaser J., Stone B. B., Groner M. C., Zinner S. H. Comparative study with enoxacin and netilmicin in a pharmacodynamic model to determine importance of ratio of antibiotic peak concentration to MIC for bactericidal activity and emergence of resistance. Antimicrob Agents Chemother. 1987 Jul;31(7):1054–1060. doi: 10.1128/aac.31.7.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaser J., Stone B. B., Zinner S. H. Efficacy of intermittent versus continuous administration of netilmicin in a two-compartment in vitro model. Antimicrob Agents Chemother. 1985 Mar;27(3):343–349. doi: 10.1128/aac.27.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner K., Höffken G., Lode H., Koeppe P., Prinzing C., Glatzel P., Wiley R., Olschewski P., Sievers B., Reinitz D. Pharmacokinetics of ciprofloxacin in healthy volunteers after oral and intravenous administration. Eur J Clin Microbiol. 1986 Apr;5(2):179–186. doi: 10.1007/BF02013983. [DOI] [PubMed] [Google Scholar]
- Bundtzen R. W., Gerber A. U., Cohn D. L., Craig W. A. Postantibiotic suppression of bacterial growth. Rev Infect Dis. 1981 Jan-Feb;3(1):28–37. doi: 10.1093/clinids/3.1.28. [DOI] [PubMed] [Google Scholar]
- Campoli-Richards D. M., Monk J. P., Price A., Benfield P., Todd P. A., Ward A. Ciprofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1988 Apr;35(4):373–447. doi: 10.2165/00003495-198835040-00003. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Post-antibiotic suppressive effect of ciprofloxacin against gram-positive and gram-negative bacteria. Am J Med. 1987 Apr 27;82(4A):58–62. [PubMed] [Google Scholar]
- Cooper B., Lawlor M. Pneumococcal bacteremia during ciprofloxacin therapy for pneumococcal pneumonia. Am J Med. 1989 Oct;87(4):475–475. doi: 10.1016/s0002-9343(89)80838-1. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Vogelman B. The postantibiotic effect. Ann Intern Med. 1987 Jun;106(6):900–902. doi: 10.7326/0003-4819-106-6-900. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C., Smith J. T. Nalidixic acid: an antibacterial paradox. Antimicrob Agents Chemother. 1975 Sep;8(3):251–261. doi: 10.1128/aac.8.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B. I., Maesen F. P., Baur C. Ciprofloxacin in the treatment of acute exacerbations of chronic bronchitis. Eur J Clin Microbiol. 1986 Apr;5(2):226–231. doi: 10.1007/BF02013995. [DOI] [PubMed] [Google Scholar]
- Dudley M. N., Mandler H. D., Gilbert D., Ericson J., Mayer K. H., Zinner S. H. Pharmacokinetics and pharmacodynamics of intravenous ciprofloxacin. Studies in vivo and in an in vitro dynamic model. Am J Med. 1987 Apr 27;82(4A):363–368. [PubMed] [Google Scholar]
- Ebert S. C., Leggett J., Vogelman B., Craig W. A. Evidence for a slow elimination phase for penicillin G. J Infect Dis. 1988 Jul;158(1):200–202. doi: 10.1093/infdis/158.1.200. [DOI] [PubMed] [Google Scholar]
- Frieden T. R., Mangi R. J. Inappropriate use of oral ciprofloxacin. JAMA. 1990 Sep 19;264(11):1438–1440. [PubMed] [Google Scholar]
- Frimodt-Møller N., Bentzon M. W., Thomsen V. F. Experimental infection with Streptococcus pneumoniae in mice: correlation of in vitro activity and pharmacokinetic parameters with in vivo effect for 14 cephalosporins. J Infect Dis. 1986 Sep;154(3):511–517. doi: 10.1093/infdis/154.3.511. [DOI] [PubMed] [Google Scholar]
- Gisby J., Wightman B. J., Beale A. S. Comparative efficacies of ciprofloxacin, amoxicillin, amoxicillin-clavulanic acid, and cefaclor against experimental Streptococcus pneumoniae respiratory infections in mice. Antimicrob Agents Chemother. 1991 May;35(5):831–836. doi: 10.1128/aac.35.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M. A., Uribe F., Moisen S. D., Fuster A. P., Selen A., Welling P. G., Painter B. Multiple-dose pharmacokinetics and safety of ciprofloxacin in normal volunteers. Antimicrob Agents Chemother. 1984 Nov;26(5):741–744. doi: 10.1128/aac.26.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogkamp-Korstanje J. A., Klein S. J. Ciprofloxacin in acute exacerbations of chronic bronchitis. J Antimicrob Chemother. 1986 Sep;18(3):407–413. doi: 10.1093/jac/18.3.407. [DOI] [PubMed] [Google Scholar]
- Höffken G., Lode H., Prinzing C., Borner K., Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985 Mar;27(3):375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D., Lietman P. S., Smith C. R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987 Jan;155(1):93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- Mordenti J. J., Quintiliani R., Nightingale C. H. Combination antibiotic therapy: comparison of constant infusion and intermittent bolus dosing in an experimental animal model. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):313–321. doi: 10.1093/jac/15.suppl_a.313. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Kumada T., Chin N. X., Mandell W. The post-antimicrobial suppressive effect of quinolone agents. Drugs Exp Clin Res. 1987;13(2):63–67. [PubMed] [Google Scholar]
- Nix D. E., DeVito J. M. Ciprofloxacin and norfloxacin, two fluoroquinolone antimicrobials. Clin Pharm. 1987 Feb;6(2):105–117. [PubMed] [Google Scholar]
- Plaisance K. I., Drusano G. L., Forrest A., Bustamante C. I., Standiford H. C. Effect of dose size on bioavailability of ciprofloxacin. Antimicrob Agents Chemother. 1987 Jun;31(6):956–958. doi: 10.1128/aac.31.6.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T. The mode of action of 4-quinolones and possible mechanisms of resistance. J Antimicrob Chemother. 1986 Nov;18 (Suppl 500):21–29. doi: 10.1093/jac/18.supplement_d.21. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Craig W. A. Kinetics of antimicrobial activity. J Pediatr. 1986 May;108(5 Pt 2):835–840. doi: 10.1016/s0022-3476(86)80754-5. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Leggett J., Turnidge J., Ebert S., Craig W. A. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J Infect Dis. 1988 Oct;158(4):831–847. doi: 10.1093/infdis/158.4.831. [DOI] [PubMed] [Google Scholar]
- Vogelman B., Gudmundsson S., Turnidge J., Leggett J., Craig W. A. In vivo postantibiotic effect in a thigh infection in neutropenic mice. J Infect Dis. 1988 Feb;157(2):287–298. doi: 10.1093/infdis/157.2.287. [DOI] [PubMed] [Google Scholar]
- Williams P. J., Hull J. H., Sarubbi F. A., Rogers J. F., Wargin W. A. Factors associated with nephrotoxicity and clinical outcome in patients receiving amikacin. J Clin Pharmacol. 1986 Feb;26(2):79–86. doi: 10.1002/j.1552-4604.1986.tb02910.x. [DOI] [PubMed] [Google Scholar]
- Wollschlager C. M., Raoof S., Khan F. A., Guarneri J. J., LaBombardi V., Afzal Q. Controlled, comparative study of ciprofloxacin versus ampicillin in treatment of bacterial respiratory tract infections. Am J Med. 1987 Apr 27;82(4A):164–168. [PubMed] [Google Scholar]
- Zhanel G. G., Hoban D. J., Harding G. K. The postantibiotic effect: a review of in vitro and in vivo data. DICP. 1991 Feb;25(2):153–163. doi: 10.1177/106002809102500210. [DOI] [PubMed] [Google Scholar]


